Abstract
Aims
To estimate the budget impact of adding tepotinib to United States (US) health plans for treating adult patients with metastatic non-small cell lung cancer (mNSCLC) harboring mesenchymal–epithelial transition exon 14 (METex14) skipping alterations.
Methods
The base-case analysis was conducted from the perspective of a hypothetical Medicare plan of 1 million members. Scenarios were analysed for other US health plans. Treatments included tepotinib, capmatinib, crizotinib, and standard of care (SoC). Patients eligible for tepotinib were estimated from published epidemiological data and literature, and real-world evidence. Clinical inputs were derived from the phase II VISION trial, US prescribing information, and published literature. Tepotinib uptake and projected testing rates for METex14 skipping alterations were based on market research. Unit costs (2020 US dollars (USD)) and resource utilization associated with drug acquisition and administration, treatment monitoring, disease and adverse event (AE) management, and subsequent treatment were derived primarily from public sources.
Results
In the base-case, 38–65 patients were eligible for tepotinib each year over the three-year time horizon. The cumulative net budgetary impact of tepotinib was –$692,541 (–2.6%); $26,531,670 in the scenario without tepotinib and $25,839,129 in the scenario with tepotinib. A negligible net budget impact was observed per member per month (PMPM) at $0.2457 and $0.2393, respectively, before and after tepotinib’s introduction. Results were most sensitive to variability in unit costs of capmatinib and tepotinib and their corresponding median treatment durations. Sensitivity and scenario analyses support the conclusion that introducing tepotinib will have minimal budgetary impact for Medicare health plans. Similar results were obtained for other US health plans.
Limitations
Assumptions and expert opinion were applied to address data gaps in key model inputs.
Conclusions
The estimated budgetary impact of tepotinib for the treatment of adult patients with mNSCLC harboring METex14 skipping alterations is minimal from the perspective of US health plans.
Introduction
Lung cancer is the most common cancer worldwide and is the leading cause of cancer-related death in the United States (US)Citation1–3. There are an estimated 2.1 million new cases globally each year, with lung and bronchial cancer representing an estimated 12.7% of all new cancer cases in the US in 2020Citation4. Most lung cancers fall into two major classes: small-cell lung cancer and non-small cell lung cancer (NSCLC), with NSCLC accounting for approximately 80–85% of casesCitation5,Citation6.
Mutations that lead to skipping of exon 14 of the mesenchymal–epithelial transition (MET) gene, hereby abbreviated as METex14, have been identified as oncogenic drivers in NSCLC, with an estimated prevalence of about 3–4%Citation7–11. Patients with NSCLC harboring METex14 skipping alterations are typically older than those without the mutation, and have a non-squamous histologyCitation12,Citation13; they tend to experience poorer prognoses than those without the mutation due to high rates of brain, bone, and liver metastasesCitation14.
Until recently, there were no therapies in the US approved specifically to treat this subset of patients, with standard of care (SoC) typically consisting of some combination of immune checkpoint inhibitors (ICIs), anti-vascular endothelial growth factor antibodies (VEGF), and/or chemotherapy. Crizotinib, a tyrosine kinase inhibitor (TKI) indicated for the treatment of patients with locally advanced or metastatic NSCLC (mNSCLC), is currently prescribed as a first-line treatment in patients who have tested anaplastic lymphoma kinase (ALK)-positive or ROS-1 positive as detected by a US Food and Drug Administration (FDA)-approved testCitation15. Crizotinib also inhibits some MET TKI mutations including METex14 skippingCitation16, and is recommended by the NCCN as a first-line or subsequent therapy option for patients with mNSCLC harboring the METex14 skipping alteration under certain circumstances (category 2A)Citation16. Still another TKI, cabozantinib, has shown promise in initial case reports and is being evaluated in clinical trials for treatment of mNSCLC patients harboring METex14 skipping alterationsCitation17–19, but is not currently recommended by the NCCN in this therapeutic contextCitation16. Accordingly, there has historically been a high unmet need for efficacious, targeted, and tolerable treatment options with a convenient dosing schedule, particularly for older patients in whom chemotherapy regimens may be unsuitable. In May 2020, capmatinib became the first TKI approved by the US FDA for adult patients with mNSCLC whose tumors have a mutation that leads to METex14 skipping alterations as detected by an FDA-approved testCitation14.
In February 2021, the US FDA approved a second TKI, tepotinib, for adult patients with mNSCLC harboring the METex14 skipping alterationsCitation20. The safety, efficacy, and tolerability of tepotinib was evaluated in the VISION study (NCT02864992), a phase II, single-arm, open-label trial of patients including those with locally advanced stage IIIB/IV NSCLC harboring METex14 skipping alterations. Although the availability of targeted treatments for these patients is anticipated to prolong survival, which will result in additional treatment costs, the management of mNSCLC imposes significant burdens on patients and payers alikeCitation21,Citation22, and it is therefore important to quantify the potential budget impact of a new therapy to better inform decision-makersCitation14,Citation23 and provide evidence to support submissions to reimbursement agenciesCitation24. To that end, this study aimed to estimate the budget impact of including tepotinib for the treatment of US adult patients with mNSCLC harboring METex14 skipping alterations.
Methods
A Microsoft ExcelFootnotei-based budget impact model (BIM) was developed in accordance with guidelines from the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) and the Society for Medical Decision Making on Good Modelling PracticesCitation25,Citation26.
Target population
The population included in the BIM was US adult patients with mNSCLC harboring METex14 skipping alterations, as detected through biomarker testing. The number of patients () was estimated from published epidemiological data, academic and grey literature, and real-world data, based on a hypothetical plan of 1 million members. Annual incidence of mNSCLC for patients <65 years and 65+, and the age composition of patients in Medicare health plans, were extracted from publicly available national databases and used to derive the number of new patients with mNSCLC for the analysisCitation4,Citation27. The proportion of frontline patients harboring the METex14 skipping alteration, and the staging distribution of NSCLC, were obtained from published literatureCitation28,Citation29, while real-world data were used to derive the ratio of newly diagnosed to recurrent frontline patients and the ratio of subsequent-line to frontline patientsCitation30. This information was combined to calculate the number of eligible patients entering the model after applying projected METex14 biomarker testing rates. The model assumes only patients who receive testing are eligible for treatment with a MET TKI (i.e. tepotinib, capmatinib, or crizotinib). Testing rates were derived from market research conducted by EMD SeronoCitation31, and driven by the anticipation that testing is likely to become increasingly common over time due to advances in testing capabilities, the proliferation of new treatments, and changing general attitudes among healthcare practitioners toward the utility of testing in the context of treatment for NSCLCCitation32. As such, testing rates of 49%, 75%, and 85% were applied in the base-case analysis for years 1, 2 and 3, respectively. Alternative assumptions around testing were explored as scenario analyses. As per the US indication for tepotinib, the base-case analysis includes all patients, irrespective of prior treatment, who are modeled as a line-agnostic aggregation of first-line (1L) and second-line or later (2L+) populations. With this approach, a new cohort of patients receiving 1L or 2L + treatment enters the model each year and is assigned to the various interventions in accordance with their respective market shares.
Table 1. Annual number of patients eligible for treatment with tepotinib in the context of a Medicare plan (line-agnostic patient population).
Treatments
Scenarios included in the model reflect current and emerging treatment alternatives for this patient population. Comparators to tepotinib included capmatinib, crizotinib, and SoC. SoC was modeled as a composite of several comparator classes and constituent treatment regimensCitation30, consisting of ICI monotherapy (atezolizumab, nivolumab, and pembrolizumab), ICI + chemotherapy ± anti-VEGF (pembrolizumab + carboplatin + pemetrexed, and atezolizumab + paclitaxel + carboplatin + bevacizumab), chemotherapy + anti-VEGF (paclitaxel + carboplatin + bevacizumab, pemetrexed + bevacizumab, docetaxel + bevacizumab, and pemetrexed + carboplatin + bevacizumab), and chemotherapy alone (docetaxel, pemetrexed, carboplatin + pemetrexed, cisplatin + pemetrexed, and carboplatin + paclitaxel). The status quo scenario included targeted treatment with capmatinib, crizotinib and SoC, while the alternative scenario also included tepotinib.
Market shares
Market shares for both the status quo and alternative scenarios are presented in . Projected shares for capmatinib, crizotinib, and SoC in the status quo were derived from market researchCitation31, while the composition of SoC was derived from real-world dataCitation30. For the alternate scenario, estimated uptake of tepotinib and shares for capmatinib were again derived from market researchCitation31, while shares for crizotinib and SoC were informed by the assumption that these would lose market share to tepotinib and capmatinib in proportion to the share they maintained in the status quo.
Table 2. Market share inputs.
Model perspective and time horizon
The base-case analysis estimated the budgetary impact of tepotinib over a three-year time horizon from the perspective of a hypothetical Medicare payer in the US consisting of 1 million members.
Model overview
The budget impact analysis conceptualizes two distinct scenarios: (1) a status quo scenario in which patients have recourse only to current treatment options (i.e. excluding tepotinib) and (2) an alternative scenario in which tepotinib is reimbursed by the Medicare plan, drawing market share from its comparators ().
Figure 1. Budget impact model structure. Abbreviations. METex14, Mesenchymal–epithelial transition exon 14; NSCLC, Non-small cell lung cancer.
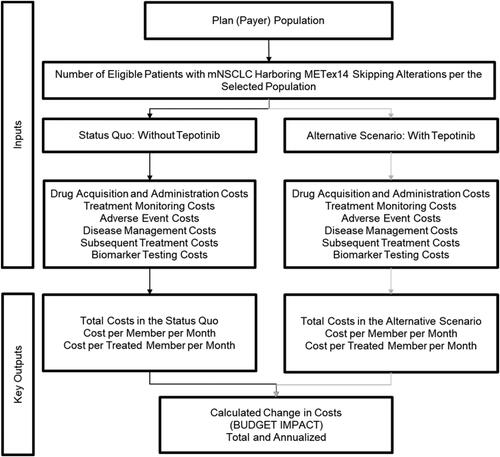
Patients with mNSCLC enter the model after biomarker testing confirms the presence of the METex14 skipping alteration, and are allocated to the available treatment options according to the market shares presented in . The model tracks patients receiving each treatment option throughout the time horizon, assuming median clinical outcomes for each option apply. Annual costs for the status quo and alternative scenarios are estimated by multiplying the number of patients receiving each option by the average cost of treatment and then aggregating over the entire treatment portfolio. The budget impact is the cost difference between the scenarios and reflects the way in which the introduction of tepotinib influences the distribution of patients across treatments.
Calculation of eligible patients for a hypothetical Medicare plan consisting of 1 million members is presented in , while analogous calculations for a hypothetical commercial plan of similar size, which is presented as a scenario analysis, is outlined in the supplementary materials (Supplementary Material SM1).
Model cost inputs
The model includes expenditures associated with drug acquisition and administration, treatment monitoring, disease and adverse event (AE) management, subsequent treatment, and biomarker testing. All costs are expressed in 2020 US dollars (USD).
The drug cost for oral tepotinib was $20,898.60 per package for a 30-day supply. All drug acquisition costs were based on wholesale acquisition cost (WAC) recorded in IBM Micromedex RedBookFootnoteii Citation33. This was done because final prices for drugs are not commonly reported and can vary across settings (e.g. due to differences in discounts and price concessions offered by manufacturers); in one methodological review of US BIMs, this observation led to the recommendation that discounts and cost-sharing be deducted from drug acquisition costsCitation34. Medication costs for tepotinib, capmatinib, crizotinib, and ICIs were calculated based on flat dosing, as per US prescribing information. For anti-VEGF agents and chemotherapies, dosing was based on mean patient body weight and surface area (65.91 kg, 1.73 m2) or glomerular filtration rate (70.42 ml/min; applied to carboplatin dosing by estimating glomerular filtration rate using the Cockcroft-Gault equation), as per the characteristics of VISION trial participantsCitation31; costs were derived by estimating the whole number of medication vials required to meet dosage requirements (Supplementary Material SM2). Unit costs associated with administration of intravenous therapies were derived from the Medicare Hospital Outpatient Healthcare Common Procedural Coding System (2020)Citation35, while overall expenditures for each regimen were determined by the duration of infusion and whether the regimen included multiple drugs (Supplementary Material SM3).
Unit costs associated with treatment monitoring and disease management were extracted from Centers for Medicare & Medicaid Services for the base-caseCitation36, while their composition was derived from a combination of published literatureCitation37,Citation38 and assumptions validated by a practicing oncologist. Monthly monitoring costs were estimated at $25.11 (Supplementary Material SM4), while monthly pre- and post-progression disease management costs were calculated to be $1,013.49 and $6,747.62, respectively (Supplementary Material SM5). It was assumed that pre- and post-progression disease management costs did not vary with the treatment received.
The model also incorporated expenditures attributable to medical resource use required to manage AEs, which were calculated by multiplying the frequency of grade 3/4 AEs occurring in at least 5% of patients, as reported in the product prescribing information or published studies, and unit costs obtained from the Healthcare Cost and Utilization Project (HCUPnet)Citation39. AE management expenditures were applied in the model as a one-time cost (Supplementary Material SM6, SM7).
Subsequent costs included all active treatments in the model and accounted for acquisition costs, frequencies, and duration of treatment. Average duration of subsequent treatments was estimated from the mean progression-free survival (PFS) for subsequent treatments observed in the VISION trial (3.0 months)Citation31. The composition of subsequent treatment could vary according to which treatment the patient received before progression, in that it was assumed patients would not be retreated with the same TKI they had previously received (where applicable) but could be administered another product from the same class of therapies (Supplementary Material SM8). Costs for subsequent treatment for tepotinib were $13,993.45, while those accrued following discontinuation from capmatinib, crizotinib and SoC were $13,900.02, $14,336.01, and $13,993.45, respectively.
The model also assumed all patients would incur one-time biomarker testing costs upon entering the model, irrespective of whether they ultimately received a MET TKI. The cost applied in the model ($2,854.73) is the average across current tests available in the US (Supplementary Material SM9)Citation40.
Sensitivity analysis
A deterministic sensitivity analysis (DSA) was conducted by testing the upper and lower bounds of individual model parameters to assess their impact on the model outcomes. Parameters incorporated into the DSA included the number of patients entering the model in each year; uptake of tepotinib in the alternative scenario; median treatment duration, PFS and overall survival (OS) for each MET TKI and each class of treatments constituting SoC, for both 1L and 2L+; additional parameters included costs associated with drug acquisition and administration, treatment monitoring, disease and AE management, and biomarker testing. All non-clinical parameters included in the DSA were varied by 20% above and below the base-case values, while clinical parameters were varied by the upper and lower bounds of the 95% confidence intervals (CIs).
Scenario analysis
The DSA was supplemented by several scenario analyses, which explored the impact of varying assumptions around rates of biomarker testing, treatment duration, and line of therapy (i.e. frontline (1L) or relapsed (2L+) patients only) from the Medicare payer perspective, as well as the budgetary impact of tepotinib from the perspective of Medicare Part D prescription drug plan (PDP) and commercial payers.
Results
Cost per course
The cost per course of therapy for selected interventions (i.e. targeted treatments, ICI monotherapy, and ICI + chemotherapy ± anti-VEGF regimens), as calculated using WAC prices, is provided in . These values include only drug acquisition costs, and are calculated by multiplying the monthly cost of therapy by the weighted treatment duration; the weights were derived with reference to the median time on treatment, the ratio of 2L + to 1L patients from the Flatiron database analysisCitation30, and utilization on both lines.
Table 3. Cost per course of therapy (wholesale acquisition cost prices).
The cost per course for the TKIs was $155,690, $172,916, and $137,190 for tepotinib, capmatinib, and crizotinib, respectively, while the cost for ICI monotherapy was $47,573 for nivolumab, $72,613 for pembrolizumab, and $46,713 for atezolizumab. Finally, the cost of a regimen consisting of pembrolizumab, carboplatin and pemetrexed was $172,127, while expenditures associated with atezolizumab, paclitaxel, carboplatin, and bevacizumab were $179,138.
Base-case analysis
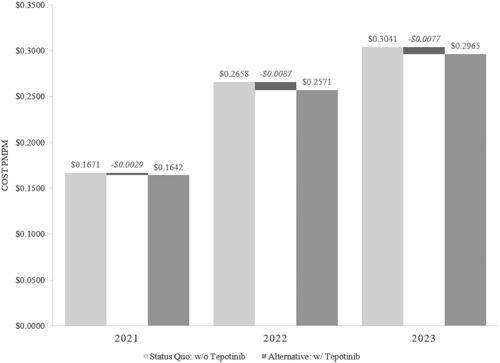
In a hypothetical Medicare health plan consisting of 1 million members, the introduction of tepotinib as a treatment for adult patients with mNSCLC harboring METex14 skipping alterations generated a cumulative net budgetary impact of –$692,541 (–2.6%) over three years with –$103,337 in year 1 (38 patients), –$313,790 in year 2 (58 patients), and –$275,414 in year 3 (65 patients). The total per member per month (PMPM) costs before and after the introduction of tepotinib were $0.2457 and $0.2393, respectively. The estimated incremental cost impact PMPM of tepotinib was –$0.0064 over the three-year period. Specifically, the budget impact PMPM of tepotinib to a Medicare health plan was estimated at –$0.0029 in year 1, –$0.0087 in year 2, and –$0.0077 in year 3. A summary of the budget impact results PMPM is presented in , while a detailed breakdown by cost category is presented in the supplementary materials (Supplementary Material SM10).
Sensitivity analysis
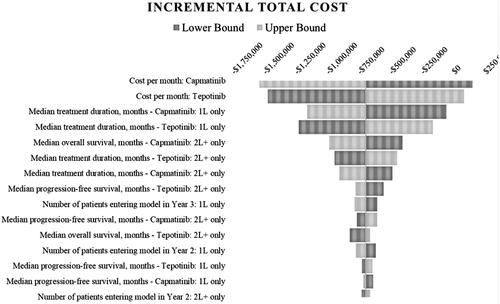
Variability in the cost per month of both capmatinib and tepotinib had the most significant impact on the model estimates of incremental cost PMPM, followed by median duration on both treatments for 1L and 2L+. Total budgetary impact ranged from $152,279 to –$1,537,360 when the monthly cost of capmatinib was decreased or increased by 20%, respectively, while incremental costs PMPM ranged from $0.0014 to –$0.0142. The opposite pattern was observed when the monthly cost of tepotinib was adjusted similarly (i.e. –$1,469,850 and $84,769 (PMPM: –$0.0136 to $0.0008)). The variability in the number of patients entering the model and survival metrics (OS/PFS) had a smaller impact on estimates of total cost. A tornado diagram illustrating these results on PMPM is presented in , while a tabular ranking of scenarios revolving around total costs, PMPM and per treated member per month (PTMPM) is included in the supplementary materials (Supplementary Material SM11). These results reflect the expectation that tepotinib’s market share in the alternative scenario will derive primarily from capmatinib.
Scenario analyses
Conservative testing rates
Conservative testing rates based upon a linear extrapolation from a published sourceCitation41 (i.e. 38%, 44%, and 51% over the three-year time horizon, vs. 49%, 75%, and 85% in the base-case) was explored. In a hypothetical Medicare health plan consisting of 1 million members, the introduction of tepotinib using these conservative testing rates resulted in a cumulative budgetary impact of –$416,953 (–2.5%) over a three-year time horizon. Results by year were –$79,084 (year 1, 29 patients), –$173,124 (year 2, 34 patients), and –$164,744 (year 3, 39 patients). The total PMPM costs prior to and after the introduction of tepotinib were $0.1553 and $0.1514, respectively; specifically, the budget impact PMPM was –$0.0022 in year 1, –$0.0048 in year 2, and –$0.0046 in year 3.
A second testing scenario assumed testing rates that were 20% lower than the base-case (i.e. 39% vs. 49% (base-case) in year 1, 60% vs. 75% (base-case) in year 2, and 68% vs. 85% (base-case) in year 3). In this scenario, the introduction of tepotinib resulted in a cumulative budgetary impact of –$554,032 (–2.6%) over the three-year time horizon. Results by year were –$82,669 (year 1, 30 patients), –$251,032 (year 2, 46 patients), and –$220,331 (year 3, 52 patients). The total PMPM cost prior to and after the introduction of tepotinib was $0.1965 and $0.1914, respectively; specifically, the budget impact PMPM was –$0.0023 in year 1, –$0.0070 in year 2, and –$0.0061 in year 3.
In general, lower assumed rates of testing attenuate the cost impact associated with the introduction of tepotinib.
Higher testing rates
Given technological progress in the diagnostics space, continuous increases in testing capacities, and a general trend toward increasing acceptance of testing as a tool for guiding optimal therapeutic decision-makingCitation32, a scenario assuming higher testing rates was conducted. This scenario assumed testing rates to be up to 20% higher than the base-case, capped at 100% (i.e. 59% vs. 49% (base-case) in year 1, 90% vs. 75% (base-case) in year 2, and 100% vs. 85% (base-case) in year 3). In this scenario, the introduction of tepotinib resulted in a cumulative budgetary impact of –$821,044 (–2.6%) over the three-year time horizon. Results by year were –$124,004 (year 1, 45 patients), –$376,548 (year 2, 69 patients), and –$320,492 (year 3, 77 patients). The total cost PMPM prior to and after the introduction of tepotinib was $0.2925 and $0.2849, respectively; specifically, the budget impact PMPM was –$0.0034 in year 1, –$0.0105 in year 2, and –$0.0089 in year 3. Higher assumed testing rates were seen to amplify the cost impact associated with the introduction of tepotinib. This occurs because testing increases the number of patients eligible to receive a TKI, including tepotinib. As introducing tepotinib reduces overall expenditures in the Medicare base-case, diverting additional patients to this intervention should further reduce spending.
Assuming treatment duration equal to PFS
In the base-case, treatment duration was equal to median time on treatment, where this information was available, and equal to median PFS otherwise. In this scenario, treatment duration was instead assumed to be uniformly equal to median PFS. The rationale for including this scenario was twofold. First, assuming treatment duration to be equivalent to PFS may be conservative as PFS is typically longer than time on treatment, which may result in overstating costs for each treatment. Second, although time on treatment is accessible for tepotinib and a small number of comparators, it was not consistently reported across comparators; accordingly, it is important to assess the implications of applying a measure of treatment duration (i.e. PFS) for which information for all comparators is available. Within the context of this scenario, the introduction of tepotinib in a hypothetical Medicare health plan consisting of 1 million members did not affect the number of eligible patients entering the model relative to the base-case. In this scenario, the cumulative budgetary impact was –$33,462 (–0.1%) over the three-year time horizon. Results by year were $12,365 in year 1, –$45,262 in year 2, and –$565 in year 3. The total PMPM cost prior to and after the introduction of tepotinib was $0.2608 and $0.2605, respectively; specifically, the budget impact PMPM was $0.0003 in year 1, –$0.0013 in year 2, and $0.0000 in year 3.
Medicare Part D prescription drug plan
Medicare’s Part D PDP is a stand-alone plan which provides coverage of outpatient prescription drugs that are found on the plan's formulary and does not include drug acquisition or administration costs associated with products administered in an inpatient setting, thereby limiting reimbursement to the three MET TKIs. This scenario assessed the impact of introducing tepotinib within the context of a PDP with all other parameters as per the base-case, where treatment duration was assumed to be equal to median time on treatment. This scenario resulted in a net budgetary impact of –$450,950 (–2.0%) over a three-year time horizon. Results by year were –$44,836 (38 patients), –$223,748 (58 patients), and –$182,367 (65 patients) in year 1, year 2, and year 3, respectively. The total costs PMPM prior to and after the introduction of tepotinib were $0.2083 and $0.2041, respectively; specifically, the budget impact PMPM was –$0.0012 in year 1, –$0.0062 in year 2, and –$0.0051 in year 3. Budgetary impact from the perspective of Medicare PDP payers is less than the base-case for Medicare because the former typically would not provide coverage for costly SoC regimens typically delivered in an inpatient setting. This eliminates any cost reductions that would otherwise accrue due to diversion of patients receiving those regimens to tepotinib.
A second scenario using Medicare’s Part D PDP was conducted which instead assumed time on treatment to be equal to median PFS. In this scenario, in a hypothetical PDP consisting of 1 million members, the introduction of tepotinib resulted in a net budgetary impact of $234,700 (1.0%) over a three-year time horizon. Results by year were $77,096, $54,315, and $103,289 in year 1, year 2, and year 3, respectively. The total PMPM costs prior to and after the introduction of tepotinib were $0.2214 and $0.2236, respectively; specifically, the budget impact PMPM was $0.0021 in year 1, $0.0015 in year 2, and $0.0029 in year 3. The results of this scenario differ significantly from the previous scenario as the median PFS is longer than the median treatment duration; this difference was observed across the tepotinib and capmatinib regimens and both 1L and 2L + treatment lines, and was most pronounced for tepotinib and in 2L + patients (8.1 months for median treatment duration, and 10.9 months for median PFS). This is particularly important given the significant ratio of 2L + to 1L patients (). With drug acquisition costs contributing to a significant portion of overall costs, a positive budgetary impact was observed.
Patient population split by 1L only and 2L + only
In the base-case, a line-agnostic patient population was modeled; all patients, irrespective of prior treatment, were modeled as a single cohort. On this basis, disease management and subsequent treatment costs for 1L are captured in 2L+.
This scenario assumes tepotinib is approved only for frontline (1L) patients but also accounts for the resultant downstream impacts, and therefore encompasses disease management and subsequent treatment costs. In a hypothetical Medicare plan consisting of 1 million members, the introduction of tepotinib resulted in a net budgetary impact of –$365,592 (–1.8%) over the three-year time horizon for the 1L patient population. Results by year were –$27,797 (21 patients), –$164,276 (32 patients), and –$173,519 (36 patients) in year 1, year 2, and year 3, respectively. The cost PMPM prior to and after the introduction of tepotinib was $0.1849 and $0.1815, respectively; specifically, the budget impact PMPM was –$0.0008 in year 1, –$0.0046 in year 2, and –$0.0048 in year 3.
A second scenario included only the 2L + patient population. In a hypothetical Medicare plan consisting of 1 million members, the introduction of tepotinib resulted in a net budgetary impact of $245,979 (2.5%) over the three-year time horizon for the 2L + patient population. Results by year were –$7,841 (17 patients), $81,714 (25 patients), and $172,106 (29 patients) in year 1, year 2, and year 3, respectively. The cost PMPM prior to and after the introduction of tepotinib was $0.0924 and $0.0947, respectively; specifically, the budget impact PMPM was –$0.0002 in year 1, $0.0023 in year 2, and $0.0048 in year 3.
The difference in results across the two populations is largely due to increased median time on treatment associated with tepotinib (8.1 months) relative to capmatinib (5.1 months) in the 2L + patient population, which in turn manifests in large differences in drug acquisition costs between the status quo and alternative scenarios.
Commercial payer perspective
This scenario examined the impact of tepotinib within the context of a US commercial health plan. Relative to the base-case, the model assumed a significantly younger population in this planCitation42, and, since incidence of NSCLC is much higher in older (65+ years) adultsCitation43, it was assumed fewer participants would be eligible for treatment with tepotinib relative to a Medicare plan (Supplementary Material SM1). In addition, this scenario often employed a different set of costs than that applied in the base-case, although it was assumed resource utilization would not materially differ (Supplementary Materials SM3–SM7). Within this context, eight patients would be eligible to receive tepotinib in year 1, while 12 and 13 patients would be eligible in years 2 and 3, respectively. The model estimated that introducing tepotinib within a commercial plan would generate a cumulative net budgetary impact of –$157,929 (–2.4%) over three years, including –$27,136 in year 1, –$73,447 in year 2, and –$57,346 in year 3. The total PMPM costs prior to and after the introduction of tepotinib were $0.0604 and $0.0589, respectively, implying a difference of –$0.0015 (–2.4%). Specifically, the budget impact PMPM was estimated at –$0.0008, –$0.0020, and –$0.0016 in year 1, year 2, and year 3, respectively (Supplementary Material SM10).
Discussion
Understanding the potential budgetary impact of introducing tepotinib – approved by the FDA in February 2021 as a treatment option in adult patients with mNSCLC harboring METex14 skipping alterations – is essential to inform formulary decisions in the context of a rapidly evolving therapeutic landscape. Accordingly, a BIM was developed in accordance with the ISPOR Good Modelling Practices GuidelinesCitation25,Citation26 to estimate the financial impact of reimbursing tepotinib. Prior to the approval of tepotinib, patients with this alteration typically received capmatinib (first FDA-approved targeted therapy for this indication), crizotinib (off-label use), or SoC, consisting of combinations of ICI monotherapy, ICI + chemotherapy ± anti-VEGF, chemotherapy + anti-VEGF, and chemotherapy alone.
In the base-case, the model estimated costs accruing to a hypothetical Medicare plan consisting of 1 million members with or without tepotinib in the market mix. The cumulative net budgetary impact was –$692,541 (or −2.6%) over three years, equivalent to a cumulative net budgetary impact of –$0.0064 PMPM. This was primarily driven by the combination of two factors. First, as indicated in , the model assumes tepotinib will gain the bulk of its market share from capmatinib. Second, although the monthly cost of tepotinib is higher than capmatinib, at the cohort level this is more than offset by differences in median treatment duration in the frontline setting (6.8 vs. 11.1 months for tepotinib and capmatinib, respectively), culminating in a lower overall cost per course of therapy ().
DSA results suggest variability in the cost of acquiring tepotinib and capmatinib had the largest impact on model outcomes, followed by variability in their respective median treatment durations, whereas the impact of variation in other parameters was comparatively more modest; however, the DSA broadly supports the base-case finding that from a budgetary standpoint, the financial impact of reimbursing tepotinib for Medicare payers is likely to be limited.
Numerous scenario analyses were also undertaken using the model. Increasing or decreasing testing rates directly impacted the number of patients eligible for treatment, and was therefore observed to amplify or attenuate tepotinib’s budgetary impact, respectively. Uniformly applying median PFS as a proxy for treatment duration inflated treatment costs for tepotinib relative to comparators for which this was already done (i.e. because median treatment duration for those comparators is unavailable), diminishing net budgetary impact relative to the base-case. Other scenarios considered the impact of reimbursing tepotinib in 1L or 2L + only or estimated the budgetary impact of tepotinib from the perspective of commercial or Medicare Part D PDP payers. Results from all scenarios aligned with expectations and supported the base-case finding that the introduction of tepotinib is unlikely to materially affect the financial position of health plans in the US.
The analyses relied upon numerous assumptions as well as data availability. Key clinical inputs such as time on treatment, PFS, OS, and AE profiles were based on values reported in US prescribing information and published clinical trials, many of which were conducted as multinational studies; however, the model assumed these to be generalizable to the US setting. Clinical inputs specific to the population of patients harboring METex14 skipping alternations do not presently exist for comparators other than tepotinib, capmatinib, and crizotinib; accordingly, data from trials in the general NSCLC population were used instead. In addition, although the model assumes treatment duration equal to median time on treatment where this information was available and PFS otherwise, in real-world treatment settings, oncologists may continue treatment even following progression, particularly where few treatment alternatives are available. However, while data gaps preclude investigating possible implications, this limitation is not expected to bias the results of the analysis, since the same consideration could apply to many of the interventions included in the study. Moreover, the approach chosen for this study was validated by a practicing oncologist. Another limitation is that estimation of subsequent treatment costs accounted only for expenditures associated with drug acquisition and did not consider any possible further impact on clinical and safety outcomes; in addition, the effectiveness of subsequent treatment was implicitly captured by OS data from the VISION trial and publicly available clinical data sources, rather than being modeled explicitly. Further to this, it is important to acknowledge the model’s limitations in capturing downstream impacts attributable to the choice of therapies in earlier treatment lines, and the resultant implications for plan budgets. For example, at present, treatment outcomes in 2L + do not depend on the class of therapies patients received in frontline. This simplifying assumption is common in budget impact analyses for new oncology agents, although it is also necessitated by a dearth of evidence surrounding clinical outcomes in relation to treatment sequences in the context of adult patients with mNSCLC harboring METex14 skipping alterations. Similarly, data gaps exist in estimates of medical resource utilization associated with disease management and treatment monitoring; therefore, these inputs were supplemented using clinical expert validation and/or assumptions, where necessary. It should also be noted that the model did not account for the availability of patient cost-sharing mechanisms, such as co-payments or coinsurance. Finally, although the results of this study may help to inform decisions regarding the allocation of scarce health care resources in the context of this indication, we acknowledge that cost-effectiveness analyses that consider outcomes alongside costs may also be of significant interest to stakeholders; accordingly, we believe this should be a priority for future research relating to the treatment of adult patients with mNSCLC harboring METex14 skipping alterations.
Conclusions
Results of the base-case analysis suggest the introduction of tepotinib had minimal financial impact associated with the treatment of adult patients with mNSCLC harboring METex14 skipping alterations from the perspective of US Medicare plans. Results of the DSA and the scenario analyses suggest this finding is robust to variation in key model parameters and may extend to US commercial and Medicare Part D PDP payers. These results are driven in part by the low prevalence of the METex14 skipping alterations, assumptions regarding projected rates of biomarker testing and the uptake of tepotinib, and differences in costs and estimated treatment duration between therapies.
Transparency
Declaration of funding
Funding for this study and article was provided by EMD Serono and Merck KGaA, and contracted with Evidera for services on this project and manuscript.
Declaration of financial/other relationships
MS, JT, AA, MM, and RS are employed by Evidera, a consulting company that has received funding from EMD Serono and Merck KGaA, the makers of TepmetkoFootnoteiii (tepotinib). AM was employed by The University of Arizona during the conduct of this study. MY and FL are employed by EMD Serono, and HV is employed by Merck KGaA. JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Author contributions
All authors contributed to the interpretation of data and results and drafting the manuscript. Additionally, AA, MM, MS, and JT contributed to the model development, conduct of analyses, and implementation of the design. All authors reviewed the final model design, data sources, and results. All authors meet the International Committee of Medical Journal Editors criteria for authorship, take responsibility for the integrity of the entirety of this work, and have given final approval to this version.
Previous presentations
A related abstract was previously presented at the 2021 American Society of Clinical Oncology Annual Meeting.
Supplemental Material
Download MS Word (146.2 KB)Acknowledgements
None reported.
Notes
i Microsoft Excel (Redmond, WA, USA).
ii IBM Micromedex RedBook (Armonk, NY, USA).
iii Tepmetko (Merck Healthcare KGaA, Darmstadt, Germany).
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386.
- Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3(4):524–548.
- SEER. National Cancer Institute – surveillance, epidemiology, and end results – cancer stat facts: lung and bronchus cancer; 2020; [cited 2020 May 4]. Available from: https://seer.cancer.gov/statfacts/html/lungb.html
- Travis WD, Asamura H, Bankier AA, et al. The IASLC lung cancer staging project: proposals for coding T categories for subsolid nodules and assessment of tumor size in part-solid tumors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol. 2016;11(8):1204–1223.
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–1260.
- Drilon A, Cappuzzo F, Ou SI, et al. Targeting MET in lung cancer: will expectations finally be MET? J Thorac Oncol. 2017;12(1):15–26.
- Jordan EJ, Kim HR, Arcila ME, et al. Prospective comprehensive molecular characterization of lung adenocarcinomas for efficient patient matching to approved and emerging therapies. Cancer Discov. 2017;7(6):596–609.
- Chen Z, Fillmore CM, Hammerman PS, et al. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14(8):535–546.
- Schrock AB, Frampton GM, Suh J, et al. Characterization of 298 patients with lung cancer harboring MET exon 14 skipping alterations. J Thorac Oncol. 2016;11(9):1493–1502.
- Rosell R, Karachaliou N. Large-scale screening for somatic mutations in lung cancer. Lancet. 2016;387(10026):1354–1356.
- Paik PK, Felip E, Veillon R, et al. Tepotinib in non-small-cell lung cancer with MET exon 14 skipping mutations. N Engl J Med. 2020;383(10):931–943.
- Wolf J, Seto T, Han JY, et al. Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med. 2020;383(10):944–957.
- Cai B, Zhou ZY, Xue W, et al. Budget impact of capmatinib for adults with metastatic non-small cell lung cancer harboring a MET exon 14 skipping mutation in the United States. J Med Econ. 2021;24(1):131–139.
- Pfizer. Highlights of prescribing information: XALKORI® (crizotinib) capsules, for oral use. US Food and Drug Administration; 2011; [cited 2021 May 14]. Available from: http://labeling.pfizer.com/ShowLabeling.aspx?id=676
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology (NCCN guidelines): non-small cell lung cancer, version 4; 2021; [updated 2021 Mar 3]. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450
- ClinicalTrials.gov. CABozantinib in non-small cell lung cancer (NSCLC) patients with MET deregulation (CABinMET); 2019; [cited 2021 Jun 4]. Available from: https://clinicaltrials.gov/ct2/show/NCT03911193
- Klempner SJ, Borghei A, Hakimian B, et al. Intracranial activity of cabozantinib in MET exon 14-positive NSCLC with brain metastases. J Thorac Oncol. 2017;12(1):152–156.
- Wang SXY, Zhang BM, Wakelee HA, et al. Case series of MET exon 14 skipping mutation-positive non-small-cell lung cancers with response to crizotinib and cabozantinib. Anticancer Drugs. 2019;30(5):537–541.
- US Food and Drug Administration. FDA grants accelerated approval to tepotinib for metastatic non-small cell lung cancer; 2020; [cited 2021 Mar 2]. Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-tepotinib-metastatic-non-small-cell-lung-cancer
- Wood R, Taylor-Stokes G. Cost burden associated with advanced non-small cell lung cancer in Europe and influence of disease stage. BMC Cancer. 2019;19(1):214.
- Migliorino MR, Santo A, Romano G, et al. Economic burden of patients affected by non-small cell lung cancer (NSCLC): the LIFE study. J Cancer Res Clin Oncol. 2017;143(5):783–791.
- Runyan A, Banks J, Bruni DS. Current and future oncology management in the United States. J Manag Care Spec Pharm. 2019;25(2):272–281.
- Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014;17(1):5–14.
- Caro JJ, Briggs AH, Siebert U, et al. Modeling good research practices—overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-1. Med Decis Making. 2012;32(5):667–677.
- Mauskopf JA, Sullivan SD, Annemans L, et al. Principles of good practice for budget impact analysis: report of the ISPOR Task Force on good research practices-budget impact analysis. Value Health. 2007;10(5):336–347.
- America's Health Insurance Plans. Medicare advantage demographics report, 2016; 2019; [cited 2020 Jun 22]. Available from: https://www.ahip.org/wp-content/uploads/MA_Demographics_Report_2019.pdf
- Drilon A, Clark J, Weiss J, et al. OA12.02 updated antitumor activity of crizotinib in patients with MET exon 14-altered advanced non-small cell lung cancer. J Thorac Oncol. 2018;13(10):S348.
- Chen VW, Ruiz BA, Hsieh MC, et al. Analysis of stage and clinical/prognostic factors for lung cancer from SEER registries: AJCC staging and collaborative stage data collection system. Cancer. 2014;120(Suppl. 23):3781–3792.
- EMD Serono. Flatiron health EHR-derived deidentified database, data on file; 2020.
- EMD Serono. Data on file; 2020.
- VanderLaan PA, Roy-Chowdhuri S. Current and future trends in non-small cell lung cancer biomarker testing: the American experience. Cancer Cytopathol. 2020;128(9):629–636.
- IBM. Micromedex® RED BOOK®; 2021. Available from: www.ibm.com
- Mauskopf J, Earnshaw S. A methodological review of US budget-impact models for new drugs. Pharmacoeconomics. 2016;34(11):1111–1131.
- Centers for Medicare & Medicaid Services. Medicare HO: CMS.gov. Hospital outpatient PPS payment by HCPCS code for CY; 2020; [cited 2021 Feb]. Available from: www.cms.gov
- Centers for Medicare & Medicaid Services. Medicare HO: CMS.gov. Physician fee schedule look-up tool. National 2020 payment amount by HCPCS code 2020; 2020; [cited 2020 Sep 23]. Available from: www.cms.gov
- Dalal AA, Guerin A, Mutebi A, et al. Treatment patterns, clinical and economic outcomes of patients with anaplastic lymphoma kinase-positive non-small cell lung cancer receiving ceritinib: a retrospective observational claims analysis. J Drug Assess. 2018;7(1):21–27.
- Graham J, Earnshaw S, Lim J, et al. Cost-effectiveness of afatinib versus erlotinib in the first-line treatment of patients with metastatic non-small cell lung cancer with EGFR exon 19 deletion mutations. J Clin Pathways. 2016;2(4):31–39.
- Agency for Healthcare and Research Quality. HCUPnet home page; 2021. Available from: https://hcupnet.ahrq.gov/#setup
- Centers for Medicare & Medicaid Services. Medicare HO: CMS.gov. Clinical laboratory fee schedule CY 2020. Q4 release 2020; [cited 2021 Jan]. Available from: www.cms.gov
- Gierman HJ, Pai N, Catasus C, et al. A retrospective three-year analysis using real-world data on uptake of broad-based NextGen sequencing panels in community oncology practices. J Clin Oncol. 2020;38(15_suppl):e13668.
- Berchick ER, Barnett JC, Upton RD. Health insurance coverage in the United States: 2018. Current population reports 2019; 2020; [cited 2020 Jun 22]. Available from: https://www.census.gov/content/dam/Census/library/publications/2019/demo/p60-267.pdf
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: incidence—SEER 9 Regs Research Data; 2019. Sub (1975–2017) 2020 [cited 2020 Jul 29]. Available from: https://canques.seer.cancer.gov/cgi-bin/cq_submit?dir=seer2017&db=1&rpt=TAB&sel=1∧0∧0∧107∧48∧0∧0∧1,22&x=Age%20at%20diagnosis∧1,22&dec=1,1,1&template=null