Abstract
Objective
To estimate the migraine-related healthcare resource utilization (HRU) and costs among patients with improved vs. worsened/stable migraine.
Methods
This was a follow-up to a retrospective, panel-based chart review conducted in France, Germany, Italy, and Spain among a panel of physicians (neurologists, headache specialists, and pain specialists) who agreed to participate in patient studies and had treated ≥10 migraine patients in 2017. Eligible physicians extracted data for up to five adults with ≥4 monthly migraine days (MMDs) who initiated a preventive treatment on or after 1 January 2013 and received physician care for ≥6 months after the date of the most recent preventive treatment initiation (index date). Based on the trajectory of migraine severity from the 1-month pre-index period to the 6-month post-index period, cohorts were classified as improved (converting from chronic to episodic or from chronic/episodic to <4 MMDs) or stable/worsened (remaining chronic/episodic or transforming from episodic to chronic) migraine. Migraine-related HRU and costs (2017 €) during the 6-month post-index period were compared between patients with improved vs. stable/worsened migraine.
Results
Overall, 470 patient charts were analyzed, with 339 classified as improved migraine and 131 classified as stable/worsened migraine. After adjusting for within-physician correlation, country, sex, and presence of comorbidities before the index date, the improved migraine cohort had significantly fewer migraine-related physician office visits (−0.81; p < .001), emergency room/accident & emergency (ER/A&E) visits (−0.67; p < .001), and hospitalizations (−0.12; p < .001) in the 6-month post-index period vs. the stable/worsened migraine cohort. Consistent with HRU patterns, the adjusted migraine-related costs for physician office visits (−€42.23; p < .05), hospitalizations (−€215.56; p < .05), and total costs (−€396.81; p < .01) in the 6-month post-index period were significantly reduced for the improved migraine cohort vs. the stable/worsened migraine cohort.
Conclusions
Over a 6-month period following initiation of preventive migraine treatment, patients with improved migraine had significantly lower migraine-related HRU and costs than those with stable/worsened migraine.
Introduction
Migraine is a distinct neurological disease that typically affects over 136 million adults across Europe [Citation1]. Evidence suggests that the prevalence of migraine is 2–3 times higher in women than in men, and it mainly affects individuals during their peak productive years (30–50 years of age) [Citation2,Citation3]. Based on the frequency, severity, and symptoms of the headache, migraine is primarily classified into an episodic migraine (EM; <15 headache days per month) and chronic migraine (CM; ≥15 headache days per month for over 3 months, of which ≥8 are migraine days) [Citation4].
The economic burden imposed by migraine is substantial, with annual costs estimated to range between €18 and €111 billion across Europe [Citation5–8]. Data from previous studies suggest that CM is associated with a higher disability burden than EM, and this difference is also reflected in terms of the societal and economic impact [Citation5,Citation9–14]. Several preventive medications (anticonvulsants, antihypertensives, antidepressants, serotonin antagonists, calcium channel antagonists, onabotulinumtoxin A [for CM], and calcitonin gene-related peptide [CGRP] monoclonal antibodies) have been used in clinical practice for treating migraine [Citation15,Citation16]. Unfortunately, many of the available preventive therapies have failed to impart the desired improvement, leading to increased resource use and cost burden on the healthcare system [Citation17].
EM has the potential to progress into a chronic form (referred to as chronification), with ∼3% (population studies) to 14% (clinic-based studies) of patients progressing from EM to CM annually [Citation18–20]. Evidence suggests that migraine chronification is reversible, and ∼26% of patients improve from CM to EM within 2 years of chronification [Citation19,Citation21,Citation22]. Several modifiable and non-modifiable factors, including depression, anxiety, sleep disorders, obesity and metabolic syndrome, hypothyroidism, allodynia, female sex, overuse of acute medication, and ineffective treatment of acute migraine, have been reported to increase the risk of migraine chronification [Citation19,Citation20,Citation23,Citation24].
Despite the potential cost implications, limited real-world studies have quantified the impact of improving migraine outcomes (i.e. reducing the number of migraine days patients experience per month) [Citation25]. To help fill this knowledge gap, the present analysis was conducted to estimate the migraine-related healthcare resource utilization (HRU) and costs associated with migraine improvement vs. migraine worsening/stability among patients in France, Germany, Italy, and Spain who had ≥4 monthly migraine days (MMDs).
Methods
Study design and data source
This study was a follow-on to a retrospective, non-interventional, panel-based chart review that collected anonymized patient-level clinical and HRU data via chart extraction between April and June 2018. The objective of the original parent study was to analyze the burden of patients with migraines in the outpatient and emergency room (ER) settings [Citation26,Citation27]. Subsequently, the protocol was amended to add the objective of quantifying and better understanding the impact of migraine on migraine-related HRU and costs. In brief, physicians (neurologists, headache specialists, and pain specialists) from France, Germany, Italy, and Spain who had treated at least 10 patients with migraines in 2017 were recruited from April to June 2018. Eligible physicians were invited to randomly select charts of up to 5 adult patients with clinically confirmed migraines who had initiated a preventive treatment on or after 1 January 2013. De-identified data were collected using electronic case report forms (developed by the study investigators), which had been pilot-tested by 1 physician from each country. Contributing physicians took ∼15–20 min to complete each chart extraction. This study was granted an exemption from institutional board review by the Western Institutional Review Board on 14 March 2018.
Study population
This study included adult patients (≥18 years of age) with ≥4 MMDs who had initiated a preventive treatment on or after 1 January 2013 and received physician care for ≥6 months after the index date. The date of the most recent preventive treatment initiation for migraine was defined as the index date. A panel of physicians contracted by the study investigators were asked to sample up to 5 patients meeting the eligibility criteria whose last name began with a randomly generated letter. If fewer than 5 patients met the eligibility criteria for a certain letter, another random letter would be generated until 5 patients were selected.
Classification of cohorts
Based on the number of monthly headache days, patients were first classified as having CM (patients with ≥15 headache days, of which ≥8 were migraine days) and EM (patients not meeting the criteria for CM). Next, based on the trajectory of migraine severity from baseline to the 6-month post-index period, these patients were stratified into the improved migraine and stable/worsened migraine cohorts using the following definitions:
Improved migraine cohort: patients having CM during the baseline period whose condition improved to EM during the post-index period. Patients having CM during the baseline period whose condition improved to having <4 MMDs during the post-index period. Patients having EM during the baseline period whose condition improved to having <4 MMDs during the post-index period.
Stable/worsened migraine cohort: patients having CM during the baseline period whose condition remained as CM during the post-index period. Patients having EM during the baseline period whose condition remained as EM (patients having ≥4 MMDs during the study period) or worsened to CM during the post-index period.
Patients with information on both the number of migraines and non-migraine headache days were considered, whereas those with no data on the number of non-migraine headache days were excluded from the analysis.
Study measures
Patient and disease characteristics
Patient demographics, such as age, sex, weight, and body mass index (BMI), were measured on the index date. Disease characteristics were measured during the baseline period (i.e. before the index date) and included employment status, comorbid conditions, history of menstrual migraines, time since initiation of preventive treatment, days of work or school missed, and loss of productivity owing to migraine. In addition, data on the average number of migraine and non-migraine headache days per month and the average duration in hours of a migraine episode and of a non-migraine headache before the index date were assessed.
Healthcare resource utilization and costs
Migraine-related HRU and costs during the 6-month post-index period following the index date were compared between the improved and stable/worsened migraine cohorts. Specific migraine-related HRU measures included physician office visits, ER/accident & emergency (A&E) visits, and hospitalizations. Migraine-related cost data for a physician’s office visit, hospitalization, and total healthcare costs were collected. The total healthcare costs were estimated as the sum of the costs of hospitalizations, outpatient visits, ER/A&E visits, specialist visits, and procedures or tests, assuming zero costs for the HRU categories with the missing number of events (i.e. those where physicians answered “unknown/not sure”). The cost associated with each migraine-related HRU category was calculated by multiplying the number of events by its local unit cost (Supplementary Table 1). All costs were expressed in euros (€) inflated to 2017 €. For France and Germany, the local unit costs were derived from the study by Bloudek et al. [Citation5], and for Italy and Spain, the local unit costs were provided by Novartis country colleagues (Supplementary Table 1).
Statistical analyses
All study variables were summarized descriptively for the overall sample and stratified as improved vs. stable/worsened migraine. Categorical variables were summarized using frequency counts and percentages, and continuous variables were summarized using means and standard deviations (SDs). In addition to unadjusted comparisons, HRU and cost outcomes during the 6 months following preventive treatment initiation were compared between the 2 groups using generalized estimating equations (GEEs) accounting for within-physician correlation (because physicians could submit up to 5 patient charts). HRU was assessed using a Poisson distribution and a log link function. For cost outcomes, Gaussian distribution and an identity link function were used. The following 2 models adjusted for different variables were used for each outcome: (1) adjusting for the country and (2) adjusting for the country, sex, and the presence of comorbidities before the index date. Predicted means for each cohort, 95% confidence intervals (CIs), predicted mean differences, and p-values were reported. Predicted mean differences represent the burden associated with a lack of migraine improvement despite preventive treatment. A p-value of <.05 was set as the threshold for statistical significance. All analyses were conducted using the Statistical Analysis System (SAS) version 9.4 and R version 3.4.0 software.
Results
Patient disposition
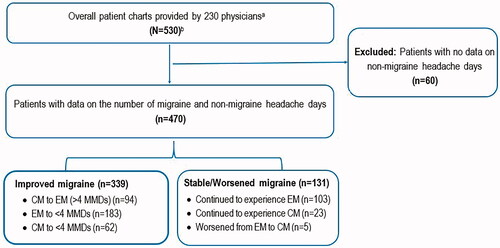
Overall, 530 patient charts (France: 160; Germany: 80; Italy: 141; Spain: 149) extracted by 230 physicians (France: 58; Germany: 35; Italy: 84; Spain: 53) were eligible for inclusion. Of these, 470 patients with data on the number of migraine and non-migraine headache days were included in the analysis. The remaining patients (n = 60) lacked information on the number of non-migraine headache days and were excluded from the analysis. Of the 470 patients analyzed, 339 were classified as having improved migraine (hereafter referred to as the improved migraine cohort), whereas 131 patients were classified as having stable/worsened migraine (hereafter referred to as the stable/worsened migraine cohort). The disposition of patients that constituted the improved and stable/worsened migraine cohorts is provided in .
Baseline characteristics
summarizes the baseline demographic and disease characteristics stratified by cohort. Barring subtle exceptions, patients across the improved and stable/worsened migraine cohorts had similar demographic and disease characteristics. The mean age of patients was 37.09 years in the improved migraine cohort and 36.92 years in the stable/worsened migraine cohort. Men were less predominant in both cohorts (improved migraine vs. stable/worsened migraine: 33.92 vs. 35.11%, respectively), and most patients were employed full-time (improved migraine vs. stable/worsened migraine: 56.05 vs. 58.75%). The most common comorbidities across both cohorts were depression (improved migraine vs. stable/worsened migraine: 16.22 vs. 19.85%), hypertension (13.86 vs. 12.21%), obesity (9.14 vs. 10.69%), and constipation (8.26 vs. 3.82%). Among the female patients (n = 309), over half had a history of menstrual migraines across both cohorts (improved migraine vs. stable/worsened migraine: 54.02 vs. 58.82%). The average time since initiation of first preventive migraine treatment was comparable across both cohorts (improved migraine vs. stable/worsened migraine: 2.26 vs. 2.17 years, respectively). In the month before the index date, patients in the improved migraine cohort experienced a higher mean number of MMDs than those in the stable/worsened migraine cohort (9.85 vs. 8.74; p < .05); the number of non-migraine headache days and the average duration of migraine and non-headache episodes were comparable. Although the percentage of patients experiencing loss of productivity at work or school because of migraine in the month before the index date was comparable (improved migraine vs. stable/worsened migraine: 75.66 vs. 74.11%), the average number of work or school days missed because of migraine was significantly higher in the stable/worsened migraine cohort compared with the improved migraine cohort (4.97 vs. 3.21 days; p < .001; ).
Table 1. Patient and disease characteristics by cohorts.
Migraine-related healthcare resource utilization and costs
Without exceptions, the unadjusted migraine-related HRU during 6 months after initiation of preventive treatment was lower among patients with improved migraine compared with those with stable/worsened migraine (). Among patients with inpatient hospitalization, the mean length of hospital stay was shorter in patients with improved migraine compared with those with stable/worsened migraine (2.79 vs. 4.00 days, respectively).
Table 2. Unadjusted migraine-related healthcare resource utilization and costs during the 6-month period stratified by cohort.
Consistent with the HRU patterns, the unadjusted migraine-related costs for each resource category (physician office visits, ER/A&E visits, and hospitalizations) and total costs during the 6-month follow-up period were significantly lower for patients with improved migraine compared with those with stable/worsened migraine ().
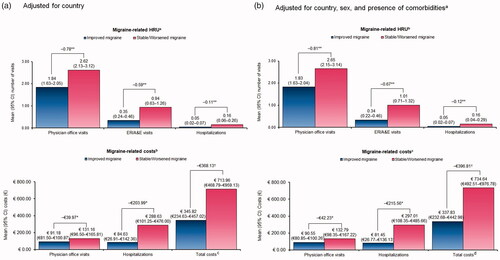
After adjusting for within-physician correlation and country, patients in the improved migraine cohort had significantly fewer office visits (−0.78; p < .001), ER/A&E visits (−0.59; p < .001), and hospitalizations (−0.11; p < .001) during the 6-month period following the initiation of migraine preventive treatment compared with patients in the stable/worsened migraine cohort. Accordingly, total costs during the 6-month period were significantly lower for patients in the improved migraine cohort compared with those in the stable/worsened migraine cohort (p < .01; ). Furthermore, after accounting for within-physician correlation, country, sex, and presence of comorbidities before the index date, patients with improved migraine had significantly fewer office visits (−0.81; p < .001), ER/A&E visits (−0.67; p < .001), and hospitalizations (−0.12; p < .001) during the 6-month period compared with those with stable/worsened migraine. In line with the trend observed with HRU, the total costs during the 6-month period were significantly lower for patients with improved migraine compared with those with stable/worsened migraine (p < .01; ).
Figure 2. Migraine-related healthcare resource utilizationa and costsb during the 6-month period stratified by cohorts. (a) Adjusted for country. CI: confidence interval; ER/A&E: emergency room/accident & emergency; HRU: healthcare resource utilization. *p < .05; **p < .001; †p < .01. aPredicted means were estimated using generalized estimating equations with a Poisson distribution and a log link function, accounting for within-physician correlation. bPredicted means were estimated using generalized estimating equations with a Gaussian distribution and an identity link function, accounting for within-physician correlation. cTotal costs include costs for outpatient visits; ER/A&E visits; hospitalizations; nurse practitioner, psychologist, psychiatrist, physiotherapy, or other specialist visits; cranial computerized topography scans; cranial and cranio-cervical magnetic resonance imaging scans; blood tests; nerve stimulator procedures; occipital nerve block procedures; electroencephalograms; and electrocardiograms. If a physician selected “unknown/not sure” for any healthcare resource item, €0 was assumed. If HRU costs were not available, costs were not included. (b) Adjusted for country, sex, and presence of comorbiditiesa. CI: confidence interval; ER/A&E: emergency room/accident & emergency; HRU: healthcare resource utilization. *p < .05; **p < .001; †p < .01. aComorbidities before the index date (yes/no). bPredicted means were estimated using generalized estimating equations with a Poisson distribution and a log link function, accounting for within-physician correlation. cPredicted means were estimated using generalized estimating equations with a Gaussian distribution and an identity link function, accounting for within-physician correlation. dTotal costs include costs for outpatient visits; ER/A&E visits; hospitalizations; nurse practitioner, psychologist, psychiatrist, physiotherapy, or other specialist visits; cranial computerized topography scans; cranial and craniocervical magnetic resonance imaging scans; blood tests; nerve stimulator procedures; occipital nerve block procedures; electroencephalograms; and electrocardiograms. If a physician selected “unknown/not sure” for any healthcare resource item, €0 was assumed. If HRU costs were not available, costs were not included.
Discussion
This study quantified the migraine-related resource and cost burden among European patients with migraine based on the trajectory of migraine severity from baseline to the 6-month post-index period. We hypothesized and confirmed that over a 6-month period following initiation of preventive migraine treatment, migraine-related HRU (physician office, ER/A&E visits, and hospitalizations) and costs were significantly lower for patients with improved migraine than for those with stable/worsened migraine.
The economic burden of migraine in Europe has been well-documented [Citation5–8,Citation26,Citation27]. Furthermore, studies have demonstrated that CM is far more disabling and costlier than EM [Citation9,Citation11]. However, little is known about the burden imposed because of migraine chronification [Citation25]. Using patient-level data abstracted by physicians, the present study assessed the migraine-related HRU and costs in patients with migraine based on the trajectory of migraine severity from baseline to the 6-month follow-up period. The results of this study showed that migraine-related HRU (physician office, ER/A&E visits, and hospitalizations) during the 6-month period following preventive treatment initiation was significantly reduced in patients whose migraine improved compared with patients with stable/worsened migraine. Accordingly, the total migraine-related costs during the 6-month period were significantly lower for patients in the improved migraine cohort compared with those in the stable/worsened migraine cohort (€337.83 vs. €734.64; Δ€396.81; p < .01). Although this study did not investigate the reason for this difference in HRU and costs between the cohorts, we speculated that these differences may be attributable to the difference in the baseline migraine severity (Migraine Disability Assessment Score) and monthly headache days, better health outcomes owing to the reduction in acute medication overuse and use of more effective preventive therapies, and patient-centered management of migraine [Citation22,Citation28,Citation29].
The results of this study are consistent with those of a recent retrospective study that assessed the economic burden and risk factors of migraine disease progression in a US population [Citation25]. Using claims-based data (2012–2016), Foster et al. reported that the all-cause and migraine-specific HRU (outpatient, inpatient, ER, and pharmacy) and costs increased significantly among patients who progressed from EM to CM compared with patients who did not progress in the 24-month post-index period (all p < .0001) [Citation25]. Moreover, the differential HRU and cost trends observed in this study are in line with current literature that reports higher resource and cost burden among patients with CM vs. those with EM [Citation5,Citation10,Citation12–14]. Data from population-based studies have shown that patients with CM use more health resources than those with EM in the US, Canada, UK, France, Germany, Italy, and Spain [Citation5,Citation10,Citation12,Citation14]. Similarly, a retrospective analysis of the electronic medical records of Italian patients with EM and CM showed that the annual direct cost per patient was 4.8-fold higher for CM vs. EM (€2037 vs. €427; p = .001) [Citation13]. Taken together, these findings provide valuable information on the economic burden associated with migraine chronification in Europe. Considering the impact of migraine chronification, effective preventive therapies that can prevent chronification are needed to reduce the resource and cost burden among patients with migraines in real-world practice [Citation17,Citation29,Citation30].
Limitations
The results of this study should be interpreted within the context of its limitations. There is a possibility that errors were introduced during chart abstraction and data entry; however, relevant logic checks were implemented to minimize such errors. The findings may be affected by inaccurate data recorded in the primary charts, recall bias, and non-random missing data. Because this was a post-hoc analysis of a previously conducted retrospective, panel-based review [Citation26,Citation27], the classifications of CM and EM during the baseline period were based on only 1 month of data, whereas the International Classification of Headache Disorders (ICHD-3) diagnostic criteria require data for >3 months. The sample size of this study was relatively small, and thus, characteristics or outcomes with low occurrence rates may not have been estimated reliably. Additionally, stratification by baseline CM and EM status was not feasible given the small sample size of patients with CM who remained stable/worsened. Although patients eligible for the study were required to have ≥4 MMDs before the index date, the number of MMDs may have varied during the study period. In addition, the index date was the date of initiation of preventive migraine treatment, and therefore, the HRU estimation might have been conservative. The data on HRU during the baseline period were not collected and therefore could not be adjusted for in the analysis. Further, the study included patients treated for migraine in France, Germany, Italy, and Spain who had ≥4 MMDs before the initiation of preventive migraine treatment. Hence, the HRU and cost estimates of this study may not be representative of patients in other countries or the whole of Europe, patients who had less frequent migraine episodes per month, or those who had not initiated preventive migraine treatment. Finally, this study does not differentiate between types and costs of preventive migraine treatments initiated by patients in the sample which could differ in efficacy and cost. Future studies should consider assessing outcomes by class of treatment, and costs, across a larger and more diverse sample of patients.
Conclusions
In summary, this real-world study demonstrated that over a 6-month period following the initiation of preventive migraine treatment, patients with improved migraine had significantly lower migraine-related HRU and costs compared with those with stable/worsened migraine. Overall, this study highlights the economic burden associated with migraine chronification and underscores the need for optimal treatment strategies that could prevent chronification and its associated economic burden.
Transparency
Declaration of financial/other interests
PV is a full-time employee of Novartis and holds shares of Novartis.
ES, EW, MLZ, and NK are full-time employees of Analysis Group, Inc.
MMP, MN, DR, ST, and PJ are full-time employees of Novartis.
MF is a full-time employee of Novartis and holds shares of Novartis.
JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
PV, ES, EW, MLZ, NK, MKP, MN, DR, ST, PJ, and MF have made substantial contributions to the analysis or interpretation of data, drafted the work or substantively revised it, approved the submitted version, and agree to be accountable for the work.
Supplemental Material
Download PDF (155.4 KB)Acknowledgements
Editorial assistance was provided by Gloria DeWalt, an employee of Analysis Group, Inc. support for this assistance was funded by Novartis.
Data availability statement
The data that support the findings of this study are available from Analysis Group, Inc., but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Analysis Group, Inc.
Additional information
Funding
References
- Stovner LJ, Nichols E, Steiner TJ, et al. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954–976.
- Steiner TJ, Stovner LJ, Katsarava Z, et al. The impact of headache in Europe: principal results of the Eurolight project. J Headache Pain. 2014;15(1):31.
- Steiner TJ, Stovner LJ, Vos T, et al. Migraine is first cause of disability in under 50s: will health politicians now take notice? J Headache Pain. 2018;19(1):17.
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211.
- Bloudek LM, Stokes M, Buse DC, et al. Cost of healthcare for patients with migraine in five European countries: results from the International Burden of Migraine Study (IBMS). J Headache Pain. 2012;13(5):361–378.
- Linde M, Gustavsson A, Stovner LJ, et al. The cost of headache disorders in Europe: the Eurolight project. Eur J Neurol. 2012;19(5):703–711.
- Olesen J, Gustavsson A, Svensson M, et al. The economic cost of brain disorders in Europe. Eur J Neurol. 2012;19(1):155–162.
- Stovner LJ, Andrée C, Eurolight Steering Committee. Impact of headache in Europe: a review for the Eurolight project. J Headache Pain. 2008;9(3):139–146.
- Agosti R. Migraine burden of disease: from the patient's experience to a socio-economic view. Headache. 2018 May;58 Suppl 1:17–32.
- Blumenfeld AM, Varon SF, Wilcox TK, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS). Cephalalgia. 2011;31(3):301–315.
- Lanteri-Minet M. Economic burden and costs of chronic migraine. Curr Pain Headache Rep. 2014;18(1):385.
- Messali A, Sanderson JC, Blumenfeld AM, et al. Direct and indirect costs of chronic and episodic migraine in the United States: a web-based survey. Headache. 2016;56(2):306–322.
- Negro A, Sciattella P, Rossi D, et al. Cost of chronic and episodic migraine patients in continuous treatment for two years in a tertiary level headache centre. J Headache Pain. 2019;20(1):120.
- Stokes M, Becker WJ, Lipton RB, et al. Cost of health care among patients with chronic and episodic migraine in Canada and the USA: results from the International Burden of Migraine Study (IBMS). Headache. 2011;51(7):1058–1077.
- Agostoni EC, Barbanti P, Calabresi P, Italian chronic migraine group, et al. Current and emerging evidence-based treatment options in chronic migraine: a narrative review. J Headache Pain. 2019;20(1):92.
- American Headache Foundation. The facts about migraine [cited 2020 Nov 23]. Available from: https://americanmigrainefoundation.org/resource-library/migraine-facts/
- Delussi M, Vecchio E, Libro G, et al. Failure of preventive treatments in migraine: an observational retrospective study in a tertiary headache center. BMC Neurol. 2020;20(1):256.
- Katsarava Z, Schneeweiss S, Kurth T, et al. Incidence and predictors for chronicity of headache in patients with episodic migraine. Neurol. 2004;62(5):788–790.
- May A, Schulte LH. Chronic migraine: risk factors, mechanisms and treatment. Nat Rev Neurol. 2016;12(8):455–464.
- Torres-Ferrús M, Ursitti F, Alpuente A, et al. From transformation to chronification of migraine: pathophysiological and clinical aspects. J Headache Pain. 2020;21(1):42.
- Buse DC, Greisman JD, Baigi K, et al. Migraine progression: a systematic review. Headache. 2019;59(3):306–338.
- Manack A, Buse DC, Serrano D, et al. Rates, predictors, and consequences of remission from chronic migraine to episodic migraine. Neurology. 2011;76(8):711–718.
- Bigal ME, Lipton RB. Migraine chronification. Curr Neurol Neurosci Rep. 2011;11(2):139–148.
- Scher AI, Midgette LA, Lipton RB. Risk factors for headache chronification. Headache. 2008;48(1):16–25.
- Foster SA, Chen C-C, Ding Y, et al. Economic burden and risk factors of migraine disease progression in the US: a retrospective analysis of a commercial payer database. J Med Econ. 2020;23(11):1356–1364.
- Vo P, Gao W, Zichlin ML, et al. Real-world healthcare resource utilization related to migraine treatment failure: a panel-based chart review in France, Germany, Italy, and Spain. J Med Econ. 2019;22(9):953–959.
- Vo P, Gao W, Zichlin ML, et al. Migraine-related healthcare resource use in the emergency department setting: a panel-based chart review in France, Germany, Italy, and Spain. J Med Econ. 2019;22(9):960–966.
- Henning V, Katsarava Z, Obermann M, et al. Remission of chronic headache: Rates, potential predictors and the role of medication, follow-up results of the German Headache Consortium (GHC) Study. Cephalalgia. 2018;38(3):551–560.
- Lipton RB, Tepper SJ, Silberstein SD, et al. Reversion from chronic migraine to episodic migraine following treatment with erenumab: results of a post-hoc analysis of a randomized, 12-week, double-blind study and a 52-week, open-label extension. Cephalalgia. 2021;41(1):6–16.
- De Matteis E, Affaitati G, Frattale I, et al. Early outcomes of migraine after erenumab discontinuation: data from a real-life setting. Neurol Sci. 2021. DOI:https://doi.org/10.1007/s10072-020-05022-z