Abstract
Aims
To study the association between initiation of first adjunctive therapy with eslicarbazepine acetate (ESL) vs. brivaracetam (BRV) on healthcare resource utilization (HCRU) and charges among patients with treated focal seizures (FS).
Materials and methods
Symphony Health’s Integrated Dataverse (IDV) claims data (1 April 2015 to 30 June 2018) were used to identify two cohorts as first adjunctive therapy with ESL or BRV following a generic anti-seizure drug (ASD). The index date was the earliest claim for a new ESL or BRV prescription. Key inclusion criteria were only 1 generic ASD in the 12 months before the index date; ≥1 medical claim with an FS diagnosis. Unit of analysis was the 90-day person-time-block. Changes in HCRU and charges were assessed using a difference-in-differences framework. Both unadjusted and adjusted analyses were performed. The adjusted model utilized person-specific fixed effects and propensity score-based weighting to control for differences in baseline covariates. Bias-corrected bootstrap confidence intervals (CIs) were calculated for charge outcomes.
Results
208 and 137 patients initiated first adjunctive therapy with ESL (43.7 years, 51.9% female) or BRV (39.3 years, 51.8% female). Patients in the ESL cohort had numerically larger reductions in all-cause and FS-related inpatient hospitalizations and outpatient visits and FS-related emergency department visits. Compared to patients initiating BRV, patients treated with ESL had significantly larger reductions in total charges (−$3,446, CI: −$13,716, −$425), all-cause (−$3,166, CI: −$13,991, −$323) and FS-related (−$2,969, CI: −$21,547, −$842) medical charges, all-cause (−$3,397, CI: −$15,676, −$818) and FS-related (−$2,863, CI: −$19,707, −$787) outpatient charges, and non-ASD-related prescription charges (−$420, CI: −$1,058, −$78).
Limitations
Claims may be missing, or miscoded; outcomes may be influenced by variables not accounted for in the analysis; only information on submitted charges was included.
Conclusions
Among patients with FS, initiation of first adjunctive therapy with ESL was associated with significantly larger reductions in medical and non-ASD-related prescriptions charges compared to BRV.
Introduction
Epilepsy is a common neurological disorder in the United States (US) with an estimated prevalence of 1.2%, including approximately 3 million adults aged 18 years or olderCitation1. Focal seizures (FS) constitute an estimated 60% of epilepsy casesCitation2.
The goal of epilepsy treatment is seizure reduction and/or freedom with minimal adverse effects. Medications include first – (e.g. phenytoin, valproate, carbamazepine [CBZ]), second – (e.g. gabapentin, lacosamide, lamotrigine [LTG], levetiracetam [LEV], oxcarbazepine [OXC], topiramate, zonisamide), and third-generation (e.g. eslicarbazepine acetate [ESL], brivaracetam, perampanel) anti-seizure drugs (ASDs)Citation3–5; third-generation ASDs are usually branded agents. Various guidelines make recommendations for initial monotherapy in adults with FS based on clinical and real-world evidenceCitation6. However, 50% of patients with epilepsy continue to experience seizures following initial monotherapy, and up to 30% are classified as refractory after at least two monotherapy regimensCitation7. In these patients, subsequent treatment strategies are substitution of the ineffective drug with another ASD, or administration of an additional ASD as adjunctive therapyCitation8. Epilepsy surgery may be an option for patients with epilepsy; however, the selection of epilepsy surgery candidates varies significantly between epilepsy centresCitation9. Third-generation ASDs are typically used in the treatment cycle as adjunctive therapyCitation10,Citation11. Third-generation ASDs are reported to have similar efficacy and several benefits compared to first- and second-generation ASDs, including fewer drug-drug interactions, fewer safety concerns, and infrequent psychiatric and cognitive adverse eventsCitation12–15, but selection criteria for third-generation ASDs as adjunctive therapies are not clearly defined.
ESL, a third-generation ASD, was first approved by the US Food and Drug Administration (FDA) in 2013 for the treatment of FS in adult patientsCitation16. Brivaracetam (BRV), a structural analog of LEV, was granted FDA approval in 2016 and is indicated for the treatment of FS in adultsCitation17. Pivotal clinical trials have demonstrated the safety and efficacy of ESL and BRV in patients with refractory epilepsyCitation12,Citation13. However, ESL is one of the few third-generation ASDs for which safety and efficacy have been investigated in a real-world clinical setting, early in the treatment cycle, in a Phase IV study in adults with FSCitation18. Although the sample size was small and the study was not powered to detect differences between patients receiving ESL as first adjunctive therapy to LEV or LTG, and patients with treatment-resistant epilepsy receiving ESL as later adjunctive therapy, findings showed that ESL was effective and well-tolerated both as the first adjunctive therapy to LEV or LTG monotherapy (n = 44) and as later adjunctive therapy in treatment-resistant patients (n = 58). ESL used as first adjunctive therapy to LEV or LTG resulted in markedly higher numerical reductions in standardized seizure frequency (SSF: 72.8%) and seizure freedom rates (25.0%) compared to ESL as later adjunctive therapy (SSF: 22.8%; seizure freedom rates: 9.6%). Treatment-emergent adverse events (TEAEs: 81% vs. 73%) and TEAEs leading to discontinuation (16% vs. 2%) were reported more frequently in those taking ESL as later versus first adjunctive therapy. Patients with epilepsy incur substantial healthcare resource utilization (HCRU) and costsCitation19. In general epilepsy populations in the US, annual total direct healthcare costs ranged from $10,192 to $47,862 per patient, and epilepsy-specific costs ranged from $1,022 to $19,749 per patientCitation20. Among the privately insured, patients with FS had significantly higher HCRU compared to matched enrollees without an epilepsy diagnosis, including 0.30 vs. 0.10 inpatient (IP) visits per patient per year, 0.69 vs. 0.30 emergency department (ED) visits per patient per year, and 14.5 vs. 8.1 outpatient (OP) visits per patient per yearCitation21.
In adults with FS, initiation of ESL as first-line (1 L) treatment or as first adjunctive therapy was associated with reductions in all-cause and FS-related HCRU and chargesCitation22,Citation23. Patients administered ESL as first adjunctive therapy experienced significant reductions in all-cause IP and all-cause and FS-related OP visits and significant increases in total prescription and ASD-related prescription charges during the follow-up periodCitation22. Compared to patients initiating a generic ASD as 1 L monotherapy, initiation of ESL was associated with significantly greater reductions in all-cause OP, ED, and FS-related OP visits, and all-cause medical, OP, non-FS-related medical, and non-ASD-related prescription charges, as well as greater relative increases in mean total prescription and ASD-related prescription chargesCitation23.
Evidence describing HCRU and cost outcomes for ESL in the 1 L and adjunctive setting is accumulating; however, comparable data for BRV are lacking, as no studies have directly compared resource use for ESL versus BRV as a first adjunctive therapy. Data on the effectiveness of BRV in patients receiving concomitant LEV or who have prior exposure to LEV are mixedCitation24. Hence, understanding differences in HCRU and charges may support decision-making by payers, providers, and patients, and reduce the economic burden associated with FS. The objective of this study was to determine the association between initiation of first adjunctive therapy with ESL compared to BRV on HCRU and charges among patients with treated FS.
Methods
Data source
Data for this analysis were extracted from Symphony Health’s Integrated Dataverse database. This is a national, administrative, open-source database that includes pharmacy claims, physician office medical claims, and hospital claims generated by commercial, Medicare, and Medicaid payers, and self-pay/uninsured patients. Symphony Health compiled longitudinal data on healthcare resource use (e.g. diagnoses, procedures), prescription fills, and charges for approximately 274 million patients. Data from 1 April 2015 to 30 June 2018 were studied. Data were de-identified in compliance with the Health Insurance Portability and Accountability ActCitation25. Using de-identified data in a study does not constitute Human Subjects Research under the United States Federal Policy for the Protection of Human Subjects (the Common Rule) 45 CFR part 46; therefore, review by an institutional review board was not requiredCitation26.
Study design
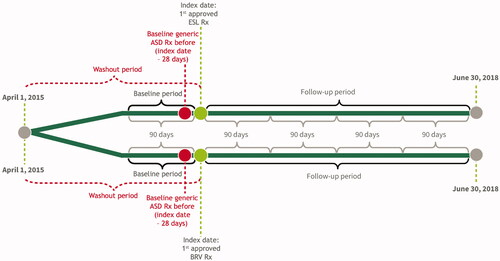
This retrospective, longitudinal cohort analysis included two cohorts of patients identified as first adjunctive therapy with ESL or BRV following a generic ASD (). A longitudinal panel data approach was used, with a person-specific “block” of 90 consecutive days as the unit of analysis. The index date was the date of the earliest claim for a new prescription (not a refill) of ESL or BRV. The baseline period was defined as the 90-day block immediately prior to the index date. The follow-up period consisted of at least 1 and up to 4 consecutive 90-day blocks immediately following the index date.
Patient population
This study included patients with treated FS and evidence of use of exactly one generic ASD prior to their index date. Patients qualified for one of two mutually exclusive cohorts: those who initiated ESL or BRV as the first adjunctive regimen to their baseline generic ASD. Patients had to satisfy the criteria for defining adjunctive therapy, including (1) ≥28 days of consecutive days with possession of a generic ASD prior to the index date; and (2) had an existing days’ supply of a generic ASD as of the index date and a subsequent claim for the same generic ASD within 30 days of exhaustion of the supply.
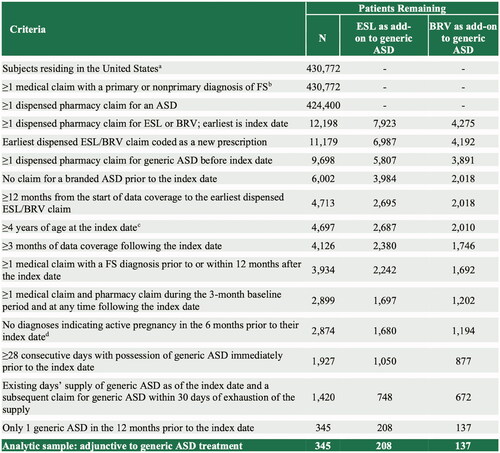
Additional sample selection criteria are described in . These included: (1) US residence; (2) ≥4 years of age as of their index date (measured assuming birth occurred on July 1 of birth year); (3) ≥1 one medical claim with an FS diagnosis code (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM]) diagnosis codes 345.4x or 345.5x or ICD-10-CM diagnosis codes G40.1x or G40.2x); (4) ≥1 dispensed pharmacy claim for an ASD; (5) ≥180 days elapsed between the start of data coverage and the index date; (6) data coverage for ≥90 days following their index date; and (7) ≥1 medical claim and ≥1 pharmacy claim (for any medication) during the 90 days prior to index date and at any time following the index date.
Figure 2. Sample selection. aExcludes patients residing in Puerto Rico and US territories, or with missing/invalid data; bDefined as ICD-9-CM codes 345.4x or 345.5x or ICD-10 codes G40.1x or G40.2x; cOnly the birth year was available; therefore, all patients were assigned a birthdate of July 1st for the purpose of calculating patient age; dDefined as ICD-9-CM codes V22.x, V23.xx, or 630-679.xx or ICD-10 codes Z33*–Z36*, or O00*–O99*. Abbreviations. ASD, anti-seizure drug; BRV, brivaracetam; ESL, eslicarbazepine acetate; FS, focal seizure; ICD-9/10-CM, International Classification of Diseases, 9th/10th Revision, Clinical Modification; LEV, levetiracetam; US, United States.
Study measures
Baseline characteristics
Demographic characteristics were assessed as of the index date and included age, gender, and Census region (North Central, Northeast, South, West).
Clinical characteristics included ASD-related characteristics and comorbidities. ASD-related characteristics were assessed on the index date and included patient copay amount, payer type (commercial, Medicaid, Medicare, other), and calendar year of the index date. Baseline comorbidities were defined as the presence of ≥1 medical claim with a diagnosis code for the respective comorbidity during the baseline period. The Charlson Comorbidity Index (CCI) was used to calculate a comorbidity score.
Healthcare resource utilization and charges in the baseline and follow-up periods
HCRU was categorized as all-cause and FS-related for IP, ED, and OP visits. Measures were constructed by patient-block during the baseline and follow-up periods. Claims were identified as IP, ED, and OP visits by their place of service. Each HCRU outcome was presented as the proportion of patients with ≥1 visit.
Charges were summed across claims for each patient block during the baseline and follow-up periods. Charges were analyzed for IP, ED, and OP visits and accumulated separately for all-cause and FS-related medical claims. In addition, charges associated with ASD-related prescription drug claims and non-ASD-related prescription drug claims were also assessed.
All claims, irrespective of their diagnosis codes, were used to identify all-cause HCRU and charges. Claims with a diagnosis of FS in any diagnosis position were used to identify FS-related HCRU and charges. The US Gross Domestic Product price index was used to inflate charges to 2018 US dollarsCitation27.
Statistical analysis
Baseline demographic and clinical characteristics were expressed as mean ± standard deviation (SD) for continuous variables and as counts and percentages for categorical variables. The differences in baseline characteristics between ESL and BRV cohorts were compared using t-tests (for continuous variables) and Chi-square tests (for categorical variables).
Changes in HCRU and charge outcomes from before to after initiation of ESL or BRV were compared using a difference-in-differences framework. Both unadjusted and adjusted analyses were conducted. An independent model was estimated for each of the HCRU and charge outcomes.
In the unadjusted model specification, a linear regression model was estimated to predict each outcome. The key explanatory variables were the cohort classification (i.e. ESL vs. BRV), an indicator variable for whether the time-block was from before or after ESL or BRV initiation (pre vs. post), and their interaction. The fitted coefficient on the interaction term was interpreted as the relative mean difference between patients who received ESL compared to BRV in the pre-post change in the outcome.
The adjusted model specification extended the unadjusted specification in two ways. First, time-invariant covariates were controlled for by adding person-specific fixed effects. Second, to adjust for differences in baseline covariates between the ESL and BRV cohorts, each observation was weighted by the odds of receiving ESL using propensity score-based weightingCitation28. Following the propensity score weighting, the balance between cohorts was assessed for each covariate via its standardized difference (Supplementary Table 1)Citation29. As with the unadjusted model specification, the fitted coefficient on the interaction between cohort and time period from the adjusted model was interpreted as the relative mean difference for patients who received ESL compared to BRV in the pre-post change in the outcome.
Table 1. Baseline demographics and clinical characteristics.
Regression standard errors were adjusted to be robust to heteroskedasticity and to account for multiple 90-day periods per individualCitation30.
Confidence intervals (CIs) were calculated for charge outcomes from a bias-corrected block bootstrap procedure with 10,000 replicatesCitation31–33.
Statistical significance was based on two-sided tests with α = 0.05. Statistical analyses were conducted using SAS software version 9.4 (SAS Institute, Cary, NC) and Stata MP software version 16.1 (StataCorp, LLC, College Station TX).
Results
Baseline characteristics
A total of 208 and 137 patients met the study inclusion criteria and initiated first adjunctive therapy with ESL or BRV, respectively. Patients’ baseline demographic and clinical characteristics are described in . Patients who initiated ESL were older than those receiving BRV (mean [SD] 43.7 [17.9] vs 39.3 [18.6] years; p = .024). Significantly more patients who initiated ESL had been prescribed LEV as their prior generic ASD (52.4% vs. 26.3%; p < .0001), while patients who initiated BRV were more likely to have been prescribed LTG (25.6% vs. 13.5%; p = .005), oxcarbazepine (11.7% vs. 5.3%; p = .03), or topiramate (9.5% vs. 3.9%; p = .032). There were no statistically statistical differences in gender, geographic region, comorbidities, payer type, patient copay amount, or CCI score between patients initiating ESL or BRV.
After weighting, standardized differences between patients who initiated ESL vs. BRV on key variables such as year of index prescription, prior generic ASD utilization, payer type, baseline medical, and prescription charges were within acceptable limits (<0.1). Supplementary Table 1 provides a summary of standardized differences before and after weightingCitation34.
Healthcare resource utilization and associated charges
Healthcare resource utilization
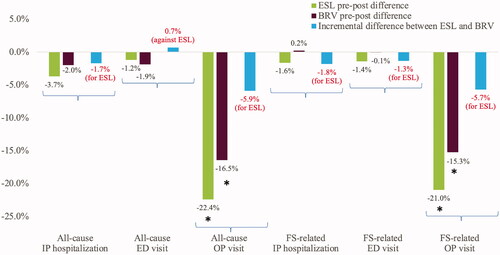
Results from the unadjusted linear regression model are presented in Supplementary Table 2. Patients treated with ESL had numerically larger unadjusted reductions per 90-day period in all-cause and FS-related IP hospitalizations and OP visits and FS-related ED visits compared to patients initiating BRV. Patients treated with BRV had numerically larger unadjusted reductions per 90-day period in all-cause ED visits.
The results from the adjusted analyses were consistent with the results from the unadjusted analyses ().
Figure 3. HCRU outcomes for patients initiating ESL compared to BRV. *Denotes p <.05, demonstrates statistically significant reductions as compared to baseline. Abbreviations. BRV, brivaracetam; ED, emergency department; ESL, eslicarbazepine acetate; HCRU, healthcare resource utilization; IP, inpatient; OP, outpatient.
Charges
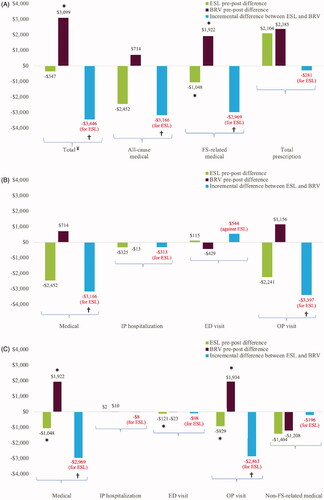
Results from the unadjusted linear regression model are presented in Supplementary Table 2. Initiation of ESL was associated with significantly larger unadjusted reductions in total charges, and all-cause and FS-related medical and OP charges compared to initiation of BRV. In adjusted analyses, initiation of ESL was associated with significantly larger reductions in total charges (−$3,446, CI: −$13,716, −$425), all-cause medical charges (−$3,166, CI: −$13,991, −$323), and all-cause OP charges (−$3,397, CI: −$15,676, −$818), and numerically larger reduction in all-cause IP hospitalizations compared to initiation of BRV. Initiation of BRV was associated with numerically larger reductions in all-cause ED charges (). Initiation of ESL was associated with significantly larger reductions in FS-related OP charges (−$2,863, CI: −$19,707, −$787) and FS-related medical charges (−$2,969, CI: −$21,547, −$842) and numerically larger reductions in non-FS-related medical charges compared to initiation of BRV ().
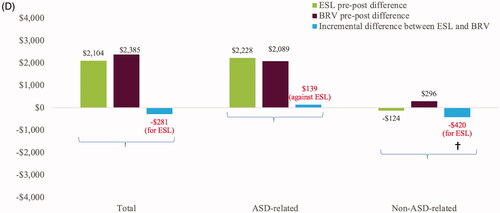
Figure 4 Charges following the initiation of ESL or BRV. (a) Total charges; (b) all-cause medical charges; (c) FS-related medical charges; (d) prescription charges. ¥Total charges are the sum of total medical and total prescription charges. *Denotes p < .05, demonstrates statistically significant reduction compared to baseline. †Denotes p < .05, demonstrates statistically significant incremental difference between ESL and BRV. Abbreviations. ASD, anti-seizure drug; BRV, brivaracetam; ED, emergency department; ESL, eslicarbazepine acetate; FS, focal seizure; IP, inpatient; OP, outpatient.
Initiation of ESL was associated with significantly larger reductions in non-ASD-related prescription charges (−$420, CI: −$1,058, −$78) and numerically smaller increases in total prescription charges compared to initiation of BRV. Initiation of BRV was associated with numerically smaller increases in ASD-related prescription charges ().
Discussion
This study suggests there may be a beneficial economic impact associated with the use of ESL as the first adjunctive therapy following a generic ASD compared to BRV in patients with FS, as demonstrated by significantly larger reductions in medical and prescription charges. These included total charges; all-cause medical and OP charges; FS-related medical and OP charges; and non-ASD-related prescription charges. Patients treated with ESL had significantly larger reductions in total charges compared to patients initiating BRV, likely driven by reductions in all-cause medical and OP charges. FS-related medical charges were also reduced, primarily driven by FS-related OP charges. Although not statistically significant, numerically larger reductions in all-cause ED charges and numerically smaller increases in ASD-related prescription charges were observed among patients treated with BRV compared to patients treated with ESL. There were no statistically significant differences in HCRU among patients treated with ESL or BRV. Numerically larger reductions in all-cause and FS-related IP hospitalizations and OP visits, and FS-related ED visits were observed among patients treated with ESL compared to patients treated with BRV. Patients initiating BRV had numerically larger reductions in all-cause ED visits compared to patients initiating ESL. Charges represent the complexity and variety of care, while HCRU represents counts of healthcare encounters (IP visits, OP hospitalizations, ED visits). Hence, charges associated with healthcare use are not necessarily linearly correlated with HCRU.
In the present study, 52.4% of patients who initiated ESL and 26.3% of patients who initiated BRV received LEV as their baseline ASD. Clinical benefits of the use of BRV in patients treated with LEV have not been conclusively demonstrated. In two Phase III studies of adjunctive BRV, patients treated with concomitant LEV and BRV had a placebo-like responseCitation35,Citation36. In a subsequent Phase III study of adjunctive BRV, patients receiving concomitant LEV were excludedCitation37. Small cohort studies have suggested that switching from LEV to BRV may be beneficial for patients experiencing behavioral adverse events associated with LEVCitation38,Citation39. Further confirmation of these results is needed, especially because a relevant percentage of patients may experience the same spectrum of psychiatric adverse effects with BRV as LEVCitation40.
In the present study, 6.7% and 5.3% of patients who initiated ESL received CBZ and OXC as their baseline ASD, respectively. Previous studies assessing transition from CBZ or OXC to ESL due to inadequate seizure control or intolerable adverse effects with these agents found ESL was efficacious and well-toleratedCitation41,Citation42. Furthermore, a real-world study evaluating HCRU among patients treated with ESL after historical use of LEV, OXC, and/or CBZ suggests that ESL initiation was associated with significant reductions in OP visits and numerical reductions in IP, IP ED, and OP ED visitsCitation43.
A previous study of early initiation of ESL as 1 L treatment or first adjunctive therapy to LEV or LTG in adults with FS was associated with a beneficial economic impact, demonstrated by significant reductions in HCRU and charges associated with specific claim typesCitation22. The add-on cohort experienced significant reductions in all-cause IP and all-cause and FS-related OP visits and significant increases in total prescription and ASD-related prescription charges during the follow-up period. The present study extends these findings by showing a reduction in HCRU and costs in patients with FS treated with ESL as the first add-on therapy following the use of any generic ASD rather than a specific agent. Inclusion of the comparative cohort of BRV allowed for an assessment of another third-generation ASD. The economic benefits associated with adjunctive ESL may be driven by the efficacy and tolerability of ESL. In an open-label, non-randomized, multicenter phase IV study, ESL as a first adjunctive therapy with LEV or LTG was associated with high reductions in median SSF (72.8%), high responder rates (62.5%), and a high proportion of patients achieving seizure freedom (25.0%)Citation18.
Total and ASD-related prescription charges for BRV and ESL increased, possibly as these are both branded agents. Initiation of ESL versus BRV was associated with a significantly larger reduction in non-ASD-related prescription charges. Although the types of non-ASDs were not examined, they may include medications used to manage neuropsychiatric disorders prevalent among patients with FS, as well as ASD-related psychiatric and behavioral side effectsCitation44. A pooled analysis of three-phase III trials of adjunctive ESL found the incidence of psychiatric and cognitive treatment-emergent adverse effects (TEAEs), but not depression and suicidality-related TEAEs, was comparable between ESL and placeboCitation15. These data may indicate lower utilization of concomitant psychiatric medications with ESL. Psychiatric side effects may be one of the many contributing factors for the increase in non-ASD-related prescription charges observed for BRV.
In the present study, patient characteristics were balanced in the patient population by using propensity score-based weighting to adjust for differences in baseline covariates between the ESL and BRV cohorts. In previous reports, patient characteristics such as age, race/ethnicity, and the presence of comorbid conditions have been associated with disparities in epilepsy treatmentCitation45. Low socioeconomic status may be another important factor that can influence access to specialized care among persons with epilepsyCitation46. In a retrospective study using 2005–2009 California State datasets, uninsured individuals and those with public insurance programs including Medicaid and Medicare had significant gaps in visits to an epilepsy provider and anti-seizure medicationsCitation45.
This study has several limitations. Given its open-source nature, the database may not capture all claims for a given patient, including claims processed through different claims transaction networks or non-electronic prescriptions that were abandoned prior to being submitted to a pharmacy and information about secondary payers. The database only contains information on submitted charges, which do not reflect paid amounts or costs faced by the provider. The database is not designed for research; it includes administrative data with clinical conditions identified using ICD-9-CM and ICD-10-CM diagnosis codes. Consequently, there is the potential for data to be missing, miscoded, or mismeasured, and there is a lack of detailed clinical data. Outcomes may have been influenced by differences in baseline and clinical characteristics, as well as differences in coverage restrictions between patients administered ESL and BRV that were not accounted for in the analysis. However, the application of the statistical methods of propensity score weighting and fixed effects may have alleviated this limitation. The relatively small sample size may have led to low statistical power, affecting the ability to find statistically significant differences between cohorts. This was an observational study that showed a beneficial economic impact associated with the use of ESL as the first adjunctive therapy following a generic ASD compared to BRV in patients with FS. Causative factors were not investigated but may include the efficacyCitation47,Citation48, tolerabilityCitation4,Citation47–49, and simplified dosingCitation50,Citation51 regimen of ESL, representing potential areas of future research. Further studies may also expand the comparator group to all third-generation ASDs. Patients who may be eligible for epilepsy surgery could represent another comparative cohort, and analysis of this patient population constitutes an interesting area of investigation. The present study focused on the use of a single generic prior to the initiation of ESL or BRV. The inclusion of multiple generics prior to initiation of ESL or BRV represents a topic of additional analyses.
Conclusion
Among patients with FS, initiation of first adjunctive therapy with ESL was associated with numerical reductions in HCRU and significant reductions in total, medical, and non-ASD-related prescription charges. These data may help clinicians make prudent prescribing decisions and enable payers to develop evidence-based utilization policies.
Transparency
Declaration of funding
Sponsorship for this study, medical writing support, and submission fee, and Fast Track accelerated publication fee were provided by Sunovion Pharmaceuticals Inc., Marlborough, MA, USA.
Declaration of financial/other interests
DM, BW, TG, AT, and GRW are employees of Sunovion Pharmaceuticals Inc. MD and AJE are employees of Medicus Economics LLC, which received funding from Sunovion Pharmaceuticals Inc. to participate in this study.
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Author contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Supplemental Material
Download MS Word (19.3 KB)Acknowledgements
Medical writing support by Jane Kondejewski, of SNELL Medical Communication Inc. Support for this assistance was funded by Sunovion Pharmaceuticals Inc., Marlborough, MA, USA.
Data availability statement
The datasets generated and/or analyzed during the current study are not publicly available due to a licensing agreement with Symphony Health’s Integrated Dataverse.
References
- Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(31):821–825.
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935-1984. Epilepsia. 1993;34(3):453–468.
- Clinical Brief. Examining the economic impact and implications of epilepsy. Am J Manag Care. 2020 [cited 2021 Apr 16]. Available from: https://ajmc.s3.amazonaws.com/_media/_pdf/A948_ClinicalBrief_pdf.pdf
- de Biase S, Nilo A, Bernardini A, et al. Timing use of novel anti-epileptic drugs: is earlier better? Expert Rev Neurother. 2019;19(10):945–954.
- Kanner AM, Ashman E, Gloss D, et al. Practice guideline update summary: efficacy and tolerability of the new antiepileptic drugs I: treatment of new-onset epilepsy: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2018;91(2):74–81.
- Glauser T, Ben-Menachem E, Bourgeois B, et al. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2013;54(3):551–563.
- Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. N Engl J Med. 2011;365(10):919–926.
- Kwan P, Brodie MJ. Epilepsy after the first drug fails: substitution or add-on? Seizure. 2000b;9(7):464–468.
- Ghaffari-Rafi A, Leon-Rojas J. Investigatory pathway and principles of patient selection for epilepsy surgery candidates: a systematic review. BMC Neurol. 2020;20(1):100.
- Mula M. Third generation antiepileptic drug monotherapies in adults with epilepsy. Expert Rev Neurother. 2016;16(9):1087–1092. Epub 2016 Jun 8.
- Santulli L, Coppola A, Balestrini S, et al. The challenges of treating epilepsy with 25 antiepileptic drugs. Pharmacol Res. 2016;107:211–219. Epub 2016 Mar 16.
- Feyissa AM. Brivaracetam in the treatment of epilepsy: a review of clinical trial data. Neuropsychiatr Dis Treat. 2019;15:2587–2600.
- Lattanzi S, Brigo F, Cagnetti C, et al. Eslicarbazepine acetate in the treatment of adults with partial-onset epilepsy: an evidence-based review of efficacy, safety and place in therapy. Core Evid. 2018;13:21–31.
- Khalegi F, Nemec II. EC. Brivaracetam (briviact): a novel adjunctive therapy for partial-onset seizures. P T. 2017;42(2):92–96.
- Andermann E, Biton V, Benbadis SR, et al. Psychiatric and cognitive adverse events: a pooled analysis of three phase III trials of adjunctive eslicarbazepine acetate for partial-onset seizures. Epilepsy Behav. 2018;82:119–127.
- Sunovion Pharmaceuticals Inc. APTIOM® (eslicarbazepine acetate) Prescribing Information. 2019 [cited 2021 Apr 16]. Available from: https://www.aptiom.com/Aptiom-Prescribing-Information.pdf
- UCB. BRIVIACT® (brivaracetam) Prescribing Information. 2018 [cited 2021 Apr 16]. Available from: https://www.briviact.com/briviact-PI.pdf
- Hixson J, Gidal B, Pikalov A, et al. Efficacy and safety of eslicarbazepine acetate as a first or later adjunctive therapy in patients with focal seizures. Epilepsy Res. 2021;171:106561.
- Allers K, Essue BM, Hackett ML, et al. The economic impact of epilepsy: a systematic review. BMC Neurol. 2015;15:245–266.
- Begley CE, Durgin TL. The direct cost of epilepsy in the United States: a systematic review of estimates. Epilepsia. 2015;56(9):1376–1387.
- Ivanova JI, Birnbaum HG, Kidolezi Y, et al. Direct and indirect costs associated with epileptic partial onset seizures among the privately insured in the United States. Epilepsia. 2010;51(5):838–844.
- Mehta D, Davis M, Epstein AJ, et al. Impact of early initiation of eslicarbazepine acetate on economic outcomes among patients with focal seizure: results from retrospective database analyses. Neurol Ther. 2020;9(2):585–598.
- Mehta D, Davis M, Epstein AJ, et al. Comparative economic outcomes in patients with focal seizure initiating first-line eslicarbazepine acetate monotherapy versus generic antiseizure drugs. Clinicoecon Outcomes Res. 2021;13:251–261.
- French J. Will brivaracetam help my patient? Only time will tell. Epilepsy Curr. 2017;17(1):35–36.
- US Department of Health and Human Services. Summary of the HIPAA privacy rule. Washington (DC): US Department of Health and Human Services. 2013 [cited 2021 Apr 16]. Available from: https://www.hhs.gov/hipaa/for-professionals/privacy/laws-regulations/index.html
- Office for Human Research Protections 2018 Requirements. 2018 common rule. Rockville (MD): Office for Human Research Protections 2018 Requirements. 2018 [cited 2021 Apr 16]. Available from: https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/revised-common-rule-regulatory-text/index.html
- US Bureau of Economic Analysis. Table 1.1.4. Price indexes for gross domestic product. Suitland (MD): US Bureau of Economic Analysis. 2021 [cited 2021 Apr 16]. Available from: https://apps.bea.gov/iTable/iTable.cfm?reqid=19&step=2
- Linden A, Adams JL. Applying a propensity score-based weighting model to interrupted time series data: improving causal inference in programme evaluation. J Eval Clin Pract. 2011;17(6):1231–1238.
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424.
- Cameron AC, Miller DL. A practitioner’s guide to cluster-robust inference. J Human Resources. 2015;50(2):317–372.
- Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Statist Med. 2000;19(9):1141–1164.
- Poi BP. From the help desk: some bootstrapping techniques. The Stata Journal. 2004;4(3):312–328.
- Wood M. Bootstrapped confidence intervals as an approach to statistical inference. Organ Res Methods. 2005;8(4):454–470.
- Stuart EA, Lee BK, Leacy FP. Prognostic score-based balance measures can be a useful diagnostic for propensity score methods in comparative effectiveness research. J Clin Epidemiol. 2013;66(8):S84–S90.
- Biton V, Berkovic SF, Abou-Khalil B, et al. Brivaracetam as adjunctive treatment for uncontrolled partial epilepsy in adults: a phase III randomized, double-blind, placebo-controlled trial. Epilepsia. 2014;55(1):57–66.
- Ryvlin P, Werhahn KJ, Blaszczyk B, et al. Adjunctive brivaracetam in adults with uncontrolled focal epilepsy: results from a double-blind, randomized, placebo-controlled trial. Epilepsia. 2014;55(1):47–56.
- Klein P, Schiemann J, Sperling MR, et al. A randomized, double-blind, placebo-controlled, multicenter, parallel-group study to evaluate the efficacy and safety of adjunctive brivaracetam in adult patients with uncontrolled partial-onset seizures. Epilepsia. 2015;56(12):1890–1898.
- Yates SL, Fakhoury T, Liang W, et al. An open-label, prospective, exploratory study of patients with epilepsy switching from levetiracetam to brivaracetam. Epilepsy Behav. 2015;52(A):165–168.
- Steinig I, von Podewils F, Möddel G, et al. Postmarketing experience with brivaracetam in the treatment of epilepsies: a multicenter cohort study from Germany. Epilepsia. 2017;58(7):1208–1216.
- Hirsch M, Hintz M, Specht A, et al. Tolerability, efficacy and retention rate of brivaracetam in patients previously treated with levetiracetam: a monocenter retrospective outcome analysis. Seizure. 2018;61:98–103.
- Mäkinen J, Rainesalo S, Peltola J. Transition from oxcarbazepine to eslicarbazepine acetate: a single center study. Brain Behav. 2017;7(3):e00634.
- Rocamora R, Peltola J, Assenza G, et al. Safety, tolerability and effectiveness of transition to eslicarbazepine acetate from carbamazepine or oxcarbazepine in clinical practice. Seizure. 2020;75:121–128.
- Williams GR, Chandra R, Wang J, et al. Healthcare resource utilization among patients with focal seizure initiating eslicarbazepine acetate after historical use of widely used first- or second-generation antiseizure drugs. Paper presented at the AES Meeting 2020; 2020 December 4–8; Seattle, WA.
- Kanner AM. Management of psychiatric and neurological comorbidities in epilepsy. Nat Rev Neurol. 2016;12(2):106–116.
- Schiltz NK, Koroukian SM, Singer ME, et al. Disparities in access to specialized epilepsy care. Epilepsy Res. 2013;107(1–2):172–180.
- Szaflarski M, Wolfe JD, Tobias JGS, et al. Poverty, insurance, and region as predictors of epilepsy treatment among US adults. Epilepsy Behav. 2020;107:107050.
- Toledano R, Jovel CE, Jimenez-Huete A, et al. Efficacy and safety of eslicarbazepine acetate monotherapy for partial-onset seizures: experience from a multicenter, observational study. Epilepsy Behav. 2017;73:173–179.
- French JA, Gazzola DM. New generation antiepileptic drugs: what do they offer in terms of improved tolerability and safety? Ther Adv Drug Saf. 2011;2(4):141–158.
- Jalihal V, Shankar R, Henley W, et al. Eslicarbazepine acetate as a replacement for levetiracetam in people with epilepsy developing behavioral adverse events. Epilepsy Behav. 2018;80:365–369.
- Kim SH, Lee H, Kim DW. Switching antiepileptic drugs to once-daily dosing regimens in epilepsy patients. Acta Neurol Scand. 2021;143(1):51–55.
- Ferrari CM, de Sousa RM, Castro LH. Factors associated with treatment non-adherence in patients with epilepsy in Brazil. Seizure. 2013;22(5):384–389.