Abstract
Aims
The MiniMed 670 G insulin pump system is the first commercially available hybrid closed-loop (HCL) insulin delivery system and clinical studies have shown that this device is associated with incremental benefits in glycemic control relative to continuous subcutaneous insulin infusion (CSII) with or without continuous glucose monitoring (CGM). The aim was to evaluate the long-term cost-effectiveness of the MiniMed 670 G system versus CSII alone in people with type 1 diabetes (T1D) in the UK.
Materials and methods
Cost-effectiveness analysis was performed using the IQVIA CORE Diabetes Model. Clinical input data were sourced from a clinical trial of the MiniMed 670 G system in 124 adults and adolescents with T1D. The analysis was performed over a lifetime time horizon and both future costs and clinical outcomes were discounted at 3.5% per annum. The analysis was performed from a healthcare payer perspective.
Results
The use of the MiniMed 670 G system led to an improvement in quality-adjusted life expectancy of 1.73 quality-adjusted life years (QALYs), relative to CSII. Total lifetime direct costs were GBP 35,425 higher with the MiniMed 670 G system than with CSII resulting in an incremental cost-effectiveness ratio (ICER) of GBP 20,421 per QALY gained. Sensitivity analyses revealed that the ICER was sensitive to assumptions around glycemic control and assumptions relating to the quality-of-life benefit associated with a reduction in fear of hypoglycemia.
Limitations
Long-term projections from short-term data are inherently associated with uncertainty but represent arguably the best available evidence in lieu of long-term clinical trials.
Conclusions
In the UK, over patient lifetimes, the incremental clinical benefits associated with the use of MiniMed 670 G system means that it is likely to be cost-effective relative to the continued use of CSII in people with T1D, particularly for those with a fear of hypoglycemia or poor baseline glycemic control.
Introduction
The worldwide burden of type 1 diabetes is substantial, with an estimated 600,900 children aged under 15 years and approximately 1,110,100 adolescents aged under 20 years living with the disease in 2019Citation1. Moreover, global incidence rates are high and increasing, with approximately 98,200 children aged under 15 years and 128,900 adolescents aged under 20 years developing the disease annually, and the UK has one of the largest numbers of new cases annually in the 0–14-year age group in EuropeCitation1. The global economic burden associated with diabetes is also significant, with an estimated burden of USD 1.3 trillion in 2015 increasing to USD 2.1 trillion in the best-case target scenarios by 2030Citation2. In the UK, the National Health Service (NHS) spending on type 1 diabetes is approximately GBP 1 billion per year, an estimated 80% of which is attributable to the treatment and management of short- and long-term diabetes-related complicationsCitation3. These complications, as well as type 1 diabetes itself, can also have a considerable impact on patients’ quality of lifeCitation4,Citation5. As healthcare systems come under increasing clinical and budgetary strain, cost-effective interventions that prove efficacious for patients while providing value for money for the healthcare payer are vital.
The ultimate treatment goal in type 1 diabetes is to mimic the normal physiologic action of the pancreas and production of insulin as closely as possible, such that blood glucose levels are maintained in the euglycemic range. This also means recognizing and correcting hyper- and hypoglycemia as quickly as possible. Self-injection of multiple daily insulins (MDI) represents traditional therapy for type 1 diabetes but is associated with a substantial self-management burden to ensure efficacy in terms of glycemic control and avoidance of hypoglycemia. Continuous advances both in terms of the recombinant insulins available and insulin delivery technology such as continuous subcutaneous insulin infusion (CSII) and subsequently sensor-augmented pump therapy (SAP) and hybrid closed-loop (HCL) systems have resulted in progressive step-wise improvements in the management of type 1 diabetes, including improved glycemic control, reduced glycemic variability and increased proportion of time spent in the euglycemic range (time in range [TIR])Citation6–8. In turn, improved glycemic control has been shown to reduce the risk for long-term micro- and macrovascular complications in landmark studies, with TIR also associated with a lower risk for diabetic retinopathyCitation9–11. However, for some people, hypoglycemia or large fluctuations in blood glucose levels may remain problematic. Moreover, anxiety over the potential sequelae of severe hypoglycemia can have a pronounced detrimental effect on quality of lifeCitation12. Despite considerable advances in therapy, the life expectancy for people with type 1 diabetes is still around 10 years below that of the general populationCitation13, suggesting that there may still be improvements that can be made in terms of helping people achieve glycemic targets while minimizing the risk of hypoglycemic events in doing so.
The MiniMedFootnotei 670 G insulin pump system was launched in 2017 and is the first commercially available HCL system, adjusting basal insulin delivery every 5 min according to real-time continuous glucose monitoring (CGM) data and a corresponding algorithm, and has been shown to provide further incremental benefits relative to CSII with or without CGMCitation14. A pivotal before and after trial of the MiniMed 670 G system, conducted in 124 adults and adolescents with type 1 diabetes showed that, over 3 months, HbA1c improved significantly from baseline and the proportion of TIR (defined as blood glucose >70–180 mg/dL) increased significantly in both adults and adolescentsCitation14. Trial results were mirrored by real-world findings, which showed that, relative to a 2-week run-in baseline period (using the MiniMed 670 G system in Manual Mode), use of the MiniMed 670 G system in Auto Mode (i.e. with automated basal insulin delivery) was associated with reduced mean glucose values and increased the proportion of TIR from 66.0% to 73.3% (p < .001)Citation15. Moreover, Duffus et al. recently demonstrated that with the MiniMed 670 G system, each 8.6-hour daily increase in time spent in Auto Mode was associated with a 5% increase in TIRCitation8.
Guidance from the National Institute for Health and Care Excellence (NICE) as well as the Association of British Clinical Diabetologists advocates that insulin pumps should be offered to all people with type 1 diabetes who have HbA1c >8.5% (69 mmol/mol) despite optimization of MDI therapy or who experience “disabling hypoglycemia” (defined as “repeated and unpredictable occurrence of hypoglycemia that results in persistent anxiety about recurrence and is associated with a significant adverse effect on the quality of life [QoL]”)Citation16,Citation17. Despite these recommendations, insulin pump use among people with type 1 diabetes is low relative to the US and other Western European countriesCitation18. In England, in 2017–18, the National Diabetes Insulin Pump Audit revealed that the use of insulin pumps in individuals with type 1 diabetes varied from <5% to >40% between individual centersCitation19. The Audit also noted that more people in England and Wales should be considered for alternative therapies such as insulin pumps when the need arisesCitation19. The MiniMed 670 G system recently became available in the UK and represents one of a number of insulin pumps currently available, but data on device uptake are lacking and the acquisition costs of novel devices may represent a barrier to uptake. However, given the benefits in glycemic control and reductions in hypoglycemia these devices can offer, initial costs associated with such interventions may be offset by the reduced risk of costly short- and long-term diabetes-related complications.
The aim of the present study was therefore to assess the long-term clinical and economic outcomes associated with switching from CSII to the MiniMed 670 G system and evaluate the cost-effectiveness of such an approach in the UK.
Methods
Model structure
Long-term cost-effectiveness analysis was performed using the IQVIA CORE Diabetes Model (CDM; IQVIA, Basel, Switzerland)Citation20–22. The model was chosen as it represents one of the most widely used, published and validated health economic models currently available for modelling the long-term outcomes of diabetesCitation21,Citation22. The CDM is a non-product-specific model that can be used to project long-term outcomes in type 1 or type 2 diabetes. Structurally, the CDM consists of a series of inter-dependent sub-models that simulate disease progression and diabetes-related complications including cardiovascular, renal, and ophthalmic complications, as well as neuropathy, foot ulcer and amputation. Long-term outcomes include life expectancy, quality-adjusted life expectancy, time-to-onset of diabetes-related complications, direct and indirect costs and incremental cost-effectiveness ratios (ICERs).
Cohort and treatment effects
Baseline demographics and characteristics of the simulated patient cohort were sourced from a single-arm non-randomized trial of the MiniMed 670 G system in 124 adults and adolescents with type 1 diabetes ()Citation14,Citation23. For inclusion in the trial, participants were required to have been using CSII (either with or without CGM) for at least 6 months prior to enrollment. The trial consisted of an initial 2-week run-in period during which all trial participants used the device in Manual Mode, wherein basal insulin delivery rates are pre-programmed. Following this, participants used the device in Auto Mode for the remaining 3-month study period. In Auto Mode basal insulin delivery is automatically adjusted every 5 min based on real-time CGM readings and glucose target levels were set at 120 mg/dL (although a temporary target of 150 mg/dL could be set during exercise).
Table 1. Baseline cohort characteristics.
By the end of the 3-month study period, mean HbA1c had decreased from 7.4% to 6.9% (a decrease of 0.5%)Citation14,Citation23. No severe hypoglycemic events (SHEs) either requiring medical assistance or not requiring medical assistance, and no ketoacidosis events were reported in people using the MiniMed 670 G system. Therefore, the event rates for SHEs requiring medical assistance, SHEs not requiring medical assistance and ketoacidosis events were set to 0 events per 100 person-years. In the CSII arm, the rate of SHEs requiring medical assistance was assumed to be 25 events per 100 patient years and the rate of SHEs not requiring medical assistance was assumed to be 65 events per 100 patient-years. The overall event rate of SHEs was sourced from Swedish data from a multinational study, wherein the total event rate in people with T1D was 90 per 100 patient yearsCitation24. Of these, it was assumed that 28% of events (i.e. 25 events per 100 patient-years) would require medical assistance, based on the findings of a Canadian study by Leiter et al.Citation25. In the CSII arm, the event rate for ketoacidosis was assumed to be 0 events per 100 patient-years.
Costs and utilities
Device acquisition costs for those using the MiniMed 670 G system, as well as for those using CSII, were based on UK list prices. Acquisition costs for the MiniMed 670 G system included the costs for the pump, cannula, reservoir, sensors (assuming the use of 52 sensors per year), and the Guardian Link 3 transmitter kit (one per year; including transmitter, charger, cleaning plugs, battery and serter). Costs associated with insulin pump training, insulin, yearly outpatient visits, insulin and self-monitoring of blood glucose (SMBG) were also included for both CSII and the MiniMed 670 G system. For both therapies, an SMBG use of 3.5 strips per day was assumed. Daily bolus insulin use was also assumed to be the same in both treatments. Direct costs for diabetes-related complications were sourced from the NICE clinical guideline NG17Citation26, NHS reference costsCitation27 and other published studies ()Citation28,Citation29. Where necessary, costs were inflated to 2018 GBP.
Table 2. Direct costs of diabetes-related complications.
Disutilities associated with diabetes-related complications and hypoglycemic events were sourced from published literatureCitation30–32. In the MiniMed 670 G system arm, a utility benefit associated with reduced fear of hypoglycemia (FoH) was incorporated into the analysis. Specifically, an annual benefit of 0.0552 was applied based on a reduction of 6.9 units on the Hypoglycemia Fear Survey (HFS) reported for people using sensor-augmented insulin pump therapy in the INTERPRET studyCitation33,Citation34as well as an earlier study reporting that a 1 unit increase in HFS score corresponded to a utility decrement of 0.008Citation12.
Sensitivity analyses
A series of one-way sensitivity analyses were performed to examine key determinants of cost-effectiveness. Firstly, the influence of baseline HbA1c was assessed in a subgroup of people with HbA1c ≥7.5% at baseline (mean HbA1c in this subgroup was 8.23% and reduction in HbA1c with MiniMed 670 G HCL system use was 0.97%). A further analysis was performed in which no reduction in HbA1c with the MiniMed 670 G system was assumed. Sensitivity analyses were also performed around the reduction in hypoglycemic events associated with the MiniMed 670 G system and also around the utility benefit associated with reduced FoH. Specifically, analyses were performed in which the hypoglycemic event rate in both arms was the same (i.e. no reduction in hypoglycemic event rate with the MiniMed 670 G system was assumed), and in which the reduction in hypoglycemic event rate with the MiniMed 670 G system was 50% of that assumed in the base case analysis. To investigate the effect of the QoL benefit associated with reduced FoH, one analysis was performed in which the utility benefit was reduced to 0.0184 (based on data published by Yeh et al.Citation35) and a second sensitivity analysis was performed in which the QoL benefit was entirely abrogated. Sensitivity analyses were also performed around treatment costs, time horizon and discount rate.
Time horizon, discount rate and perspective
The analysis was performed from the perspective of the health care payer (UK NHS). Future costs and clinical outcomes were discounted at a rate of 3.5% per annum in line with UK guidanceCitation36. The time horizon used in the analysis was that of patients’ lifetimes.
Results
In the base case analysis people in the MiniMed 670 G system arm were projected to have a mean quality-adjusted life expectancy of 14.09 quality-adjusted life years (QALYs) compared with 12.36 QALYs for people on CSII, resulting in an incremental gain of 1.73 QALYs with the MiniMed 670 G system (). Total lifetime costs were higher for people in the MiniMed 670 G system arm (GBP 192,720 versus GBP 157,295 for people in the CSII arm), which was driven primarily by higher treatment costs in the MiniMed 670 G system arm (GBP 110,400 versus GBP 59,468). The projected improvement in quality-adjusted life expectancy combined with higher costs resulted in an ICER of GBP 20,421 per QALY gained. Analysis of the cost-effectiveness acceptability curve (constructed using data from a scatterplot of pairs of incremental costs and effects, wherein the proportion of data points below a given willingness-to-pay threshold is determined) showed that at a willingness-to-pay threshold of GBP 20,000 per QALY gained the likelihood of the MiniMed 670 G system being considered cost-effective relative to CSII was 45.1%; however, this figure increased to 99.8% at a willingness-to-pay threshold of GBP 30,000 per QALY gained ().
Figure 1. Cost-effectiveness acceptability curve for MiniMed 670 G system versus CSII. Abbreviations. CSII, Continuous subcutaneous insulin infusion; QALY, Quality-adjusted life-year.
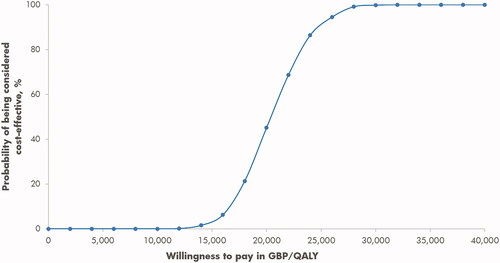
Table 3. Summary findings from the base case analysis.
The improved glycemic control associated with the use of the MiniMed 670 G system was projected to delay the onset of diabetes-related complications relative to CSII. The most pronounced delays included those reported for neuropathy and microalbuminuria, the onset of which was projected to be delayed by a mean of 2.70 years and 2.34 years, respectively, for the MiniMed 780 G system versus CSII.
Sensitivity analyses revealed that the cost-effectiveness of the MiniMed 670 G system was sensitive to changes in assumptions relating to the QoL benefit associated with reduced FoH and the reduction in hypoglycemic event rate as well as baseline HbA1c. In the base case analysis, a utility benefit of 0.0552 was assumed. If this was reduced to 0.0184, quality-adjusted life expectancy in the MiniMed 670 G system arm was reduced to 13.39 QALYs, compared with 14.09 QALYs in the base case, which resulted in the ICER increasing to GBP 36,316 per QALY gained (). Similarly, if the QoL benefit was entirely abrogated, quality-adjusted life expectancy was reduced further to 13.04 QALYs and the ICER increased to GBP 55,012 per QALY gained. In terms of severe hypoglycemic events, if no reduction in hypoglycemia event rate was assumed with the MiniMed 670 G system, quality-adjusted life expectancy in this arm was reduced to 13.59 QALYs and the ICER subsequently increased to GBP 37,955 per QALY gained. The ICER was also influenced by baseline HbA1c. In a subgroup analysis in people with HbA1c ≥7.5% (mean HbA1c 8.23%) the ICER was reduced to GBP 12,892 per QALY gained. Findings were also sensitive, although to a lesser extent, to changes in treatment costs and the time horizon of the analysis (). In particular, as diabetes is a long-term progressive disease, clinical benefits were most pronounced over longer time horizons.
Table 4. Summary findings of sensitivity analyses.
Discussion
The findings of the current analysis suggest that in the UK, over a long-term time horizon, the use of the MiniMed 670 G system in people with type 1 diabetes is likely to be considered cost-effective relative to CSII. In the base case analysis, the ICER for the MiniMed 670 G system relative to CSII was GBP 20,421 per QALY gained, which is in line with the commonly cited threshold range for the UK of GBP 20,000–30,000 per QALY gainedCitation37. The findings of the present analysis concur with those of another similar analysis conducted for the Swedish setting. Jendle et al.Citation38 also utilized clinical input data from Garg et al.Citation14 and Bergenstal et al.Citation23 and reported that in Sweden long-term use of the MiniMed 670 G system was associated with an ICER of SEK 164,236 per QALY gained (EUR 15,296 per QALY gained using August 2019 exchange rates) compared with CSII. However, it should be noted that the present analysis was performed from the payer perspective and does not capture the potential economic benefits associated with any impact on indirect costs such as lost productivity due to long-term complications or hypoglycemic events. Moreover, the present analysis did not evaluate the budget impact of introducing an HCL device, and future studies elucidating this may help to further inform healthcare decision-makers—particularly with regards to additional funding for diabetes technologies being achieved through greater use of more affordable biosimilar insulins in the UK.
The findings of the sensitivity analyses indicated that baseline HbA1c and the QoL benefit associated with reduced FoH were key determinants of outcomes. In a subgroup analysis of people with a mean baseline HbA1c of 8.23%, the ICER was reduced GBP 12,892 per QALY gained. Figures from the 2017–2018 UK National Diabetes Insulin Pump Audit report indicated that, in England and Wales, 34.4% of people with type 1 diabetes using an insulin pump achieve HbA1c ≤7.5% (compared with 27.0% people not using an insulin pump)Citation19 which suggests that some people may benefit from the further optimization of therapy that switching to a HCL system could provide. The rationale for the inclusion of a utility for reduced FoH with the 670 G system was based on published evidence, indicating that FoH is relatively common in people with type 1 diabetes and even extending to the parents of children with type 1 diabetesCitation39. As well as having a psychological impact, FoH has also been reported as being a barrier to an active lifestyle for children with type 1 diabetesCitation40. Although FoH may be most pronounced for SHEs owing to the severity of possible sequelae, even non-severe events can invoke a fear of future events. In one multinational survey, 39% of people who experienced a non-severe hypoglycemic event in the previous 4 weeks reported a high level of fear of future daytime hypoglycemic events, and 50% reported a high level of fear of future nocturnal hypoglycemic eventsCitation41. In the current analysis, the QoL benefit assumed for reduced FoH was based on data from the INTERPRET study of SAP published in 2013Citation33,Citation34. These findings were supported by a more recent UK-based study that showed that the use of CGM (with or without insulin pump use) significantly reduced FoH in both adolescents with type 1 diabetes and their parentsCitation42. The MiniMed 670 G system was the first approved automated insulin delivery system available. As such, the increased sophistication of features including the automated adjustment of basal insulin delivery and prevention of both high and low glucose levels can further alleviate FoH in some users. Moreover, the use of insulin pumps in people with type 1 diabetes where QoL is compromised due to FoH is in line with NICE recommendations, where insulin pumps are recommended for those with disabling hypoglycemia that results in “persistent anxiety about recurrence”Citation16. Given these factors, the use of a utility benefit in the MiniMed 670 G arm of the analysis was considered appropriate.
Additionally, evidence from surveys suggests that there is a high level of user satisfaction with HCL systems including the MiniMed 670 G system primarily due to improved blood glucose controlCitation43,Citation44. Users have also reported more intangible benefits including a reduction in anxiety and improved confidence with disease managementCitation44. Individual narratives from UK-based users of the MiniMed 670 G system include instances where users feel the device has resulted in increased independence and allowed them to feel “more normal”Citation45. In particular, within the context of helping to prevent nocturnal hypoglycemic events UK-based users/caregivers have described RT-CGM systems specifically as “fantastic,” a “no brainer” and “life-changing”Citation46,Citation47. Additionally, whilst the user experience of HCL systems has been very positive, some UK-based individuals have expressed frustration at the lack of uniformity in terms of NHS commissioning policy and funding for medical devices including CGM and insulin pumps and the fact that this represents a barrier to uptake. In one recent analysis limited to the use of CGM in the UK, around one-third of people using CGM (without or without an insulin pump) were self-fundingCitation46 and the proportion of people with type 1 diabetes using insulin pumps varies considerably between different treatment centersCitation19. Despite the strong clinical evidence documenting the clinical benefits of CSII and the further incremental benefits of SAP and HCL systems, which incorporate CGM, insulin pump use for some specialist centers is below 5%Citation19. While it is difficult to capture some of these intangible benefits in diabetes modelling, the present analysis provides evidence that, even without these benefits, further reimbursement of an HCL system would provide both improved health outcomes for patients and value for money for the NHS in the UK. These findings are of particular importance for pediatric and adolescent individuals with type 1 diabetes, for whom HCL systems could provide crucial benefits in terms of glycemic control and TIR shortly after diagnosis to avoid substantial morbidity and early mortality later in lifeCitation9–11.
A limitation of the study, inherent to all long-term cost-effectiveness analyses, was the uncertainty associated with long-term projections of short-term clinical data. That acknowledged, projections of short-term data are an essential tenet of long-term health economic modeling, and it remains one of the best available options for informing decision making in absence of long-term clinical trial data. Projection of outcomes over patients’ lifetimes is also recommended in guidelines for computer modeling of diabetes interventionsCitation48. In addition, uncertainty was limited by using a validated diabetes model and by conducting sensitivity analyses around model inputsCitation20–22. However, the clinical input data used to inform the modelling analysis were sourced from a non-randomized before-after study, and important parameters such as TIR, regarded in one recent online survey as having a “big impact” on daily life, were not incorporated (as the IQVIA CORE Diabetes Model does not contain such functionality)Citation49. However, data from randomized controlled trials are limited and the impact of increased TIR on QoL is still uncertain. Therefore, more data are needed to validate any association between the use of closed-loop systems, increased TIR and improved QoL. Indeed, although evidence is emerging to suggest that increased TIR may confer clinical and QoL benefits, the available studies are either observational, cross-sectional or post-hoc analyses of the DCCT dataset and long-term longitudinal data exploring the relationship between TIR and complications that could be utilized in health economic modeling studies are still lacking. Consequently, at this stage, the long-term implications of improved TIR are yet to be definitively established. Allied to this, the present analysis did not capture any clinical or economic benefits associated with any reduction in minor hypoglycemic events and the possible impact of increased TIR with using a closed-loop system on QoL and utilities. Although minor hypoglycemic events are not associated with medical resource utilization, and are therefore of limited concern from the perspective of the payer, they can have a negative impact on people’s day-to-day activities including school, work and sleepCitation50. As the impact of minor hypoglycemic events was not captured here, it is possible that the findings of the current analysis may be conservative.
Conclusions
The findings of the current analysis suggest that the use of the MiniMed 670 G system is associated with incremental clinical benefits relative to CSII and over a lifetime time horizon is likely to represent good value for money compared to CSII from the perspective of the UK NHS.
Transparency
Declaration of funding
Funding for this manuscript was provided by Medtronic International Trading Sàrl, Tolochenaz, Switzerland.
Declaration of financial/other relationships
SR is a current employee of Vyoo Agency, which has received consulting fees from Medtronic.
MIB, ZO, SdP and OC are current employees of Medtronic, which manufactures the MiniMed 670 G insulin pump system.
JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Author contributions
All authors contributed equally to the conception and design of this study.
SR performed the analysis and interpreted the output data.
All authors contributed to the drafting of the paper and revising it critically for intellectual content.
Previous presentations
The results of this manuscript have not been previously presented elsewhere.
Acknowledgements
Editorial assistance was provided by Ossian Health Economics and Communications, Basel, Switzerland.
Notes
i MiniMed is a trademark of Medtronic, Northridge, CA, USA.
References
- Patterson CC, Karuranga S, Salpea P, et al. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: results from the international diabetes federation diabetes Atlas. Diabetes Res Clin Pract. 2019;157:107842.
- Bommer C, Sagalova V, Heesemann E, et al. Global economic burden of diabetes in adults: projections from 2015 to 2030. Dia Care. 2018;41(5):963–970.
- National Institute for Health Research Horizon Scanning Research and Intelligence Centre. MiniMed 670G Hybrid closed loop system for type 1 diabetes. 2017. Available at: http://www.io.nihr.ac.uk/wp-content/uploads/migrated/MiniMed-670G-HCL-for-type-1-diabetes-FINAL.pdf [cited 2020 Oct 19]
- Raymakers AJN, Gillespie P, O’Hara MC, et al. Factors influencing health-related quality of life in patients with type 1 diabetes. Health Qual Life Outcomes. 2018;16(1):27.
- Rosner B, Roman-Urrestarazu A. Health-related quality of life in paediatric patients with type 1 diabetes mellitus using insulin infusion systems. A systematic review and meta-analysis. PLOS One. 2019;14(6):e0217655.
- Akturk HK, Giordano D, Champakanath A, et al. Long-term real-life glycaemic outcomes with a hybrid closed-loop system compared with sensor-augmented pump therapy in patients with type 1 diabetes. Diabetes Obes Metab. 2020;22(4):583–558.
- Lepore G, Scaranna C, Corsi A, et al. Switching from suspend-before-low insulin pump technology to a hybrid closed-loop system improves glucose control and reduces glucose variability: a retrospective observational case-control study. Diabetes Technol Ther. 2020;22(4):321–325.
- Duffus SH, Ta’ani ZA, Slaughter JC, et al. Increased proportion of time in hybrid closed-loop "auto mode" is associated with improved glycaemic control for adolescent and young patients with adult type 1 diabetes using the MiniMed 670G insulin pump. Diabetes Obes Metab. 2020;22(4):688–693.
- Nathan DM. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Dia Care. 2014;37(1):9–16.
- Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Mortality in type 1 diabetes in the DCCT/EDIC versus the general population. Diabetes Care. 2016;39(8):1378–1383.
- Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Dia Care. 2019;42(3):400–405.
- Currie CJ, Morgan CL, Poole CD, et al. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin. 2006;22(8):1523–1534.
- Huo L, Harding JL, Peeters A, et al. Life expectancy of type 1 diabetic patients during 1997–2010: a national Australian registry-based cohort study. Diabetologia. 2016;59(6):1177–1185.
- Garg SK, Weinzimer SA, Tamborlane WV, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2017;19(3):155–163.
- Stone MP, Agrawal P, Chen X, et al. Retrospective analysis of 3-month real-world glucose data after the minimed 670g system commercial launch. Diabetes Technol Ther. 2018;20(10):689–692.
- National Institute for Health and Care Excellence. Technology appraisal guidance TA151. Continuous subcutaneous insulin infusion for the treatment of diabetes mellitus. Available at: https://www.nice.org.uk/guidance/ta151/chapter/1-Guidance [cited 2020 Oct 19].
- Association of British Clinical Diabetologists. Standards of care for management of adults with type 1 diabetes. 2016. Available at: http://www.diabetologists-abcd.org.uk/Position_Papers/Type_1_standards_of_care.pdf [cited 2020 Oct 19].
- Sherr JL, Hermann JM, Campbell F, et al. T1D exchange clinic network, the DPV initiative, and the national paediatric diabetes audit and the royal college of paediatrics and child health registries. Use of insulin pump therapy in children and adolescents with type 1 diabetes and its impact on metabolic control: comparison of results from three large, transatlantic paediatric registries. Diabetologia. 2016;59(1):87–91.
- National Diabetes Insulin Pump Audit. 2017–2018. England and Wales, 8 August 2019. Available at: https://files.digital.nhs.uk/DF/2ACADD/National%20Diabetes%20Insulin%20Pump%20Audit%202017-18%20Report.pdf [cited 2020 Oct 19].
- Palmer AJ, Roze S, Valentine WJ, et al. The CORE diabetes model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20(sup1):S5–S26.
- Palmer AJ, Roze S, Valentine WJ, et al. Validation of the CORE diabetes model against epidemiological and clinical studies. Curr Med Res Opin. 2004;20(Suppl 1):S27–S40.
- McEwan P, Foos V, Palmer JL, et al. Validation of the IMS CORE diabetes model. Value Health. 2014;17(6):714–724.
- Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316(13):1407–1408.
- Östenson CG, Geelhoed-Duijvestijn P, Lahtela J, et al. Self-reported non-severe hypoglycaemic events in Europe. Diabet Med. 2014;31(1):92–101.
- Leiter LA, Yale J-F, Chiasson J-L, et al. Assessment of the impact of fear of hypoglycemic episodes on glycemic and hypoglycemia management. Can J Diabetes. 2005;29:186–192.
- National Institute for Health and Care Excellence. National Clinical Guideline Center. 2015. Clinical guideline NG17, type 1 diabetes in adults: diagnosis and management. Available at: https://www.nice.org.uk/guidance/ng17/evidence/appendices-hu-pdf-435400240 [cited 2020 Oct 19].
- NHS reference costs. 2020. Available at: https://improvement.nhs.uk/resources/reference-costs/#rc1718 [cited 2020 Oct 19]
- Guest JF, Fuller GW, Vowden P. Diabetic foot ulcer management in clinical practice in the UK: costs and outcomes. Int Wound J. 2018;15(1):43–52.
- Hammer M, Lammert M, Mejías SM, et al. Costs of managing severe hypoglycaemia in three European countries. J Med Econ. 2009;12(4):281–290.
- Beaudet A, Clegg J, Thuresson PO, et al. Review of utility values for economic modeling in type 2 diabetes. Value Health. 2014;17(4):462–470.
- Evans M, Khunti K, Mamdani M, et al. Health-related quality of life associated with daytime and nocturnal hypoglycaemic events: a time trade-off survey in five countries. Health Qual Life Outcomes. 2013;11(1):90.
- Marrett E, Radican L, Davies MJ, et al. Assessment of severity and frequency of self-reported hypoglycemia on quality of life in patients with type 2 diabetes treated with oral antihyperglycemic agents: a survey study. BMC Res Notes. 2011;4(1):251.
- Nørgaard K, Scaramuzza A, Bratina N, et al. Sensor-augmented pump therapy in real-life: patients reported outcomes results of the INTERPRET observational study. Abstract. Berlin: EASD; 2012. p. 1058.
- Nørgaard K, Scaramuzza A, Bratina N, et al. Interpret Study Group. Routine sensor-augmented pump therapy in type 1 diabetes: the INTERPRET study. Diabetes Technol Ther. 2013;15:273–280.
- Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med. 2012;157(5):336–347.
- National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. Section 5 The reference case. 2013. Available at: https://www.nice.org.uk/process/pmg9/chapter/the-reference-case#discounting [cited 2020 Oct 19]
- McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics. 2008;26(9):733–744.
- Jendle J, Pöhlmann J, de Portu S, et al. Cost-effectiveness analysis of the MiniMed 670G hybrid closed-loop system versus continuous subcutaneous insulin infusion for treatment of type 1 diabetes. Diabetes Technol Ther. 2019;21(3):110–118.
- Driscoll KA, Raymond J, Naranjo D, et al. Fear of hypoglycemia in children and adolescents and their parents with type 1 diabetes. Curr Diab Rep. 2016;16(8):77.
- Jabbour G, Henderson M, Mathieu ME. Barriers to active lifestyles in children with type 1 diabetes. Can J Diabetes. 2016;40(2):170–172.
- Fulcher G, Singer J, Castañeda R, et al. The psychosocial and financial impact of non-severe hypoglycemic events on people with diabetes: two international surveys. J Med Econ. 2014;17(10):751–761.
- Ng SM, Moore HS, Clemente MF, et al. Continuous glucose monitoring in children with type 1 diabetes improves well-being, alleviates worry and fear of hypoglycemia. Diabetes Technol Ther. 2019;21(3):133–137.
- Grando MA, Bayuk M, Karway G, et al. Patient perception and satisfaction with insulin pump system: pilot user experience survey. J Diabetes Sci Technol. 2019;13(6):1142–1148.
- Farrington C. Psychosocial impacts of hybrid closed-loop systems in the management of diabetes: a review. Diabet Med. 2018;35(4):436–449.
- Diabetes Times. Teenager receives first commercial artificial pancreas. 2018. Available at: https://diabetestimes.co.uk/teenager-receives-first-commercial-artificial-pancreas/ [cited 2020 Oct 19]
- Pickup JC, Ford Holloway M, Samsi K. Real-time continuous glucose monitoring in type 1 diabetes: a qualitative framework analysis of patient narratives. Diabetes Care. 2015;38(4):544–550.
- Diabetes.co.uk Medtronic’s closed-loop MiniMed 670G now available on NHS in some areas. Available at: https://www.diabetes.co.uk/news/2019/mar/medtronic's-closed-loop-minimed-670g-now-available-on-nhs-in-some-areas-99233687.html [cited 2019 Aug 12].
- American Diabetes Association Consensus Panel. Guidelines for computer modeling of diabetes and its complications. Diabetes Care. 2004;27:2262–2265.
- Runge AS, Kennedy L, Brown AS, et al. Does time-in-range matter? Perspectives from people with diabetes on the success of current therapies and the drivers of improved outcomes. Clin Diabetes. 2018;36(2):112–119.
- Brod M, Christensen T, Thomsen TL, et al. The impact of non-severe hypoglycemic events on work productivity and diabetes management. Value Health. 2011;14(5):665–671.
