?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aims
This study was designed to describe health care resource utilization (HCRU) of patients with metastatic colorectal cancer (CRC) or gastric cancer to test the hypothesis that greater treatment variability would be associated with increased HCRU.
Methods
A retrospective observational study using Marketscan claims data was conducted. Eligible patients had a first diagnosis of metastatic CRC or gastric cancer between 2004 and 2015 and must have received systemic anti-cancer therapy after diagnosis. Treatment variability was measured using the Herfindahl-Hirschman Index (HHI). HHI scores were stratified by quartile. HCRU variables were evaluated throughout the follow-up period and described by 6-month periods. Chi-square test was used for categorical variables and ANOVA for continuous variables.
Results
A total of 55,403 CRC and 9,073 gastric cancer patients were eligible. First-line HHI scores ranged from 0.1304–0.2778 for CRC and 0.0383–0.1778 for gastric cancer by state of residence. Statistically significant differences by HHI quartiles for HCRU in CRC included hospitalizations (p = 0.0003), ER visits (p < 0.0001), ER visits leading to hospitalization (p < 0.0001), and supportive care (all agents studied, p < 0.01). For gastric cancer, significant differences by HHI quartile were observed for ER visits (p = 0.002) and selected supportive care (G-CSF, erythropoiesis-stimulating agents, bisphosphonates, nutritional support, and antiemetics, each p < 0.05). No consistent increasing or decreasing trends were observed across the quartiles for either cohort.
Limitations
Large sample sizes could lead to statistical significance without being clinically meaningful. High treatment heterogeneity in the gastric cancer cohort and lack of a homogeneous quartile for comparisons limited the ability to evaluate HCRU by different levels of treatment variability.
Conclusions
Statistically significant relationships were observed between treatment variability as measured by HHI and increased HCRU, but no consistent directional trends in HCRU variables were observed. Therefore, this study failed to reject the null hypothesis of equivalent HCRU by level of treatment variability.
Introduction
Gastrointestinal cancers together account for 333,680 new cancer cases and 167,790 cancer deaths each year in the United StatesCitation1. The most common of these is colorectal cancer (CRC), which accounts for 147,950 (44.3%) of those new cases and 52,300 (31.7%) of all gastrointestinal cancer deaths. Gastric cancer is a much less common disease (27,600 patients diagnosed per year, only 8.3% of all gastrointestinal cancers) but is associated with poor survival. While 64.6% of patients with CRC are alive 5 years after diagnosis, only 32.0% of patients with gastric cancer are alive at that time. Of note, the survival of patients diagnosed with metastatic disease is much lower; both CRC and gastric cancer patients diagnosed with metastatic disease have poor long-term outcomes (5-year survival rates of 14.3 and 5.5%, respectively)Citation2.
There are opportunities for early detection of CRC through colonoscopy, which is recommended among those with no elevated risk starting at age 50, while no population-based screening strategy exists for gastric cancer in the US. This may in part explain why only 22% of patients with CRC are diagnosed with metastatic disease, whereas 36% of all gastric cancers are first identified after the disease has already metastasizedCitation2.
Patients diagnosed with metastatic CRC benefit from more standardized treatment strategies and clear recommendations for systemic therapies as compared to the care for patients with metastatic gastric cancerCitation3,Citation4. While tailoring care to patients and their unique biomarkers is critical to ensure patients receive optimal care, the presence or absence of these biomarkers direct patient care to specific treatment strategies. According to the National Comprehensive Cancer Network (NCCN) Guidelines in Oncology, all patients with metastatic/unresectable CRC should undergo genomic testing using next-generation sequencing (NGS)Citation3. Patients with BRAF, NRAS, or KRAS mutations are recommended against receiving panitumumab or cetuximab, and patients with high microsatellite instability or mismatch repair genes (MSI-H/MMR) or NTRK will also influence treatment choice. Therefore, the treatment strategies for patients with metastatic CRC are tailored and streamlined to disease characteristics and strong evidence supports a relatively limited set of NCCN-recommended treatment regimens in the first-line setting.
In gastric cancer, however, only rare genomic biomarkers have been identified and there are few targeted therapies currently available for patient care. First-line therapy for patients with advanced gastric cancer generally consists of a variety of combinations of platinum and/or fluoropyrimidine agents and if the tumor overexpresses HER2, trastuzumab should be added to the regimenCitation4. HER2 may be detected by immunohistochemistry (IHC), which while it will not detect the variety of genomic alterations as NGS-based testing, is sufficient for most patients in the absence of targeted therapy or known biomarkers. NCCN Guidelines recommend targeted (e.g. IHC, polymerase chain reaction [PCR]) and not broad-based genomic testing for most patients with gastric cancerCitation4.
Real-world observations of how these very different gastrointestinal cancers are managed in routine clinical practice appear to be associated with these guidelines and available treatments. In CRC, real-world data demonstrate that more than 90% of all patients with metastatic disease are treated with one of three first-line regimensCitation5. Unlike CRC, there is considerable variability in the treatment patterns of patients with metastatic gastric cancer in the US, demonstrated by the high number and low proportion of use of treatment regimens across lines of therapyCitation6–8. These studies have shown more than 200 unique regimens utilized in the first-line treatment of patients with metastatic gastric cancer, with no single regimen utilized by more than 17% of patientsCitation6.
There is a lack of a clear and optimal treatment for patients with metastatic gastric cancer versus for those with CRC.
These very different gastrointestinal cancers provide an opportunity to explore the relationship between health care resource utilization (HCRU) and treatment variability as measured by the Herfindahl-Hirschman Index (HHI)Citation9. This study was therefore designed to evaluate the HCRU among patients in the setting of homogeneous care (CRC) and in highly heterogeneous care (gastric cancer), to evaluate the hypothesis that greater treatment heterogeneity is associated with increased HCRU.
Methods
Database and cohort selection
The IBM Marketscan Commercial and Medicare Supplemental Databases (formerly and at the time of study, Truven Marketscan Databases) were used for this study. These administrative claims data are patient-level de-identified data obtained from employers, health plans, hospitals, and Medicare programs. These data include health insurance claims across the continuum of care (including inpatient, outpatient, pharmacy and other carve-out services) from a variety of fee-for-service, preferred provider organizations and capitated health plans. These databases are fully complaint with US privacy laws and regulations, and as de-identified data do not qualify as human subjects research and are therefore exempt from these institutional review board requirementsCitation10. Research using Marketscan data have been widely published since the early 1990s.
Patients were eligible for this study who were adults (age 18 or older) diagnosed with CRC (ICD-9-CM 153.x, 154.0, or 154.1 or ICD-10-CM C18.x, C19.x, C20.x) or gastric cancer (ICD-9-CM 153.x, 154.0, or 154.1 or ICD-10-CM C18.x, C19.x, C20.x) between January 1, 2004 and December 31, 2015. To qualify as a diagnosis, two unique codes for the cancer must have been observed on two different dates in the database. Patients with both codes for CRC and gastric cancer were excluded to ensure cohorts were mutually exclusive. Patients with evidence of ICD codes for gastrointestinal stromal tumor or lymphoma were excluded, as these diseases frequently also utilize CRC and gastric cancer ICD codes in the billing process. Additionally, patients were required to have one or more codes indicating disease metastasis (ICD-9 197.x-199.1 or ICD-10 C78.x-C80.0) or have no evidence of a surgical resection or gastrectomy procedure to exclude resectable or earlier stage disease. As a result, these cohorts were limited to patients with advanced or metastatic disease (simply termed CRC and gastric cancer hereafter). The earliest observation of a code for CRC or gastric cancer was considered to be the index diagnosis date. Eligible patients were required to have evidence of receiving systemic anti-cancer therapy on or after the index diagnosis. The treatment regimens and lines of therapy were identified using standard published algorithms for each disease, respectively, consolidating fluoropyrimidines (capecitabine and fluorouracil) and two platinum agents (cisplatin and carboplatin)Citation11. Descriptive statistics were used to evaluate baseline (at index diagnosis) characteristics and treatment patterns for the CRC and gastric cancer cohorts, respectively.
Treatment variability
Treatment variability was calculated by US state of patient residence as recorded in the database. Each patient was assigned the state HHI score in which they resided, and data were summarized by US Census geographical region (Northeast, North Central, South, or West) using the following formula to calculate the HHI scoreCitation9,Citation12:
where si is the proportion of regimen i in the line of therapy, and N is the number of regimens. Thus, in the line of therapy with two regimens that each have 50% of the line of therapy, the HHI equals 0.502 + 0.502 = 0.5000. Scores above 0.2500 are considered very homogeneous, 0.1500–0.2500 moderately homogeneous, scores below 0.1500 reflect heterogenous treatment patterns, and any score below 0.0100 is complete fragmentationCitation13. A difference of 0.1000 can be considered a meaningful difference. Patients with unknown state of residence were excluded from all HHI-related analyses.
Health care resource utilization (HCRU) variables
HCRU variables included hospitalizations (proportion of patients hospitalized, number of hospitalizations, duration of stay, and 30-day readmissions), emergency room (ER) visits (proportion of patients with ER visits, number of ER visits, and ER visits that resulted in a hospital admission), supportive care (granuloctye colony-stimulating factor [G-CSF], erythropoiesis-stimulating agent [ESA], bisphosphonate, antiemetic, or opioid use), nutritional support, and hospice care. HCRU was descriptively evaluated in 6-month intervals.
Due to potential differential follow-up, HCRU was summarized in 6-month intervals for patients with claims data during each period, respectively, starting with the initiation of first-line therapy. Each 6-month period was continued for each patient until death or disenrollment from the health care plan. To avoid capturing periods without treatment differentially between 6-month periods, if a patient was not receiving treatment at the start of the subsequent 6-month period, the time period for HCRU assessment was initiated at the time point at which treatment was re-initiated, which may allow for gap periods without HCRU data reported during treatment-free intervals. HCRU was assessed for four sequential 6-month periods starting with the initiation of first-line therapy.
Relationship between treatment variability and HCRU variables
Patients were grouped by quartile of HHI scores for first-line therapy. Chi-square tests were used to compare categorical HCRU variables by patients in the lowest vs highest HHI quartile; analysis of variance (ANOVA) was used for continuous HCRU variables. Specific HCRU variables as defined above were investigated for the relationship with HHI score throughout the study period. All analyses were conducted with the CRC and gastric cancer cohorts independently using SAS 9.2 (SAS Institute, NC).
Results
A total of 55,403 patients with CRC and 9,073 patients with gastric cancer were eligible for inclusion in this study (). In both cohorts, the majority were male (51.1% CRC; 64.2% gastric cancer). The mean age at diagnosis was 62 years in both cohorts. Patients were similarly located across the US in both cohorts, with the majority of patients in the North Central and South regions.
Table 1. Study cohorts.
Treatment regimens were available for 55,207 patients with CRC in the first-line setting. The most common treatments received in the first-line setting were single-agent fluoropyrimidines (n = 13,208, 23.9%), fluoropyrimidines plus oxaliplatin (FOLFOX/CAPEOX, n = 9,612, 17.4%), and FOLFOX/CAPEOX plus bevacizumab (n = 5,339, 9.7%). Unclassified drugs only (all drugs in the regimen were coded using nonspecific or generic codes) were reported for 7,104 (12.9%) and could represent any set of drugs. Second-line treatments were observed among 42.3% (n = 23,334), with the most common regimens again FOLFOX/CAPEOX (n = 4,503, 19.3%), fluoropyrimidines (n = 2,061, 8.8%), and fluoropyrimidines plus irinotecan (FOLFIRI/CAPEIRI, n = 1,890, 8.1%). Similar to first line, regimens listing only unclassified drugs were recorded among 2,720 (11.7%) patients in the second line.
Treatment regimen data were available for 9,029 patients with gastric cancer in the first-line setting. The most frequently observed regimens were fluoropyrimidines (n = 1,058, 11.7%), FOLFOX/CAPEOX (n = 884, 9.8%), and platinum (carboplatin or cisplatin) plus paclitaxel (n = 722, 8.0%). Exclusively unclassified drugs were recorded among 826 (9.2%) patients in the first-line setting. Second-line treatments were observed among 4,496 (49.8%) patients. Of those, 448 (10.0%) received fluoropyrimidines, 312 (6.9%) received FOLFOX/CAPEOX, and 189 (4.2%) received FOLFIRI/CAPEIRI. Exclusively unclassified drugs were recorded among 413 (9.2%) patients.
Treatment variability
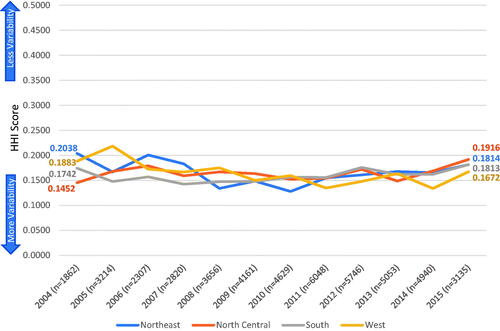
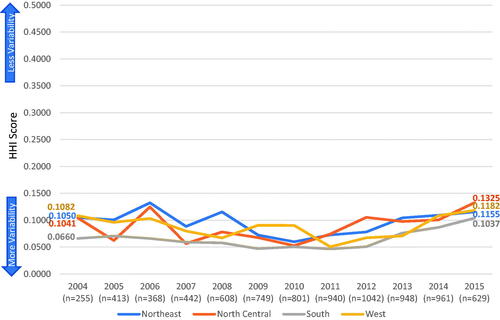
Of all the eligible patients, 47,571 (86.2%) with CRC and 8,156 (89.9%) with gastric cancer had both sufficient geographic location and treatment regimen details to be included in the HHI score calculations. HHI scores ranged from 0.1304–0.2778 for CRC and 0.0383–0.1778 for gastric cancer by state in the first-line setting. Regional treatment variability is summarized in and by year of index diagnosis. Treatment patterns across all time periods evaluated were moderately homogeneous for CRC and were heterogeneous for gastric cancer across all regions. For patients diagnosed in 2004, scores ranged from 0.1452–0.2038 for CRC and 0.0660–0.1082 for gastric cancer. Patients diagnosed in 2015 had comparable HHI scores (ranging from 0.1672–0.1916 for CRC and 0.1037–0.1325 for gastric cancer). None of the differences between years of diagnosis met the threshold change of 0.1000 for meaningful differences.
Health care resource utilization (HCRU) variables
Health care resources utilized by 6-month time periods are provided in (CRC) and (gastric cancer). Hospitalization, ER visits, antiemetics and opioids were each utilized by more than 30% of all patients during each 6-month period. The rate of hospital admission from an ER visit ranged from 17–20% for CRC and 25–32% for gastric cancer patients. The number of patients during each subsequent 6-month period was reduced due to the decreasing number of patients with claims recorded over the 2-year period presented. The reasons for lack of observation were not recorded but were all associated with either the end of the database or disenrollment from the health plan. Death dates were not available due to privacy concerns; however, some claims did record death as a reason for hospital discharge; these rates were relatively low (1.9–2.7% for CRC and 4.1–5.8% for gastric cancer) across each 6-month observation period.
Table 2. Health care resource utilization, colorectal cancer.
Table 3. Health care resource utilization, gastric cancer.
Relationship between treatment variability and HCRU variables
For the CRC cohort, there were statistically significant differences in HCRU variables by quartile of HHI scores for all variables except patients who died in the hospital, duration of hospital stay, 30-day readmissions, and ER visits within the last 30 days of life, all of which had low rates of occurrence in all cohorts (). There was no visible pattern of increase or decrease and the values did not appear to have large clinically meaningful differences. For the gastric cancer cohort, there were significant differences in supportive care medication use (G-CSFs, ESAs, nutritional support, bisphosphonates, and antiemetics) by HHI quartile (all p < 0.05), but no clear directional trends were observed ().
Table 4. Heath care resource utilization by quartile of treatment variability, colorectal cancer.
Table 5. Heath care resource utilization by quartile of treatment variability, gastric cancer.
Discussion
This study explored the hypothesis that greater variability in treatment would be associated with more health care resources utilized. The findings led us to fail to reject the null hypothesis of no difference for most HCRU variables. While there were multiple significant differences observed in the CRC cohort, the large sample size could have led to statistical significance that does not reflect clinically meaningful differences. The numeric differences did not follow an increasing or decreasing pattern but rather appeared to simply vary between quartiles. However, in gastric cancer where sample sizes are smaller, there were significant differences among a number of supportive care HCRU variables measured. Many of these appeared to have a pattern that suggests lower resource use among patients with high heterogeneity, but the lack of a truly homogeneous quartile for comparison limits the ability to conduct analyses to specifically test the hypothesis in the gastric cancer cohort. All quartiles in the gastric cancer cohort represented high treatment variability and were similar (did not vary by more than 0.1000). Additionally, the application of HHI at the state level (cannot be calculated for a single patient) to the relationship of patient-level HCRU (versus state-level HCRU) may have further introduced a level of bias. HCRU summarized across the state versus patient level would likely have resulted in wide confidence intervals, but similar units of study may be worth investigating in studies where the HHI can be calculated at a smaller unit, such as by clinic site. The Marketscan database did not contain a study site variable that could definitively assign the HHI score to a clinic practice site. Furthermore, the database does not contain disease stage which is one of the major determinants of treatment approaches. The inclusion of different stages of disease in a single cohort remains a risk that cannot be fully addressed. To reduce this risk of variability between patients in the study cohort, all patients were required to have non-resectable (estimated by the lack of surgical codes) or metastatic (requiring evidence of metastatic disease codes) disease. While patients with metastatic disease are likely to truly reflect a stage IV diagnosis, the absence of these codes cannot determine the absence of metastatic disease. Therefore, patients may have been excluded entirely who in fact did have metastatic disease, and it is unclear how many patients included due to unresectable disease had metastases. This uncertainty in disease stage, while addressed by restricting the use of diagnostic and procedural codes, remains a limitation of using claims dataCitation14.
The strengths of this study include a very large study cohort size with longitudinal data over multiple years. The patient-level details of treatments and HCRU provide a rich source of data to explore these relationships. The level of HCRU among patients included in this study is high. Approximately one-third of all patients with CRC required at least one hospitalization during the first two 6-month periods after diagnosis, and more than one-third of patients with gastric cancer required at least one hospitalization during each of the four 6-month periods reported in this study. Approximately half of all patients received opioid medications, and more than three-quarters of patients received antiemetic medications. Both CRC and gastric cancer are diseases that are challenging for the patient and require complex supportive care. It would not be expected that these measures of HCRU should or would be reduced, as these treatments will help patients manage disease symptoms and toxicities of the chemotherapy regimens that are recommended for their care. Additionally, in the analyses that included the duration of follow-up, many of these medications could be used apart from the treatment regimen in the supportive care of patients nearing the end of life. However, as the recommendations for the care of patients with these diseases have changed over time, it would be expected that HCRU most likely to be unplanned, such as hospitalizations and ER visits, may be reduced. A more focused evaluation by type of HCRU is recommended in subsequent research. Similarly, the duration of follow-up could influence analyses that evaluated the entire follow-up period. While there was no evidence in this study of different durations of follow-up, future research must consider that these are aggressive diseases, and HCRU could be influenced by different survival outcomes across groups if the duration of time for which data are collected is not considered in the analysis.
Treatment guidelines were updated multiple times during the study period. However, the variability of treatment did not vary by year. The actual drugs used in the study time period are similar to those used today. Despite multiple changes, the most common drugs observed in this study remain recommended therapies in the 4.2020 version of the NCCN Guidelines for colorectal cancer (FOLFOX/CAPEOX with or without bevacizumab) and in the 3.2020 version of the guidelines for gastric cancer (FOLFOX/CAPEOX, which is a preferred regimen, and fluoropyrimidines, both with level 2 A recommendations). Due to lack of clinical data such as results of biomarker testing in the Marketscan database, the appropriateness of these treatments cannot be evaluated in the context of biomarker-positive/wild-type disease. However, this study also demonstrates the longstanding treatment regimens in place for both these cancers over time, which may have played a role in the lack of changes in treatment variability observed in this study. Future research may wish to explore the role of guideline-consistent versus guideline-inconsistent regimens and HCRU to evaluate other factors that may be important beyond heterogeneity.
This study was unable to definitively answer the primary research question, and further work is needed to investigate the relationship between HCRU and treatment variability. Ideally, one would wish to assign HHI scores at a smaller level than US state. Each state contains a wide variety and types of practices, yet given the lack of variables to narrow to the site level, patients were assigned by state. It is highly likely that each US state, despite being assigned a single HHI score, actually represents a variety of homogeneous and heterogeneous settings. At this time, databases are limited that contain sufficient information to conduct such analyses. As real-world data sources continue to evolve, this study should be conducted where site-level information as well as complete HCRU are collected. It is important to note that the issues surrounding heterogeneity are a health care system issue in the standardization of treatments through guidelines and pathway systems. Heterogeneity itself has little bearing on a decision for a specific provider, but rather applies to the care recommendations in population-based health care decision-making.
Conclusions
Despite the limitations of this study, this study provides a strong summary of the HCRU among patients diagnosed with advanced or metastatic colorectal or gastric cancer throughout four 6-month periods after diagnosis. While there may be a relationship between treatment heterogeneity and HCRU, the findings from this study are inconclusive. Recommendations are made for further investigation of this important research question.
Transparency
Declaration of funding
The study was funded by Eli Lilly and Company.
Declaration of financial/other relationships
LMH, AML, and YEZ are employees of Eli Lilly and Company. AML is a shareholder of Eli Lilly and Company stock. YF is an employee of Syneos Health.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
An earlier version of this work was presented at the International Society of Gastrointestinal Oncology, October 2017 as an oral presentation.
Acknowledgements
None
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA A Cancer J Clin. 2020;70(1):7–30.
- SEER. Cancer Stat Facts 2020. [December 2, 2020]. Available from: https://seer.cancer.gov/statfacts/
- NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Colon Cancer V4.2020 2020. [December 3, 2020]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
- NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Gastric cancer V3.2020 2020. [December 3, 2020]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
- McLean J, Rho YS, Kuruba G, et al. Clinical practice patterns in chemotherapeutic treatment regimens for metastatic colorectal cancer. Clin Colorectal Cancer. 2016;15(2):135–140.
- Abrams T, Hess LM, Zhu YE, et al. Predictors of heterogeneity in the first-line treatment of patients with advanced/metastatic gastric cancer in the US. Gastric Cancer. 2018;21(5):738–744.
- Hess LM, Cui ZL, Wu Y, et al. Patient experience after receiving a diagnosis of gastric cancer in the USA. J Gastrointest Cancer. 2018;49(1):25–34.
- Paulson S, Hess LM, Chatterjee A, et al. The impact of a clinical decision support system for advanced gastric or gastroesophageal junction cancer. J Clin Pathways. 2020;6(10):55–64.
- Rhoades SA. The Herfindahl-Hirschman index. Fed Res Bull. 1993;79:188.
- HHS. Human Subject Regulations Decision Charts: 2018 Requirements 2020. [March 10, 2021]. Available from: https://www.hhs.gov/ohrp/regulations-and-policy/decision-charts-2018/index.html#c1.
- Hess LM, Li X, Wu Y, et al. Defining treatment regimens and lines of therapy using real-world data in oncology. Future Oncol. 2021;17(15):1865–1877.
- Cutler DM, Morton FS. Hospitals, market share, and consolidation. JAMA. 2013;310(18):1964–1970.
- Wright JD, Chen L, Accordino M, et al. Regional market competition and the use of immediate breast reconstruction after mastectomy. Ann Surg Oncol. 2019;26(1):62–70.
- Whyte JL, Engel-Nitz NM, Teitelbaum A, et al. An evaluation of algorithms for identifying metastatic breast, lung, or colorectal cancer in administrative claims data. Med Care. 2015;53(7):e49–e57.