Abstract
Objective
Hemophilia A is the second most common bleeding disorder causing patients to have lifelong follow-up and treatment. Despite being a rare disease, hemophilia A has a high economic burden on individuals and the public. The purpose of this study was to estimate the total disease cost of hemophilia A in Turkey.
Materials and Methods
Data used in this analysis were collected through literature review, including studies conducted in Turkey in December 2018. A disease burden analysis was performed by modeling hemophilia A-related costs among patients, their relatives, and the social security system. Two expert panels were held to evaluate real-world data sources and to provide further information. All direct medical and non-medical costs were calculated annually from the Social Security Institution of the Republic of Turkey perspective, while indirect costs were estimated from the patient and community perspective.
Results
For the calendar year of 2018, the number of hemophilia A patients in Turkey were estimated to be 5,055, with an average weight of 64.7 kg. The average annual direct medical, direct non-medical, and indirect costs of hemophilia A were calculated as €93,268 ($109,286; ₺502,717), €2,533 ($2,968; ₺13,655), and €7,957 ($9,323; ₺42,888) per patient, respectively, with a total annual cost of €103,759 ($121,578; ₺559,259). For the management of patients with inhibitors (4.9%), the average annual total cost was calculated to be €325,439 ($381,330; ₺1,754,117) per patient. The total annual disease burden of hemophilia A in 2018 was estimated to be about €524 million ($614 million; ₺2.82 billion), which corresponded to 1.6% of the total health expenditure in Turkey.
Conclusion
The most important reason hemophilia A has a significant economic burden in Turkey is that replacement therapy is expensive. The major cost contributor was identified as factor replacement therapy. With inhibitor development, the average annual cost increased more than 3-fold.
Introduction
Hemophilia A is an X chromosome-linked bleeding disorder characterized by the deficiency of clotting factor VIII (FVIII). Hemophilia A has a recessive genetic pattern and is almost always seen in males. The general incidence of hemophilia A is 17.1 (14.8–19.3) in 100,000 male birthsCitation1. Bleeding episodes involving joints and surrounding soft tissue constitutes the hallmarks of the disease and might lead to arthropathy in the long-term if insufficiently treatedCitation2. Randomized clinical trials and real-world evidence have demonstrated that prevention prevents joint bleeding and deterioration of joint status, and primary prevention with FVIII has, therefore, been recognized as the standard of care for individuals with severe hemophilia A in countries with adequate resources. Prophylactic therapy also reduces the incidence of central nervous system bleeds (intracranial and spinal hematoma), which are less common than joint bleeding but much more life-threatening. Additional advantages of prophylaxis vs. on-demand treatment include reduced hospitalizations and absenteeism from school or work, greater participation in social activities and, overall, improved health-related quality-of-lifeCitation3.
In developed countries, hemophilia A patients are expected to have a lifespan similar to that of the general male populationCitation4. Given the overall increase in life expectancy, anticipated new treatment modalities, including gene therapy, and a substantial cost associated with factor replacement, analysis of the economic burden of hemophilia A, is of importance for making reimbursement policy decisionsCitation5,Citation6.
The general health insurance system of the Social Security Institution of Turkey covers all citizens regardless of age or employment status. Private insurances in Turkey constitute less than 5% of all healthcare-related expenditureCitation7, while Social Security Institution covered 78% of all healthcare expenditure in 2018Citation8. The sustainability of this public reimbursement model is therefore crucial with evidence-based guidance. The objective of this study was to provide an analysis of direct and indirect costs related to hemophilia A in Turkey from a social security perspective.
Materials and methods
Data sources
National tables and study reports were used where possible. The number of hemophilia A patients in 2018 was estimated based on the World Federation of Hemophilia 2010–2016 surveysCitation9–15 and Turkish Statistical Institute population information and projectionsCitation16,Citation17. Percent distributions of hemophilia A severity and prophylactic or on-demand factor replacement regimens along with annual rates of bleeding episodes, surgeries, and other hemophilia-related complications were calculated with previously reported results of pediatric and adult population studies in TurkeyCitation18–26. Additional data were obtained from studies reporting findings in patients with inhibitorsCitation27. Patients’ weight was estimated using the Turkish Statistical Institute national health surveys and available literature on national pediatric anthropometry findings and age distribution of hemophilia A patientsCitation14,Citation28,Citation29. Rates of surgical procedures for the management of hemophilic arthropathy were estimated per year based on the available national study findingsCitation21–23,Citation30,Citation31.
Healthcare practice service costs were calculated using the Official Health Notification (1 February 2019) of the Social Security InstitutionCitation32. These services include inpatient and outpatient diagnostic tests and imaging, consultation, consumables, and surgical and medical intervention procedures. Medicine reimbursement costs were obtained from the registries and lists of the Turkish Medicine and Medical Device Agency and RxMedia Pharma databaseCitation33,Citation34. National hemophilia diagnosis and treatment guideline recommendations were used for schedules of factor treatmentCitation35. Factor utilization information for 2017 and 2018 in Turkey was obtained from IQVIA (Danbury, CT). Data regarding survival, employment, retirement, and wage and pension payments were obtained from the statistical annals 2017–2019 of the Social Security InstitutionCitation36. Two panels with six local experts in the field were held in January 2019 to assess available data sources and provide practical insight and disease characteristics of hemophilia A patients in routine clinical practice. In addition to the fact that the participants of the expert panel were members of the Turkish Pediatric Hematology Association, and they were physicians who treat hemophilia in tertiary hospitals, the epidemiological data from the literature were also in the expert panel. Four of the experts are also the authors of this article. The last search for data sources was carried out in March 2019.
Definitions
The severity of hemophilia A was defined according to plasma levels of FVIII as mild (>5 to <40%), moderate (1–5%), and severe (<1%)Citation37. The Social Security Institution reimburses prophylactic factor replacement (up to 4,500 IU/week) in severe hemophilia A and patients with more than three bleeding episodes per monthCitation32. Bleeding episodes and surgeries were classified as minor or major following national guidelinesCitation38,Citation39. Prevention was not expected to be applicable in mild cases. Immune tolerance induction (ITI) treatment (50 IU/kg three times per week for a maximum duration of 1 year) is reimbursed for patients under the age of 11 years with a plasma FVIII activity ≤1% and an inhibitor level ≤10 Bethesda units/mLCitation32.
Cost analysis
Direct medical cost assessment was performed using the methodology provided by Cowley et al.Citation40. Indirect disease-related costs were calculated for labor absenteeism of adult patients and caregivers, disability pensions, and early retirement or deathCitation41,Citation42. Other direct costs were calculated for intercity travel and daily payments, the requirement of a professional caregiver, transportation, accommodation, meals during travels to healthcare facilities, and out-of-pocket medical expensesCitation41,Citation42. Mean values were calculated with available data sources for the calendar year of 2018. Cost calculations and analyses in TRY (₺) currency were carried out with Microsoft Excel 2016 (Microsoft Corporation; Redmond, WA). All monetary figures in Turkish, EU, and US currencies for international readership were also given (the 2018 exchange rate was 1€ = 5.39₺; 1$ = 4.60₺)Citation43.
Sensitivity analysis
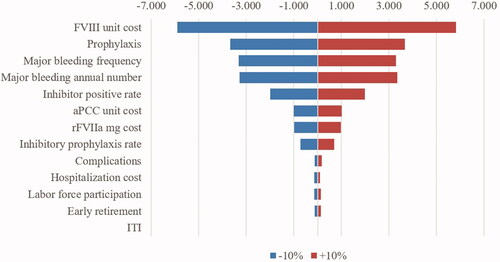
To observe the effect of the variables included in the calculation on the analysis, the reflections of the ±10% changes in the values used in the basic analysis were investigated. A tornado graphic was used to compare the effects of one-way sensitivity analysis.
Results
Epidemiology and patient characteristics
The prevalence of hemophilia A in Turkey was previously reported to be 8.9–12.5/100,000 in the male populationCitation9–15. The number of hemophilia A patients in 2018 in Turkey was estimated to be 5,055. The number of adult patients was calculated to be 3,148 (62.3%). The average age was estimated to be 25, and the mean patient weight was 64.7 kg. The number of patients and mean weight estimates per age group are presented in .
Table 1. Number of hemophilia A patients and mean patient weights in Turkey, 2018.
Hemophilia A severity was mild in 30%, moderate in 15%, and severe in 55% of patients. Of all hemophilia A patients, 52.3% were estimated to be on prophylactic FVIII treatment, 4.9% to be patients with inhibitor, and 2.6% to be with inhibitor and on prophylactic regimens with bypassing agents. Prophylactic treatment was found to represent 86% and 33% of factor replacement regimens in severe and moderate disease, respectively. Rates of radiosynovectomy, arthroscopic synovectomy, and arthroplasty in 1 year were calculated to be 5.1%, 1.6%, and 5.3%, respectively. The annual mean number of bleeding episodes was four in patients on prophylaxis treatment, whereas it was seven bleeding episodes in patients treated on-demand. It was estimated that 59.9% and 22.1% of hemophilia A patients would annually develop a minor and major bleeding episode, respectively. Annual rates of hospitalization requirements and bleeding episodes are presented in .
Table 2. Estimates for annual hospitalization requirements and bleeding rates of hemophilia A patients.
Direct medical costs
Clotting factors and other medications
Management of a major bleeding episode or major surgery was found to require 960 IU/kg of factor VIII, which corresponds to an average cost of €12,874 ($15,085; ₺69,393) per major episode/surgery for a treatment duration of 10 days. The average cost of factor replacement for a minor bleeding episode or surgery was calculated to be €2,337 ($2,738; ₺12,596) for a treatment duration of 2–3 days.
The annual mean number of prophylactic infusions was calculated to be 127.3 per patient, with a mean factor dose of 30 IU/kg (maximum 4,500 IU per infusion). Cost of factor VIII was found to be €0.32/IU (0.37 $/IU; 1.71 ₺/IU) and average prophylactic factor replacement cost per patient was calculated to be 63,227 €/year (74,086 $/year; 340,796 ₺/year).
For the prophylactic management of patients with inhibitors, it was calculated that aPCC was used in 85% of patients and rFVIIa in 15%. The average bypassing agent dose was estimated to be 85 units/kg every other day, and the average cost was found to be 0.95 €/unit (1.11 $/unit; 5.10 ₺/unit). The annual average cost of prophylactic treatment in a hemophilia A patient with inhibitor was calculated to be €319,663 ($374,561; ₺1,722,981). rFVIIa was utilized in 97.6% of the cases with inhibitors for the management of bleeding episodes. The average bypassing agent cost was calculated as €19,162 ($22,452; ₺103,281) for every bleeding episode or surgery. ITI treatment was found to be applicable in 0.3% of hemophilia A patients as per reimbursement criteria. Cost of annual ITI treatment was calculated to be €61,542 ($72,111; ₺331,710). Additional annual average cumulative medication costs of tranexamic acid, desmopressin, analgesics, topical antiallergic medications, and fibrin sealant were estimated to be €55.12 ($61.07; ₺280.9) per patient. Mean annual total cost of medications administered to a hemophilia A patient was calculated to be €91,858 ($107,634; ₺495,116).
Management of further complications
Surgeries, rehabilitation procedures, and medical treatments for seizure neuropathic pain, and chronic hepatitis were considered for the cost calculation of hemophilia A-related complications other than inhibitor development. The cumulative cost was 660 €/year (773 $/year; 3,558 ₺/year) on average per patient.
Other medical costs
Additional average costs of admissions and other medical procedures were calculated to be 853 €/year (1,000 $/year; 4,600 ₺/year) for a patient with inhibitor and 836 €/year (980 $/year; 4,508 ₺/year) for a patient on prophylactic regimen.
Direct non-medical costs
Intercity travel and daily payments
Patients in Turkey are eligible for a daily payment from the Social Security Institution for intercity travels required for medical care. It was estimated that 53% of hemophilia A patients received medical care within their home city, while 47% needed transportation to a medical facility in another city. The total intercity travel and daily payments for hemophilia A patients were estimated to be 58,408 €/year (68,439 $/year; 314,820 ₺/year).
Informal and professional caregivers
It was estimated that 55% of hemophilia A patients were accompanied by an informal caregiver during their hospital visits. Cumulative payment loss of caregivers was calculated to be 2,007,513 €/year (2,352,282 $/year; 10,820,497 ₺/year). The expert panel estimated that 5% of hemophilia A patients require a full-time professional caregiver. The average professional caregiver cost was found to be 361€/month (423 $/month; 1,945 ₺/month) and the overall cost of caregiver wage was calculated to be 606,234 €/year (710,348 $/year; 3,267,600 ₺/year).
Transportation, meals, and accommodation
The average weighted one-way travel distance was calculated to be 485 km. The transportation cost of hemophilia A patients was estimated to be 5,967,747 €/year (6,992,643 $/year; 32,166,158 ₺/year).
On average, a hemophilia A patient requiring intercity transportation to a medical facility was estimated to have spent 36 days on outpatient visits, necessitating 2 days of hotel stay. Cumulative cost of meals was calculated to be 31,959 €/year (37,448 $/year; 172,260 ₺/year) and accommodation cost was 89,927 €/year (105,370 $/year; 484,704 ₺/year).
Out-of-pocket expenses
Patients in Turkey need to pay a portion of their healthcare expenses, subject to service and type of healthcare facilities and medication requirements. Total cost of out-of-pocket expenses of hemophilia A patients was calculated to be 1,905,707 €/year (2,232,991 $/year; 10,271,760 ₺/year).
Indirect costs
Early retirement and disability pension
The average retirement age in Turkey was found to be 52.6 years, and the average pension was 358 €/month (419 $/month; 1,927 ₺/month). It was estimated that 9% of hemophilia A patients would retire early at the mean age of 41.0 years, resulting in an average productive loss of 12 years per patient. Cost of pension payments and tax revenue loss was calculated to be 3,265 €/year (3,826 $/year; 17,601 ₺/year) per an early retired hemophilia A patient and cumulative public cost was 15,959,861 €/year (18,700,794 $/year; 86,023,651 ₺/year).
Nine percent of hemophilia A patients were estimated to receive a disability pension per social security laws. The total annual cost of disability pension payments to hemophilia A patient was estimated to be €729,685 ($855,000; ₺3,933,002).
Labor absenteeism
The overall employment rate of hemophilia A patient was estimated to be 53%. The mean number of working days missed for an adult patient or a parent of a pediatric patient due to the medical management of hemophilia A was calculated to be 98.4 days/year. When calculated based on the legal minimum wage, the cumulative public cost of labor absenteeism was calculated to be 19,692,640 €/year (23,074,637 $/year; 106,143,331 ₺/year). Cumulative payment loss of employed hemophilia A patients was calculated to be 437,166 €/year (512,245 $/year; 2,356,326 ₺/year).
Early death
While it was expected that the average life expectancy of a hemophilia A patient would be similar to the overall national life expectancy, hemophilia A patients with inhibitors would have a 1.6-times higher risk of death. On average, a patient with an inhibitor was expected to lose 15 years of lifespan. A hemophilia A-related early death was expected to result in a 25% decline in family income. Overall cost of income loss due to early deaths was estimated to be 2,855,691 €/year (3,346,125 $/year; 15,392,174 ₺/year).
Sensitivity analysis
The variables that had the highest impact on the results were determined as the FVIII unit cost, the rate of patients treated with prophylaxis, the incidence and annual incidence of major bleeding and the inhibitor positive patient rate, respectively, in one way sensitivity analysis. aPCC and FVIIa unit costs were found to be similarly effective in the analysis, followed by the rate of prophylaxis and frequency of complications used in inhibitor-positive patients ().
Total cost of hemophilia A
The total direct medical cost of hemophilia A was found to be 93,268 €/year (109,286 $/year; 502,717 ₺/year) per patient, 75,120 €/year (88,021 $/year; 404,896 ₺/year) per patient on prophylactic therapy, and 310,682 €/year (364,038 $/year; 1,697,574 ₺/year) per patient with inhibitor, whilst total direct non-medical cost was 2,533 €/year (2,968 $/year; 13,655 ₺/year) and indirect cost was 7,957 €/year (9,323 $/year; 42,888 ₺/year) per patient. Overall average annual cost of hemophilia A was estimated to be €103,759 ($121,578; ₺559,259) per patient, of which €95,802 ($112,255; ₺516,371) (93%) was for direct costs, while €91,806 ($107,573; ₺494,835) (88.5%) was the cost of factor VIII and bypassing agents (). Besides, the annual cost of hemophilia A stratified by severity is shown in .
Table 3. Annual average direct and indirect costs of hemophilia A per patient (2019).
Table 4. Annual cost of hemophilia A stratified by severity (2019).
The total annual economic burden of hemophilia A in 2018 was estimated to be €524,499,645 ($614,576,758; ₺2,827,053,087), corresponding to 1.6% of the estimated total health expenditure in Turkey.
Discussion
This study was conducted to analyze the annual direct and indirect costs of hemophilia A in Turkey. To the best knowledge of the authors, this is the first comprehensive economic analysis of hemophilia A management in Turkey, and the overall cost was estimated to be over €524 million ($614 million; ₺2.82 billion). As a comparison, the economic burden of cardiovascular diseases in Turkey, affecting 3.4 million patients in 2016, was calculated to be €9.2 billion Intercity travel and daily paymentsCitation44. This comparison indicates that the total cost of hemophilia A per patient was about 38-times higher. Hemophilia A-related direct medical cost per patient was also found to be more than 250-times the average health expenditure per capita of about €359 ($420; ₺1,935) in 2018Citation8. Labor and school absenteeism, early retirement, and complications were also considered a considerable social burden on patients.
Previous reports from Europe have highlighted the high cost associated with optimal hemophilia A management. While these studies differ in their cost calculation models and included expense items, the annual medical cost of hemophilia A was usually found to be within a range of €40,000–100,000 per patientCitation45–47. In studies that have included direct and indirect cost analyses, clotting factor concentrates accounted for up to 90% or more of overall hemophilia A-related costsCitation46–51.
The BURQoL-RD study on hemophilia provides an update of patients’ QoL, and for the first time in Italy, offers an estimation of average total unit cost from the social perspective. This study also shows a significant relationship between age and QoL and costs other than drugs. Assuming a society perspective, the estimated mean annual total cost per patient in 2012 is €117,732. Drugs represent 92% of total costsCitation52.
Our findings are in line with these reports that the overall average cost per patient associated with hemophilia A was about €103,759 ($121,578; ₺559,259) per year, and expenses on clotting factors represented 88.5% of it. It should be noted that medical costs other than medications accounted for only 1.5% of direct medical costs. This finding should be interpreted within the dominant public nature of both healthcare and reimbursement systems in Turkey, with relatively low prices associated with hospital services and the absence of high physician charges.
Our results indicate that medical treatment of hemophilia A with inhibitors is more than 4-times more costly than the cost of hemophilia A prophylaxis without inhibitor development. Similarly, higher costs for hemophilia A with inhibitors were reported repeatedlyCitation6,Citation46,Citation47,Citation53–57. More frequent bleeding episodes, higher cost of bypassing agents, and potential eligibility for ITI therapy could explain the additional expenses associated with hemophilia A with inhibitor developmentCitation27,Citation47,Citation55.
Direct non-medical costs were found to be 2.4% of the total cost in our study. Transportation was the highest non-medical direct cost. This could be explained by the finding that about half of all hemophilia A patients needed to travel for healthcare services, probably with the intent of access to specialist care, with long travel distances. This finding is consistent with earlier reports indicating that transportation is the major non-medical direct cost for hemophilia A patientsCitation49. Labor absenteeism was the highest indirect cost of hemophilia A, a finding which is also similar to previous resultsCitation48,Citation49.
Optimal clinical management of hemophilia A should be considered the desired achievement to optimize costs and decrease disease burden on patients and caregivers. Prophylaxis should be initiated in severe hemophilia A, and bleeding episodes should be prevented rather than treated with factor replacement in emergency services. Less frequent bleeding episodes, in turn, would decrease absenteeism and burden on the social security system. Increased factor costs associated with prevention could be balanced by avoiding direct and indirect costs of managing bleeding episodes and complicationsCitation58,Citation59. The non-economic benefit of this approach would be improved quality-of-life for patients along with their caregivers and familiesCitation60,Citation61. Compliance with factor replacement regimens must be optimized as well to fully actualize these expected benefits. Compliance should be the key aspect of patient training programsCitation62,Citation63. Training should additionally cover education on lifestyles, psychosocial needs, and the living environment of the patient and caregiver. Multidisciplinary training environments where social service specialists, psychologists, nurses, physiotherapists, dentists, and clinicians work together should be established. This multidisciplinary approach would also help avoid caregiver burnoutCitation64.
Our study has several strengths as well as limitations. We used observational or official data representing Turkey for the main parameters of patient demographics, disease characteristics, and cost calculations where available. While we did not undertake a patient-level data collection, available data sources were deemed sufficient to draw a reliable picture of hemophilia A management in Turkey. Estimates from the expert panels were limited to subgroup features, daily living and occupational activities, and associated calculations. From the social security and public reimbursement system perspective, we consider that this methodology provided accurate findings of average cost per patient and national economic impact. An additional economic evaluation of patient subgroups could be performed in the future with prospective designs using metrics such as disability-adjusted life years. With the introduction of new treatment options for hemophilia A, comparative cost-effectiveness analyses would be of value for making rational reimbursement decisions and optimizing treatment-related budget effects. Alternative reimbursement models, such as risk-sharing agreements with the pharmaceutical industry, could benefit from lower medication costs.Citation65
Conclusion
While classified as a rare disease, the economic impact of hemophilia A is significant on the social security system of Turkey. The key driver of cost is clotting factor replacement, and inhibitor development drastically increases the overall cost. We hope that our findings will contribute to patient support, resource allocation, and reimbursement strategies in Turkey to better hemophilia A patients experiences.
Transparency
Declaration of funding
Roche Müstahzarları Sanayi A.Ş. provided a study grant for medical writing support, which was provided by Pleksus Clinical Research, Istanbul, Turkey. Roche or Pleksus has not influenced the content of this study.
Declaration of financial/other relationships
MCA has received research funding from Pfizer, Roche, Novartis, and Bayer, and is a member of the board of directors/speaker’s bureau/advisory committee for Roche, Takeda, Pfizer, Novo Nordisk, Novartis, Sanofi-Genzyme, CSL Behring, Octapharma, and Bayer.
KK has received research funding from Pfizer, Novo Nordisk, and Roche, and is a consultant and member of the speaker’s bureau/advisory committee for Roche, Novo Nordisk, Pfizer, Bayer, Takeda, Behring, Sanofi, and Sobi.
BA is a consultant and member of the speaker’s bureau/advisory committee for Novo Nordisk, Roche, CSL-Behring, Bayer, Pfizer, Takeda, and Sobi.
CB is a consultant and member of the speaker’s bureau/advisory committee for NovoNordisk, Roche, CSL-Behring, Bayer, Pfizer, Takeda, and Sobi.
SM and EO have no relevant financial or other relationships to disclose.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
This article was presented at ISPOR Europe 2019 as an abstract presentation (ISPOR Europe, November 2019, Copenhagen, Denmark Vol:22, Suppl.3, Pages A1–A2, S403–S940).
Acknowledgements
Ayşe Esra Aydın provided the medical writing support, MD, from Pleksus Clinical Research, Istanbul, Turkey and was funded by Roche Müstahzarları Sanayi A.Ş.
References
- Iorio A, Stonebraker JS, Chambost H, et al. Establishing the prevalence and prevalence at birth of hemophilia in males: a Meta-analytic approach using national registries. Ann Intern Med. 2019;171(8):540–546.
- Zimmerman B, Valentino LA. Hemophilia: in review. Pediatr Rev. 2013;34(7):289–294.
- Aledort L, Mannucci PM, Schramm W, et al. Factor VIII replacement is still the standard of care in haemophilia A. Blood Transfus. 2019;17(6):479–486.
- Mannucci PM, Iacobelli M. Progress in the contemporary management of hemophilia: the new issue of patient aging. Eur J Intern Med. 2017;43:16–21.
- Peters R, Harris T. Advances and innovations in haemophilia treatment. Nat Rev Drug Discov. 2018;17(7):493–508.
- Guh S, Grosse SD, Mcalister S, et al. Health care expenditures for medicaid-covered males with haemophilia in the United States, 2008. Haemophilia. 2012;18(2):276–283.
- Tunc O, Kiyak M. Private health insurance in Europe and Turkey. JEFA. 2015;2:409–425.
- Turkish Statistical Institute. Health expenditure statistics, 2018.
- World Federation of Hemophilia. Report on the annual global survey 2010. Montreal, Quebec, December 2011.
- World Federation of Hemophilia. Report on the annual global survey 2011. Montreal, Quebec, December 2012.
- World Federation of Hemophilia. Report on the annual global survey 2012. Montreal, Quebec, December 2013.
- World Federation of Hemophilia. Report on the annual global survey 2013. Montreal, Quebec, November 2014.
- World Federation of Hemophilia. Report on the annual global survey. 2014. Montreal, Quebec, October 2015.
- World Federation of Hemophilia. Report on the annual global survey. 2015. Montreal, Quebec, October 2016.
- World Federation of Hemophilia. Report on the annual global survey. 2016. Montreal, Quebec, October 2017.
- Turkish Statistical Institute. 2018 address-based population registration system results, 2019.
- Turkish Statistical Institute. Turkey mid-year population projection. 2013.
- Keklik Karadağ F. Evaluation of socio demographic factors and comorbidities in the patiens with hemophilia at Ege Adults Hemophilia Unit [Dissertation]. Ege University, School of Medicine, Internal Medicine Department; 2017.
- Karaman K, Akbayram S, Garipardıç M, et al. Diagnostic evaluation of our patients with hemophilia A: 17-year experience. Turk Pediatri Ars. 2015;50(2):96–101.
- Çelebi F. Research of morbidity and bleeding areas of hemophilia and von Willebard disease [dissertation]. Ankara: Hacettepe University Medical School; 2016.
- Deniz A. Quality of life in pediatric and young adult hemophilia patients with factor level ≤1 [dissertation]. Kocaeli: Kocaeli University; 2017.
- Sezgin M. Life quality of children with hemophilia and radiological scores of their arthropathy [dissertation]. Samsun: Ondokuz Mayıs University; 2016.
- Demir B. In patients wi̇th hemophilia musculoskeletalinvestigation of changes [dissertation]. Erzurum: Atatürk University, 2014.
- ERbaş Yıldız M. Evaluation of the effect of treatment and sedentary lifestyle of haemophiliacs on metabolic condition and renal functions [dissertation]. Istanbul: İstanbul University, Cerrahpaşa Faculty of Medicine; 2011.
- Zengin O. The relatıonshıp between frequency and ıntensıty of bleedıng ın hemophılıa patıents and hemophılıc arthropathy, radıologıcal assessment of arthropathy and ıts socıo-economıc ımpacts [dissertation]. Gaziantep: Gaziantep University; 2012.
- Turkish Maternal, Child and Adolescent Health Institute. Hemophilia report, 2018.
- Kavakli K, Yesilipek A, Antmen B, et al. The value of early treatment in patients with haemophilia and inhibitors. Haemophilia. 2010;16(3):487–494.
- Turkish Statistical Institute. Turkey health survey, 2016.
- Neyzi O, GüNöz H, Furman A, et al. Türk çocuklarında vücut ağırlığı, boy uzunluğu, baş çevresi ve vücut kitle indeksi referans değerleri. Çocuk Sağlığı ve Hastalıkları Dergisi. 2008;51:1–14.
- Zeydanoğlu A. Aynı cerrah tarafından diz artroplastisi yapılan genel anestezi uygulanan hemofili hastalarında kronik ağrı gelişim sıklığının araştırılması [dissertation]. İzmir: Ege University; 2017.
- Polat Kelle A. The effectiveness of radiosynovectomy in chronic hemophilic synovitis [dissertation]. Adana: Çukurova University; 2010.
- Social Security Institution. Health practice notice, 01 February 2019.
- Ministry of Health Turkish Medicines and Medical Devices Agency. Drug price list, 7 Mart 2019.
- RxMediaPharma. Version 2019, 19.0.53. Izmir, 7 March 2019.
- Turkish Society of Hematology. National guideline for diagnosis and treatment of hemophilia. Ankara: Turkish Society of Hematology; 2017.
- Social Security Institution. Statistics annuals, 2017–2019.
- Blanchette VS, Key NS, Ljung LR, et al. Subcommittee on factor VIII, factor IX and rare coagulation disorders of the scientific and standardization committee of the international society on thrombosis and hemostasis. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12(11):1935–1939.
- Turkish Society of Hematology. Hemorrhage prevention in adult hemophilia – treatment guideline. Ankara: Turkish Society of Hematology; 2012.
- Turkish Society of Hematology. Preparation for surgery in hemophilia – diagnosis and treatment guide. Ankara: Turkish Society of Hematology; 2011.
- Cowley P, Bodabilla L, Musgrove P, et al. Content and financing of an essential national package of health services, global assessments in the health sector. Geneva: World Health Organization; 1994.
- Neumann PJ, Sanders GD, Russell LB, et al. (Eds). Cost-effectiveness in health and medicine. New York: Oxford University Press; 2016.
- Angelis A, Lange A, Kanavos P. Using health technology assessment to assess the value of new medicines: results of a systematic review and expert consultation across eight european countries. Eur J Health Econ. 2018;19:123–152.
- Central Bank of Turkey Exchange Rates 16.07.2018. Available from: https://www.tcmb.gov.tr/kurlar/201807/06072018.xml
- Balbay Y, Gagnon-Arpin I, Malhan S, et al. Modeling the burden of cardiovascular disease in Turkey. Anatol J Cardiol 2018;20:235–240.
- Nerich V, Tissot E, Faradji A, et al. Cost-of-illness study of severe haemophilia a and B in five french haemophilia treatment centres. Pharm World Sci. 2008;30(3):287–292.
- Rocha P, Carvalho M, Lopes M, et al. Costs and utilization of treatment in patients with hemophilia. BMC Health Serv Res. 2015;15:484.
- Café A, Carvalho M, Crato M, et al. Haemophilia A: health and economic burden of a rare disease in Portugal. Orphanet J Rare Dis. 2019;14(1):211.
- O'Hara J, Hughes D, Camp C, et al. The cost of severe haemophilia in Europe: the CHESS study. Orphanet J Rare Dis. 2017;12(1):106–113.
- Keshavarz K, Bordbar M, Hashemipoor Z, et al. Economic burden of hemophilia a and B: a case in Iran. Hematology. 2020;25(1):149–155.
- Cavazza M, Kodra Y, Armeni P, et al. Social/economic costs and quality of life in patients with haemophilia in Europe. Eur J Health Econ. 2016;17(Suppl 1):53–65.
- Dundar S, Zülfikar B, Kavakli K, et al. A cost evaluation of treatment alternatives in mild-to-moderate bleeding episodes in haemophilia patients with inhibitors in Turkey. J Med Econ. 2005;8(1–4):46–54.
- Kodra Y, Cavazza M, Schieppati A, et al. The social burden and quality of life of patients with haemophilia in Italy. Blood Transfus. 2014 Apr;12(Suppl 3):S567–S75.
- Valentino LA, Pipe SW, Tarantino MD, et al. Healthcare resource utilization among haemophilia a patients in the United States. Haemophilia. 2012;18(3):332–338.
- Rocino A, Franchini M, Coppola A. Treatment and prevention of bleeds in haemophilia patients with inhibitors to factor VIII/IX. J Clin Med. 2017;6(4):46.
- Chen SL. Economic costs of hemophilia and the impact of prophylactic treatment on patient management. Am J Manag Care. 2016;22:s126–33.
- Rocino A, Cortesi PA, Scalone L, et al. Immune tolerance induction in patients with haemophilia a and inhibitors: effectiveness and cost analysis in an European Cohort (The ITER Study)). Haemophilia. 2016;22(1):96–102.
- Abbonizio F, Giampaolo A, Coppola A, et al. Therapeutic management and costs of severe haemophilia a patients with inhibitors in Italy. Haemophilia. 2014;20(4):e243–e250.
- Berntorp E, Shapiro AD, White G. Modern haemophilia care. Lancet. 2012;379(9824):1447–1456.
- Chen SL. Economic costs of hemophilia and the impact of prophylactic treatment on patient management. Am J Manag Care. 2016;22(5 Suppl):s126–s133.
- Schwartz CE, Powell VE, Eldar-Lissai A. Measuring hemophilia caregiver burden: validation of the hemophilia caregiver impact measure. Qual Life Res. 2017;26(9):2551–2562.
- Lorenzato CS, Santos RB, Fagundes GZZ, et al. Haemophilia experiences, results and opportunities (HERO study) in Brazil: assessment of the psychosocial effects of haemophilia in patients and caregivers. Haemophilia. 2019;25:640–650.
- Lindvall K, Colstrup L, Loogna K, et al. Knowledge of disease and adherence in adult patients with haemophilia. Haemophilia. 2010;16:592–596.
- Hoefnagels JW, Fischer K, Bos RAT, et al. A feasibility study on two tailored interventions to improve adherence in adults with haemophilia. Pilot Feasibility Stud. 2020;6(1):189.
- Escobar MA, Brewer A, Caviglia H, et al. Recommendations on multidisciplinary management of elective surgery in people with haemophilia. Haemophilia. 2018;24(5):693–702.
- Gürsoy K. An analysis of public pharmaceutical policy, pricing and spending in Turkey. Journal of Social Security. 2016;6:225–243.