?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aim
To analyse the cost-effectiveness of MEP with standard of care (SoC) versus other anti-IL-5 therapies approved for the treatment of severe eosinophilic asthma (SEA) patients, within the Spanish National Health System (NHS) perspective.
Methods
A Markov model with a 4-week cycle length was used to compare MEP with BEN and RES as therapies added to SoC in the management of SEA, in terms of cost per QALY gained and incremental cost-effectiveness ratio (ICER). Costs (€2019) were obtained from public sources, while utilities and transition probabilities were retrieved from literature, e.g. network meta-analysis. Continuation criteria for biological treatment and reduction of oral corticosteroids (OCS) was set at 50% minimum reduction of exacerbation rate. Adverse events related to chronic OCS use included diabetes, osteoporosis, cataracts, acute myocardial infarct, and peptic ulcer. The analysis was performed over a 5-year time horizon from the National Healthcare System (NHCS) perspective, with a yearly discount rate of 3% applied to both costs and QALYs. Probabilistic sensitivity analysis and univariate deterministic sensitivity analysis were performed to address uncertainty around the cost-effectiveness results.
Results
On top of SoC, the model indicates that MEP is dominant (lower cost, higher benefit) compared to BEN and RES: For BEN and RES, respectively, treatment with MEP had a point estimate of 0.076 and 0.075 additional QALYs, and savings of €3,173.47 and €7,772.95 per patient. The findings were robust to variation as estimated using sensitivity analysis.
Conclusions
MEP is a cost-effective treatment in comparison with BEN and RES added to SoC for patients with SEA in the Spanish setting.
Introduction
With the highest prevalence of chronic respiratory diseasesCitation1, asthma is described by the Global Initiative for Asthma (GINA) as a “heterogeneous disease, usually characterized by chronic airway inflammation. It is defined by the history of respiratory symptoms such as wheeze, shortness of breath, chest tightness and cough that vary over time and intensity, together with variable airflow limitation”Citation2. With an estimated 358 million cases worldwide in 2015Citation1, rates of asthma have been observed to increase with adaptation of modern lifestyles, urbanicity, and ageing populations, and are therefore expected to continue growing over the coming yearsCitation3. Combined with an expected rise in the direct and indirect costs associated per patientCitation4, the burden of asthma, both on patients and society, is large and growing.
Asthma is a complex heterogeneous condition, in which exacerbations, severity, and frequency depend on patient characteristics and asthma phenotypeCitation4,5. Effective and safe treatment, particularly for severe asthma, is recognized as an unmet needCitation6. Severe asthma with inadequate control is associated with high healthcare resources consumptionCitation5,6 and substantial indirect costsCitation7. Direct costs related to asthma management are mostly driven by medication costs, which are attributable to 45–84% of direct costs in Europe, and 51–68% in North AmericaCitation8. Severe asthma patients have been estimated to accrue annual costs ranging between €7,411 and €10,199 per patient in SpainCitation5.
Severe eosinophilic asthma (SEA) is a phenotype of asthma characterized by eosinophilic inflammation, which affects ∼5% of asthma patients and up to 50% of severe asthma patientsCitation9. Eosinophils, a type of white blood cells, cause lung inflammation in this subgroup of asthma patients, increasing the risk of suffering an exacerbation. SEA is identified in primary care based on a measured historic blood eosinophil count of ≥ 150–300 cells/µLCitation10. Due to persistent airflow limitation, distal inflammation with air trapping and continuous exacerbations, SEA leads to poor asthma controlCitation11,12.
Asthma management aims at limiting exposure to asthma triggers, to control and prevent the frequency and severity of exacerbations and to improve patients’ quality-of-life (QoL). Optimal asthma treatment requires to correct identification of the relevant asthma phenotype for a proper control of the patients’ condition. Due to its refractory nature, severe asthma requires treatment with high doses of inhaled corticosteroids (ICS) in addition to a long-acting β2-agonist (LABA), leukotriene modifier or theophylline and/or continuous or near continuous systemic corticosteroids as background therapyCitation6,12. Despite combined therapy, SEA remains difficult to treatCitation6, implies continuous or intermittent high-dose ICS treatment, and may require emergency room visits and hospitalizationCitation13.
Currently, SEA patients who continue to suffer multiple exacerbations can benefit from three different anti-interleukin-5 (anti-IL-5) therapies: Mepolizumab (MEP), Benralizumab (BEN), and Reslizumab (RES)Citation14–16. Interleukin-5 (IL-5), a T-cell-derived cytokine, acts as a mediator in eosinophil activationCitation13. As IL-5 levels are strongly correlated with asthma severity in SEA patientsCitation17, these biologic therapies offer a superior asthma control by reducing eosinophilsCitation18. Anti-IL-5 therapies added to standard of care (SoC) have established efficacy in SEA patients, have no serious safety concerns in clinical trials, and have demonstrated favorable benefit–risk profilesCitation13. Biologic treatments for severe asthma may also reduce the need for complementary therapy with continuous systemic corticosteroids, which often lead to several adverse events, the most frequently reported of which include diabetes, osteoporosis, cataract, acute myocardial infarction, and peptic ulcersCitation19,20. Evidence favors anti-IL-5 add-on treatment as they reduce exacerbation rates and hospitalizations at a similar level of efficacy. Guidelines in Spain recommend the use of MEP, BEN, and RES in those cases of severe eosinophilic asthma, specificallyCitation21, MEP and BEN for patients with eosinophils ≥500 cells/µL and those with <500 cells/µL but who have suffered more than two severe exacerbations in the last year requiring at least two oral or systemic corticosteroid cycles or increasing maintaining corticosteroids for at least 3 days; or patients with more than one exacerbation requiring hospital admission, ICU admission, or mechanical ventilation; the same restrictions are applied for the use of RES in those patients with eosinophils 400–500cells/µLCitation22–24.
Several indirect treatment comparisons have been carried out in order to determine differences in costs and effectiveness among available anti-IL-5 treatmentsCitation12,18,25,26. The study carried out by Busse et al.Citation11 reports that MEP was associated with significantly greater improvements in exacerbations and asthma control in comparison with RES or BEN in patients with similar blood eosinophil counts. However, there is a lack of comparative evidence regarding the economic impacts of these therapiesCitation27.
The main objective of this study was to estimate the economic and health-related impact of three anti-IL-5 treatments added to SoC in the management of adult SEA patients in Spain through an estimation of direct costs and quality-adjusted life years (QALYs) over a 5-year time horizon.
Methods
Study design
A cost-effectiveness analysis (CEA) was performed to compare both MEP with BEN and RES as add-ons to the SoC in SEA patients. All these three anti-IL-5 therapies are indicated for add-on, long-term treatment in SEA patientsCitation10.
The incremental cost-effectiveness ratio for both scenarios was estimated for a 5-year time horizon. The analysis was carried out from the Spanish NHCS perspective and only direct healthcare costs were considered. In accordance with the main Spanish guidelines for economic evaluationCitation28, the analyses used a yearly discount rate of 3% for both costs and QALYs, and a cost-effectiveness threshold of €30,000 to evaluate resultsCitation29.
Model structure
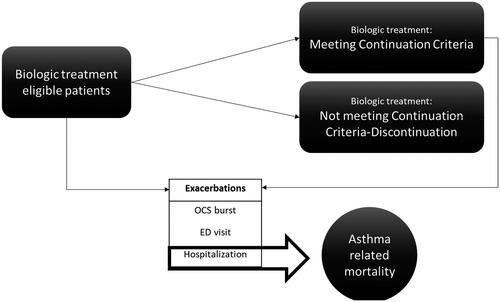
A 4-health state Markov model with a 4-week cycle length was used to simulate costs and QALY gains for a 5-year horizon ()Citation30. The model distinguishes between clinical response to anti-IL-5 (i.e. continuation criteria met or not met); mortality related to asthma and all-cause; and three types of exacerbation events requiring (1) OCS burst, (2) emergency department (ED) visit, and (3) hospitalization. Transitions between on-treatment health states and asthma-related death were caused by hospital exacerbations. All remaining deaths were considered as non-asthma related.
Population
According to the approved label, MEP is indicated for SEA in adults, adolescents, and children aged 6 years and older. MEP trials target population had a blood eosinophil count of ≥150 cells/µL at baseline or ≥300 cells/µL in the 12 months prior to screening. In contrast, BEN and RES are indicated only for adults, and the clinical trials for BEN and RES had baseline blood eosinophil counts in patients ≥300 cells/µL (as primary analysis) and ≥400 cells/µL, respectively. Consequently, two scenarios were analyzed:
Patients with blood eosinophil ≥ 300 cells/µL: MEP + SoC versus BEN + SoC.
Patients with blood eosinophil ≥ 400 cells/µL: MEP + SoC versus RES + SoC.
Age and sex distributions were obtained from the Quirce et al.Citation31 study (n = 134) that analysed SEA patients aged ≥18 in Spain, mean age (95% CI) 54.07 years old (51.55–56.59), of whom 63.4% were female and 36.6% were male.
Probabilities
Efficacy and safety data about MEP came from three pivotal studies: DREAMCitation32, MENSACitation33, and SIRIUSCitation34, relative efficacy of MEPO versus BEN and RES was based on the published network meta-analysis, that was conducted due to the absence of head-to-head trials directly comparing MEP versus BEN or RES comparing licensed doses of these treatments (). The relative efficacy of MEP, BEN, and RES in severe asthma was assessed by synthesizing available RCT evidence via a common comparator (placebo). The primary efficacy outcomes were clinically significant exacerbation reduction and asthma control. Here, clinically significant exacerbations were defined as exacerbations requiring oral/systemic corticosteroids, or at least a doubling of existing dose for maintenance OCS, and/or hospitalization and/or emergency room treatment. Asthma control was measured with the Asthma Control Questionnaire (ACQ). In addition, lung function, measured as change from baseline pre-bronchodilator FEV1, was included as a secondary outcomeCitation11. The proportion of patients with ≥50% exacerbation reduction was based on MENSA results (78.3% for ≥300 cells/µL and 78.2% for ≥400 cells/µL)Citation33. Rate of clinically significant exacerbation for patients meeting continuation criteria (mCC) with MEP was 0.26 (SD 0.51) for ≥300 cells/µL and 0.16 (SD 0.71) for ≥400 cells/µLCitation33. The clinically significant exacerbation rate was adjusted from the reduction factor observed for all patients with MEP and mCC (50% for ≥300 cells/µL and 29.7% for ≥400 cells/µL); for BEN and RES, an equivalent reduction with the same proportion was assumed. Once the number of exacerbations for each comparator was estimated, a distribution among the exacerbation type was done (OCS burst, ED visit, and hospitalization) ()Citation33.
Table 1. Clinical data.
The probability of adverse events related to OCS was estimated according to the risk profile of the patients. An ad hoc analysis was carried out in the Clinical Practice Research DatalinkCitation35 following a logistic model adjusted by dose intensity, previous dose, age, gender, diabetes diagnosis, smoker status, high BMI, hypertension diagnosis, dyslipidemia diagnosis, impaired glucose tolerance, and/or non-steroidal anti-inflammatory drugs use. The probability of suffering adverse events according to the patient characteristics was then estimated.
Cataract: it was assumed that 61.9% of the patients were males, 5.7% diabetic patients, and 19.5% were smokers.
Acute Myocardial infarction: assumptions, 62.1% males, 6.0% diabetic patients, 19.1% smokers, 25.9% with high BMI, 31.9% hypertensive, and 17.8% had dyspepsia.
Gastroduodenal ulcer: assumptions, 62.1% males, 25.9% with high BMI, of whom 4.7% with non-steroidal anti-inflammatory drugs use.
Osteoporosis: assumptions, 60.4% males and 42.5% age ≥60.
Diabetes without complications: assumptions, 62.4% males, 0.1% impaired glucose tolerance, and 22.8% high BMI.
Mortality
Asthma related mortality adjusted by age was obtained from Watson et al.Citation36. Based on National Review of Asthma Deaths (NRAD), asthma-related mortality was only linked to hospital exacerbationCitation37. All-cause mortality was obtained from 2017 from the Spanish Statistics InstituteCitation38.
Costs
Costs (expressed in €2019) were obtained from the Spanish General Council of Official Pharmaceutical Colleges for drug costCitation39, diagnosis-related group (DRG) for event costCitation40, and Spanish cost database e-salud ()Citation41. Use of resources came from a clinical trial and were validated in an expert panel.
Table 2. Costs of drugs, monitoring, exacerbations, and adverse events.
For the pharmacological costs of the biologic treatments, both posology and treatment schemes were considered (ex-factory price). For MEP, the mean daily dose was 100 mg, for BEN the dose was 30 mg, independently of the stage of induction or maintenance; and for RES a mean of 1.98 vials of 100 mg and 1.47 vials of 25 mg (a mean of 234.75 mg) was usedCitation43–45. Additionally, the posology for BEN considered was an induction phase of three doses every 4 weeks and then a maintenance phase every 8 weeks. The annual discontinuation probability for biologic add-on therapy was assumed at 10% for all three drugs and constant throughout time based on expert opinion. Biologic treatment duration was set at 5 years, in a conservative approach. Continuation criteria for biological add-on therapy were set at 50% reduction in exacerbations according to an expert panel.
SoC treatment was 100% fluticasone (0.0328 µg/day), 100% salmeterol (200 mg/day), 56.1% salbutamol (800 µg/day), 49.7% anti-leukotriene (10 mg/day), and 16.0% theophyllines (400 mg/day). From the study from Sicras-Mainar et al.Citation46, it was assumed that 30.2% of the patients maintained OCS treatment. Prednisolone dose was dependent on the approach: 13.20 mg/day if it was administered as an SoC, and 9.24 mg/day if it was administered as a biologic. To estimate the OCS dose in the patients treated with a biologic, an adjustment derived from the median OCS reduction of MEP versus SoC (50% reduction) was appliedCitation34,47.
One medical visit a year was assumed for the administration of biological treatment and a monthly nurse visit for MEP and RES. For BEN, it was one medical visit and six nurse visits the first year and 5.5 nurse visits the following years, according to the treatment scheme. Likewise, one medical visit and three nurse visits a year were considered for SoC. In all the cases, time for administration by the physician/nurse was 10 minutes. Medical visit cost was €242.04/h and nurse visit cost was €26.55/hCitation41.
Data of resource use to manage exacerbations came from an internal analysis of MENSA and DREAMCitation32,33. Costs of telephone call (€18.74), home day visit (€65.80), home night visit (€123.93), practice visit (€38.22), outpatient attendance (€242.04), A&E attendance not admitted (€151.21), and hospitalization (length of stay cost per day adjusted to 10.9 days: €3,827.64) were taken into account. Additionally, the total rescue OCS for exacerbations was 350 mg for OCS burstCitation48, 491 mg for ED visit (141 mg during EDCitation48 and 350 mg after visitCitation48) and 759 mg for hospitalization (409 mg during hospitalization and 350 mg after hospitalizationCitation48,Citation49).
Quality-of-life
Health-related Quality-of-Life of asthma symptoms are based on St. George’s Respiratory Questionnaire data collected in the MENSA trial, mapped to EQ-5D ()Citation33,50. Utilities for MEP and SoC came from MENSACitation33,50 according to the subgroups of patients.
Table 3. Utility values.
Differences in utility between MEP, BEN, and RES was estimated based on published differences in change from baseline ACQ score in the network meta-analysis.Citation11 A model selection step, including fractional polynomials up to the 2nd order (i.e. two terms), was performed to select a model which best predicts utility using ACQ-5 scores, using data from MUSCACitation51. Published differences and associated 95% confidence intervals between anti-IL5 treatmentsCitation11 were then used to calculate the difference in utility between treatments using bootstrapping, which consider both the uncertainty in the published differences in ACQ between treatments and the uncertainty in the model coefficients. The best fitting model, according to the described methods, in the primary population and most sub-populations was a 2nd order polynomial as follows: Mean (SD) improvement in utility of MEP compared to BEN and RES were 0.023 (0.011) and 0.022 (0.009).
Exacerbation disutilities came from the Lloyd et al.Citation52 study with the length of the MENSA study (12.680 days for OCS burst, 10.410 days for ED visit, and 20.7 days for hospitalization)Citation33. We assumed that ED visit disutilities are the same as for OCS bursts.
Results were calculated in terms of cost per QALY gained and incremental cost-effectiveness ratio (ICER).
Sensitivity analysis
Deterministic and probabilistic sensitivity analyses were conducted to assess the robustness of the model results. Deterministic univariate sensitivity analyses (UDSA) were carried out by modifying all the parameters within a ±20% range. Additionally, different time horizons and populations (sex and age) were evaluated.
A probabilistic sensitivity analysis (PSA) was carried out, with 2,000 cohorts simulated. This analysis allows the evaluation of the model results for the diverse typologies of patients, considering the distribution of each of the parameters in its natural range and the existing correlation. Used distributions were beta or Dirichlet for probabilities, lognormal for rates, gamma for resource use and costs, and beta for utilities.
All data inputs and model structure were validated by Spanish clinical experts in the field.
Results
Patients with blood eosinophil ≥300 cells/µL
In a 5-year time horizon, patients were treated with MEP for an average of 3.23 years, and with BEN 3.23 years (). This reflects as asthma-related mortality of 0.92% for MEP and 1.10% for BEN. Additionally, a 17% reduction in the number of exacerbations was observed, that corresponded to a reduction of 0.64 exacerbations with MEP. Specifically, OCS exacerbations were 2.66 for MEP and 3.19 for BEN; ED exacerbations were 0.2 for MEP and 0.24 for BEN, and hospitalizations 0.34 for MEP and 0.41 for BEN.
Table 4. Results of the cost-effectiveness analysis at 5 years.
Regarding the QoL over the 5-year time horizon, MEP resulted in 3.406 QALYs compared to 3.33 QALYs for BEN (). This improvement is caused by better utility because of stronger efficacy in improving asthma control (3.43 QALYs for MEP, and 3.36 for BEN), and disutilities for exacerbations are greater for BEN, −0.0164 vs −0.0137 for MEP.
Costs in MEP patients accounted for €47,706.59, and €50,880.06 for BEN patients over the period, with 89% related to intervention costs (). Savings from MEP are associated with reductions on intervention costs (€2,956), hospitalization (€269), OCS bursts (€27), and ED visits due to exacerbation (€8). However, a discrete increase in monitoring treatment costs (€85), and SoC costs (€2) was also observed.
In short, considering that MEP costs are lower (€3,173), and the QALYs and LY are similar or larger, MEP results were dominant vs BEN (). With a time horizon set to lifetime, MEP was still dominant: MEP costs raised to €65,864.80, and BEN costs up to €69,009.58; QALYs to 12.873 for MEP and 12.780 for BEN.
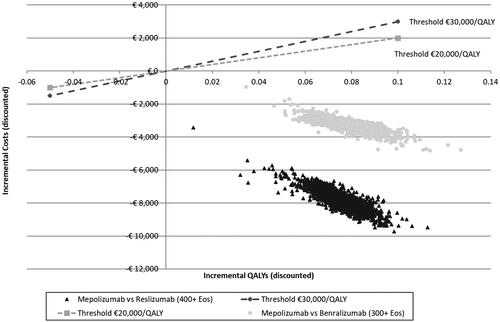
The univariate sensitivity analysis indicated a MEP mCC exacerbation rate of 50% (0.0101–1.3860), BEN overall RR exacerbation (0.41–0.86), MEP discontinuation (6.43–14.24%), BEN mCC RR exacerbation (0.3326–0.7228), and SoC overall exacerbation rate (1.0758–1.8693), were the parameters primarily influencing the results. MEP was dominant across all these analyses (Supplementary Figure S1). Probabilistic analysis verified MEP dominance for thresholds of €20,000/QALY and €30,000/QALY ().
Patients with blood eosinophil ≥400 cells/µL
In the comparison between patients treated with MEP or RES, over a 5-years’ time horizon, patients were treated with MEP for an average of 3.23 years and with RES for 3.22 years (); reflecting an asthma related mortality of 0.95% for MEP and 1.19% for RES. Moreover, the number of exacerbations were reduced by 20.5% for those being treated with MEP corresponding to 0.849 incremental exacerbations. OCS exacerbations were 2.71 for MEP and 3.42 for RES; ED exacerbations 0.2 for MEP and 0.25 for RES; and hospitalizations 0.35 for MEP and 0.44 for RES.
Regarding QoL over a 5-year time horizon, the differences between MEP and RES increased slightly, with 3.373 QALYs for MEP vs 3.298 QALYs for RES (); caused by more patients on mCC health state (2.37 QALYs for MEP and 2.30 for RES) and that disutilities for exacerbations related to RES are higher than those for MEP (−0.0176 vs −0.0140).
Costs for MEP patients in the aforementioned period were €47,739.84 and €55,512.79 for RES patients, being 90% of it, intervention costs (). MEP savings were related to reductions in intervention costs (€7,367), hospitalization for exacerbations (€362), OCS burst (€35), and ED visits for exacerbation (€11). However, there was a slight increase in costs for SoC costs (€3), monitoring treatment costs (€0.5), and OCS related AE costs (€0.04).
Considering that MEP’s costs are lower (€7,773), and QALYs and LY are similar or greater, MEP is dominant vs RES (). If the time horizon is set to a lifetime, MEP will remain dominant: MEP costs increase to €67,298.27 and RES costs to €75,029.34; QALYs were 12.616 for MEP and 12.518 for RES.
Univariate sensitivity analysis indicated that the parameters with greatest influence on the results were MEP exacerbation rate for mCC patients 50% (0.0011–1.0668), MEP discontinuation (6.43–14.24%), patients with ≥50% exacerbation reduction (66.36–88.03%), and RES overall RR exacerbation rate (0.40–0.62). MEP dominance was robust to all tested variance (Supplementary Figure S2). Probabilistic analysis verified MEP dominance for ICER thresholds of €20,000/QALY and €30,000/QALY ().
Scenario analysis
All the scenarios were consistent with the previous results, MEP was dominant over RES and BEN for both sexes, all age groups tested (mean age 65, 75, 85, and 95) for both patient’s profiles (≥300 cells/µL and ≥400 cells/µL) (Supplementary Tables S1–6).
Discussion
Economic evaluations have proven to be fundamental tools to guide resource allocation efficiently along with improving the public health in a resource-constrained healthcare system.Citation54–56 Given the general increase in the pressure of health services worldwide,Citation57 economic evaluations are essential to decision-making in the publicly-funded healthcare benefits, particularly for health conditions that impose substantial socioeconomic burden, such as SEA.Citation54,Citation55,Citation57 In this CEA, the economic value of MEP and the comparators BEN and RES were assessed for the Spanish healthcare setting.
As the Busse et al.Citation11 analysis indicated, anti-IL5 biologic therapies for SEA patients, MEP, BEN, and RES, all significantly reduced asthma exacerbations and improved overall asthma control. However, the indirect comparison of MEP vs BEN and RES concluded that MEP offered greater improvements both in SEA exacerbations and control in patients with similar blood eosinophil counts. Taking into account the ITC and the results of the model, we observed a reduction in OCS therapy for the patients being treated with MEP, as was recently demonstrated by Llanos et al.Citation58 in a real-world retrospective study of 346 patients. Reduction in OCS therapy leads to down-stream benefits in terms of reductions in OCS-related adverse events.Citation13
Additionally, the beneficial response to biologic therapies appears to increase with higher blood eosinophil levels. This is of great importance, as patients with higher eosinophil levels have a higher frequency of exacerbations and comparatively poorer disease control and this implies higher costs for the national healthcare system.Citation13
In this CEA, the disutilities linked to exacerbations and health states and asthma-related mortality have been handled as recommended by McQueen et al.Citation27 in their systematic literature review of cost-effectiveness analyses for biological asthma treatments. Reflecting the chronic nature of asthma, and in accordance with recommendations by the same authors, data for a 5-year time horizon is presented as the main case, with results for 10-years and a lifetime horizon as sensitivity analyses.
In this study MEP was found to be dominant when compared with BEN and RES, with more QALYs gained and lower costs in the different time horizons evaluated (5-, 10-, and a lifetime horizon).
The main strength of this study is that the analysis was conservative in order to obtain a lower bound for improvements associated with MEP use. However, the study has limitations, for instance, the study assumed 100% adherence to SOC, but in the real-world, adherence to standard therapy may be inadequate can impact SEA control.Citation59 In 2019, the EMA’s human medicines committee (CHMP) approved the self-administering method of MEP in SEA patients.Citation60 This decision may result in a reduction of administration costs for this treatment arm, which would result in MEP being more cost-effective. These results highlight the necessity to prioritize MEP vs other biologic treatments due to its efficiency for the healthcare system in Spain, moreover, from the social point of view, this treatment improves clinical outcomes which imply an improvement in patients’ QoL. This data should be considered cautiously as the unit costs and QoL are country-dependent.
Conclusions
The CEA model results suggest that MEP as an add-on therapy to SoC is a cost-effective alternative for Spanish SEA patients, with better clinical outcomes and lower cost than BEN and RES.
Transparency
Declaration of funding
This study was funded by GlaxoSmithKline [Study code: HO-19-19968].
Declaration of financial/other interests
AG and SY are GSK employees. SY is GSK shareholder. EM was a GSK employee when the study was conducted. GVDW is an employee of Pharmerit International and received scientific consultancy fees from GSK. FJGB has received speaker fees, consulting fees, or research grants from ALK, AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Gebro Pharma, GlaxoSmithKline, Laboratorios Esteve, Menarini, Munipharma, Novartis, Rovi, Roxall, Stallergenes-Greer, and Teva. JLI has received fees as scientific advisor, participant of projects, and/or talks from AstraZeneca, Bayer, Boehringer Ingelheim, Chiesi, GSK, Grifols, Menarini, Novartis, Orion, Pfizer, Sandoz, and Teva. XM has received fees as a speaker, scientific advisor, and participant of clinical studies from AstraZeneca, Boehringer Ingelheim, Chiesi, Faes Farma, GSK, Menarini, Mundifarma, Novartis, and Teva.
A peer reviewer on this manuscript has disclosed that they have performed consulting, served on advisory boards, or received travel reimbursement from Amphastar, AstraZeneca, Boehringer Ingelheim, Chiesi, Connect Biopharma, GlaxoSmithKline, Mylan, Novartis, Sunovion, and Theravance. They have also conducted multicenter clinical research trials for some 40 pharmaceutical companies. The peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Supplemental Material
Download MS Word (66.2 KB)Acknowledgements
Editorial support in the form of development of the initial draft, collating author comments, editorial suggestions to draft versions of this paper, assembling tables and figures, copyediting, and referencing was provided by Mónica Cerezales and Carlos Crespo from Axentiva solutions and was funded by GSK.
References
- Soriano JB, Abajobir AA, Abate KH, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706.
- Global Initiative for Asthma. Global strategy for asthma management and prevention. Fontana-on-Lake (WI); 2020.
- Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy. 2004;59(5):469–478.
- Global surveillance, prevention and control of chronic respiratory diseases. A comprenhensive approach. Geneva: Switzerland; 2007.
- Melero Moreno C, Quirce S, Huerta A, et al. Economic impact of severe asthma in Spain: multicentre observational longitudinal study. J Asthma. 2019(8);56:861–871.
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373.
- Puig-Junoy J, Pascual-Argenté N. Socioeconomic costs of asthma in the European Union, United States and Canada: a systematic review. Rev Esp Salud Publica. 2017;91:e201703025.
- Souliotis K, Kousoulakou H, Hillas G, et al. Direct and indirect costs of asthma management in Greece: an expert panel approach. Front Public Heal. 2017;5:67.
- Skolnik NS, Carnahan SP. Primary care of asthma: new options for severe eosinophilic asthma. Curr Med Res Opin. 2019;35(7):1309–1318.
- Bakakos A, Loukides S, Bakakos P. Severe eosinophilic asthma. J Clin Med. 2019;8:1375.
- Busse W, Chupp G, Nagase H, et al. Anti-IL-5 treatments in patients with severe asthma by blood eosinophil thresholds: indirect treatment comparison. J Allergy Clin Immunol. 2019;143(1):190–200.e20.
- Global Initiative for Asthma. Pocket guide for asthma management and prevention for adults and children older than 5 years old. A pocket guide for health professional updated 2019. Fontana-on-Lake (WI); 2019.
- Buhl R, Humbert M, Bjermer L, et al. Severe eosinophilic asthma: a roadmap to consensus. Eur Respir J. 2017;49(5):1700634.
- European Medicines Agency. European Medicines Agency. Assessment report on extension(s) of marketing authorisation. Nucala. Procedure No. EMEA/H/C/003860/X/0018. [Internet]. 2019. Available from: https://bit.ly/37aRrJh. 2015.
- European Medicines Agency. Cinqaero: EPAR – product information. 2016.
- European Medicines Agency. Fasenra: EPAR – product information. 2018.
- Castillo JR, Peters SP, Busse WW. Asthma exacerbations: pathogenesis, prevention, and treatment. J Allergy Clin Immunol Pract. 2017;5(4):918–927.
- Cabon Y, Molinari N, Marin G, et al. Comparison of anti-interleukin-5 therapies in patients with severe asthma: global and indirect meta-analyses of randomized placebo-controlled trials. Clin Exp Allergy. 2017;47(1):129–138.
- Waljee AK, Rogers MAM, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. 2017;357:j1415.
- Bloechliger M, Reinau D, Spoendlin J, et al. Adverse events profile of oral corticosteroids among asthma patients in the UK: Cohort study with a nested case-control analysis. Respir Res. 2018;19(1):75.
- Sociedad Española de Neumología y Cirugía Torácica. GEMA 5.0. Guía española para el manejo del asma. 2020.
- Agencia Española de Medicamentos y Productos Sanitarios M de S. Informe de posicionamiento terapéutico de benralizumab (Fasenra®) como tratamiento adicional en el asma grave no controlada eosinofílica [Internet]. 2019. Available from: https://www.aemps.gob.es/medicamentosUsoHumano/informesPublicos/docs/IPT-benralizumab-Fasenra-asma_EPOC.pdf?x74012.
- Agencia Española de Medicamentos y Productos Sanitarios M de S. Informe de posicionamiento terapéutico de reslizumab (Cinqaero®) como tratamiento adicional en el asma eosinofílica grave no controlada [Internet]. 2018. Available from: https://www.aemps.gob.es/medicamentosUsoHumano/informesPublicos/docs/IPT-reslizumab-Cinqaero-asma_EPOC.pdf?x42633.
- Agencia Española de Medicamentos y Productos Sanitarios M de S. Informe de posicionamiento terapéutico de mepolizumab (Nucala®) como tratamiento adicional en el asma eosinofílica refractaria grave [Internet]. 2016. Available from: https://www.aemps.gob.es/va/medicamentosUsoHumano/informesPublicos/docs/IPT-mepolizumab-Nucala-asma_EPOC.pdf.
- Henriksen DP, Bodtger U, Sidenius K, et al. Efficacy, adverse events, and inter-drug comparison of mepolizumab and reslizumab anti-IL-5 treatments of severe asthma – a systematic review and meta-analysis. Eur Clin Respir J. 2018;5(1):1536097.
- Cockle SM, Stynes G, Gunsoy NB, et al. Comparative effectiveness of mepolizumab and omalizumab in severe asthma: an indirect treatment comparison. Respir Med. 2017;123:140–148.
- McQueen RB, Sheehan DN, Whittington MD, et al. Cost-effectiveness of biological asthma treatments: a systematic review and recommendations for future economic evaluations. Pharmacoeconomics. 2018;36(8):957–971.
- López-Bastida J, Oliva J, Antoñanzas F, et al. Spanish recommendations on economic evaluation of health technologies. Eur J Health Econ. 2010;11(5):513–520.
- Sacristán JA, Oliva J, Del Llano J, et al. ¿Qué es una tecnología sanitaria eficiente en España? Gac Sanit. 2002;16(4):334–343.
- National Institute for Health and Care Excellence. Mepolizumab for treating severe refractory eosinophilic asthma. Technology appraisal guidance [TA431]. 2017.
- Quirce S, Melero C, Huerta A, et al. Economic impact of severe asthma exacerbations in Spain: multicentre observational study. J Asthma. 2021;58(2):207–212.
- Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659.
- Ortega HG, Liu MDC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207.
- Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197.
- Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol. 2015;44(3):827–836.
- Watson L, Turk F, James P, et al. Factors associated with mortality after an asthma admission: a national United Kingdom database analysis. Respir Med. 2007;101(8):1659–1664.
- Levy ML. The national review of asthma deaths: what did we learn and what needs to change? Breathe. 2015;11(1):14–24.
- Instituto Nacional de Estadística (INE). Population mortality tables for Spain by year, province, sex, age, and functions. 2018.
- Base de Datos de Medicamentos. Consejo General de Colegios Oficiales de Farmacéuticos [WWW Document]. n.d.
- Ministerio de Sanidad C y BSG de E. Registro de Actividad de Atención Especializada, Conjunto Mínimo Básico de Datos [WWW Document]. [cited 2020 Aug 4]. Available from: https://pestadistico.inteligenciadegestion.mscbs.es/publicoSNS/comun/ArbolNodos.aspx?idNodo=23606
- Gisbert R, Brosa M. Health costs and cost-effectiveness ratios database: eSalud. Barcelona: Oblikue Consulting, S.L.; 2007.
- Crespo C, Brosa M, Soria-Juan A, et al. Direct cost of diabetes mellitus and its complications in Spain (SECCAID Study: Spain estimated cost Ciberdem-Cabimer in Diabetes). Av en Diabetol. 2013;29(6):182–189.
- ANEXO I Ficha Técnica o Resumen de las Características del producto, Nucala. FT_1151043001. European Medicines Agency.
- ANEXO I Ficha Técnica o Resumen de las Características del producto, Fasenra. FT_1171252001. European Medicines Agency.
- ANEXO I Ficha Técnica o resumen de las características del producto, Cinqaero. FT_1161125001. European Medicines Agency.
- Sicras-Mainar A, Capel M, Navarro-Artieda R, et al. Real-life retrospective observational study to determine the prevalence and economic burden of severe asthma in Spain. J Med Econ. 2020;23(5):492–500.
- SIRIUS Statistical Report, Data on file. n.d.
- Global Initiative for Asthma. Global strategy for asthma management and prevention. 2015.
- Edmonds M, Milan S, Camargo CJ, et al. Early use of inhaled corticosteroids in the emergency department treatment of acute asthma. Cochrane Database Syst Rev. 2012;12:CD002308.
- Starkie HJ, Briggs AH, Chambers MG, et al. Predicting EQ-5D values using the SGRQ. Value Health. 2011;14(2):354–360.
- Chupp G, Bradford E, Albers F, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5(5):390–400.
- Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim Care Respir J. 2007;16(1):22–27.
- Sullivan PW, Slejko JF, Sculpher MJ, et al. Catalogue of EQ-5D Scores for the United Kingdom. Med Decis Making. 2011;31(6):800–804.
- Chisholm D, Evans DB. Economic evaluation in health: saving money or improving care? J Med Econ. 2007;10(3):325–337.
- Bodrogi J, Kaló Z. Principles of pharmacoeconomics and their impact on strategic imperatives of pharmaceutical research and development. Br J Pharmacol. 2010;159(7):1367–1373.
- Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford: Oxford University Press; 2005.
- OECD. Health at a glance 2019: OECD indicators. Paris: OECD Publishing; 2019.
- Llanos J-P, Ortega H, Bogart M, et al. Real-world effectiveness of Mepolizumab in patients with severe asthma: an examination of exacerbations and costs. JAA. 2020;13:77–87.
- Ilic AD, Zugic V, Zvezdin B, et al. Influence of inhaler technique on asthma and COPD control: a multicenter experience. COPD. 2016;11:2509–2517.
- European Medicines Agency. Assessment report on extension(s) of marketing authorisation. Nucala. Procedure No. EMEA/H/C/003860/X/0018; 2019.