Abstract
Aims
V114, a 15-valent pneumococcal conjugate vaccine (PCV15) currently approved in adults in the US, contains the 13 S. pneumoniae serotypes in PCV13 and two additional serotypes, 22 F and 33 F, which are important contributors to residual PD. This study quantified the health and economic burden of pediatric invasive pneumococcal disease (IPD) associated with V114 serotypes in eight countries in Europe.
Materials and methods
A Markov model estimated V114-type IPD cases and costs in hypothetical unvaccinated birth cohorts from Denmark, France, Germany, Italy, Norway, Spain, Switzerland, and the UK over 20 years. Inputs were obtained from published literature. IPD cases and costs were calculated for three time periods using time-specific epidemiological data: (a) pre-PCV7; (b) pre-PCV13; and (c) post-PCV13. Costs were estimated from a societal perspective (2018 Euros) and discounted at 3%.
Results
The model estimated that 4,649 IPD cases in the pre-PCV7 period, 3,248 cases in the pre-PCV13 period, and 958 cases in the post-PCV13 period were attributable to V114 serotypes. Total discounted costs associated with V114 serotypes were €109.1 million (pre-PCV7 period), €65.7 million (pre-PCV13 period), and €18.7 million (post-PCV13 period).
Limitations
Post-meningitis sequelae, acute otitis media, and non-bacteremic pneumonia were not considered. Direct non-medical costs were not included. Conclusions on effectiveness of V114 or added value over existing infant vaccination programs cannot be drawn.
Conclusions
IPD cases and costs were estimated in hypothetical birth cohorts in eight European countries followed for 20 years during three time periods. Serotypes included in V114 were associated with significant morbidity and costs in pre-PCV7, pre-PCV13, and post-PCV13 periods. Future pediatric pneumococcal vaccines should maintain protection against serotypes in licensed vaccines while extending coverage to additional serotypes to ensure reductions in IPD burden are maintained.
Introduction
Streptococcus pneumoniae (pneumococcus) is a gram-positive bacterium that causes invasive pneumococcal disease (IPD)Citation1,Citation2. In Europe, IPD is associated with substantial disease burdenCitation1,Citation3,Citation4, with an incidence of approximately 14.5 cases per 100,000 infants aged <1 year of age and five cases per 100,000 children aged 1–5 years of age in 2017Citation4. Bacteremia and bacteremic pneumococcal pneumonia are common manifestations of IPD. While meningitis is less common, case fatality rates (CFRs) can be as high as 12.9%Citation5,Citation6. Meningitis also has an associated risk of developing sequelae such as deafness or developmental delay in childrenCitation7,Citation8. IPD is associated with high direct medical costs as well as considerable indirect costs related to lost productivity among parents of children with pneumococcal diseases and among children due to premature deathCitation9,Citation10.
To address the disease and economic burden of pneumococcal disease, a pneumococcal conjugate vaccine (PCV) containing seven S. pneumoniae serotypes (PCV7) was introduced in infant immunization schedules in many European countries in the early 2000sCitation3 (). Despite a substantial reduction in the number of IPD cases in Europe following the introduction of PCV7, a subsequent increase in the incidence of disease caused by non-vaccine serotypes was observed; this necessitated the development of new vaccines containing additional serotypesCitation23. A 10-valent PCV (PCV10; SYNFLORIXFootnotei) and a 13-valent PCV (PCV13, PREVNAR13Footnoteii) were introduced in Europe in 2009, and resulted in further reductions in IPDCitation23. However, since then, the incidence of disease attributable to non-vaccine serotypes has risen, with some serotypes, such as serotypes 3 and 19 A persistingCitation4,Citation23,Citation24. Consequently, a number of next-generation PCVs are now in late-stage clinical development for pediatric use, including a 15-valent PCV (PCV15; V114; VAXNEUVANCEFootnoteiii) and a 20-valent PCV (PREVNAR20)Citation25, both recently approved for use in adults in the USCitation26,Citation27.
Table 1. Year of PCV introduction in European countries.
V114 contains all PCV13 serotypes (serotypes 1, 3, 4, 5, 6 A, 6 B, 7 F, 9 V, 14, 18 C, 19 A, 19 F, and 23 F) and two additional serotypes, 22 F and 33 FCitation28,Citation29. Serotypes 22 F and 33 F are two of the most common non-PCV13 serotypes that cause IPD in European childrenCitation11,Citation14,Citation30–34. In addition, 33 F has a high invasive disease potential compared with other non-PCV13 serotypesCitation35. To support V114 recommendation decisions, evidence of the potential health and economic value of a V114 pediatric vaccination program is needed. This study aimed to quantify the health and economic burden of IPD attributable to the 15 serotypes in V114 in hypothetical unvaccinated birth cohorts from eight European countries (Denmark, France, Germany, Italy, Norway, Spain, Switzerland, and the UK) that introduced PCV7 into routine infant vaccination schedules followed later by the introduction of PCV13. This study aimed to estimate the number of IPD cases and costs attributable to the 15 serotypes in V114 across three time periods, pre-PCV7, pre-PCV13, and post-PCV13, using time-specific epidemiological data, in order to emphasize the need to maintain the reduction in and address the current IPD burden.
Methods
IPD cases and costs attributable to V114 serotypes were calculated prior to PCV7 introduction. In order to account for the impact of PCVs on disease burden and serotype distribution, IPD cases and costs after introduction of PCV7 but prior to PCV13 introduction (for the six additional serotypes in PCV13), and after introduction of PCV13 (for serotypes 22 F and 33 F), were also calculated.
Analytical approach
This analysis was conducted using a Markov state transition model, which simulated clinical events and societal costs associated with V114 serotypes over a 20-year time horizon. Because IPD is an infectious disease, a dynamic transmission model would have been the preferred analytical approach, although static Markov models are also acceptableCitation36,Citation37.
Model structure
The structure of the model was similar to that used in a previously published health economic evaluation of PCV13Citation38.
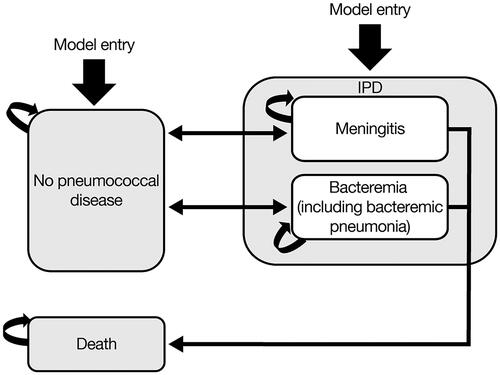
Three health states were included in the model: no pneumococcal disease, IPD (pneumococcal meningitis or bacteremia, including bacteremic pneumococcal pneumonia), and death (). The model was run on an unvaccinated birth cohort at risk of developing IPD. Over the time horizon of the model, the likelihood of an infant developing IPD differs by age. The model tracked the birth cohorts up to 20 years of age or until death, whichever occurred earlier, with a cycle length of 1 year. The model assumed that IPD events were mutually exclusive; hence, infants could experience only one IPD event during each model cycle. However, over the course of the time horizon, each infant could experience more than one IPD event. Following IPD episodes, infants could recover and return to the no pneumococcal disease health state. Infants with IPD also faced a risk of death (depending on the specific manifestation of IPD and age).
The analysis was based on the societal perspective, and, hence, included direct medical costs and indirect costs from productivity losses in children linked to premature death, and in caregivers of children with IPD. Because a Markov model was used, this study did not take into account transmission dynamics of pneumococcal infections from person-to-person, and did not include post-meningitis sequelae or non-IPD manifestations (such as acute otitis media and non-bacteremic pneumococcal pneumonia).
Model inputs
Target population
The target population of the analysis included cohorts of unvaccinated newborns from eight European countries that introduced PCV7 followed by PCV13 into routine infant vaccination schedules (Denmark, France, Germany, Italy, Norway, Spain, Switzerland, and the UK). Cohort sizes were estimated based on publicly available national statistics for 2018. The total cohort consisted of 3.2 million newborns (657,076 from the UKCitation39, 758,590 from FranceCitation40, 787,523 from GermanyCitation41, 439,747 from ItalyCitation42, 369,302 from SpainCitation43, 61,476 from DenmarkCitation44, 55,120 from NorwayCitation45, and 87,851 from SwitzerlandCitation46).
Epidemiologic inputs
Time-specific epidemiological data from the pre-PCV7 period, pre-PCV13 period, and post-PCV13 period (specific years depending on the history of PCV introduction for each country, as shown in ) were retrieved from published literature and surveillance reports. Age-specific IPD incidenceCitation11,Citation19,Citation21,Citation30,Citation32,Citation33,Citation47–51, distributionCitation11,Citation12,Citation14,Citation19–21,Citation30–34,Citation52–57, and proportions of IPD that were meningitis and bacteremia (including bacterial pneumonia)Citation6,Citation12,Citation30,Citation33,Citation47,Citation48,Citation50,Citation51,Citation54,Citation58–61 were based on published literature and publicly available reports, with the exception of IPD incidence and serotype distribution for Switzerland, which was obtained from the Swiss Ministry of Health (personal communication) (). CFRs for meningitis and bacteremia were also taken from published literatureCitation3,Citation5,Citation6,Citation13,Citation32,Citation62–65. These were the most recent CFRs available and were applied across all periods to reflect current access to medical care and treatment options.
Table 2. Epidemiological inputs.a
Cost inputs
The total costs per episode of IPD comprised direct medical costs and indirect costs (), including productivity losses due to premature death among children, and productivity losses among adult caregivers of children with IPD, expressed in 2018 Euros and discounted at 3%. Direct costs were obtained from the published literatureCitation5,Citation61–63,Citation66–68 and inflated to 2018 Euros using consumer price indices for healthcareCitation81–88. The human capital approach was utilized to determine indirect costsCitation89. Age-specific life expectancy values taken from life tables and age-specific average income per year were used to determine lost life-time income associated with premature death. In turn, the number of work days missed multiplied by daily wage rates and labor force participation rates in 2018 were applied to measure work loss in parents caring for children with pneumococcal diseaseCitation62,Citation66,Citation69–74,Citation76–80. Numbers of work days missed was calculated based on the length of hospital stay associated with bacteremia and meningitis episodes from published literatureCitation59,Citation62,Citation66.
Table 3. Cost inputs (2018 Euros).a
Model verification and validation
The validity of the model was confirmed by comparing its structure with that of previously published models during collaboration with experts. In addition, a number of tests were used in the model to ensure internal validity and verify the resultsCitation90. Tests were used to check for all three major types of model errors – logic, mechanical, and omission. As an example for verifying the model logic, the total number of persons in each health state at each cycle for each age was ensured to be equal to the size of the population at that age. Mechanical tests were also included to ensure that the right inputs were used in all calculations. Omission tests were used to ensure that the theoretical model structure and its components were all adequately represented in the calculations.
Model outputs
Estimated outcomes included number of IPD cases and deaths and overall total, direct, and indirect costs attributable to vaccine-type serotypes in the pre-PCV7, pre-PCV13, and post-PCV13 periods.
Sensitivity analysis
A one-way sensitivity analysis was conducted to assess the impact of uncertainties of key model parameters and assumptions on model results in the pre-PCV7 period. IPD incidence, CFR, and cost data were varied by ±20%, and the discount rate was varied from 0–5%. Given the key parameters used, the sensitivity analyses focused on the results for the pre-PCV7 period only. This is because the qualitative results (i.e. the positive/negative impact of the key parameters on the outcomes) were similar across the time periods.
Results
Clinical events
IPD cases by serotype
The model estimated that 4,649 IPD cases in the pre-PCV7 period, 3,248 cases in the pre-PCV13 period, and 958 IPD cases in the post-PCV13 period were attributable to V114 serotypes. The majority of IPD cases in the pre-PCV7 period were attributable to PCV7 serotypes (3,374 cases; 73%) ().
Table 4. IPD cases attributable to V114 serotypes in the pre-PCV7, pre-PCV13, and post-PCV13 periods.
The six additional serotypes in PCV13, but not in PCV7, increased from 1,170 cases in the pre-PCV7 period, to 2,544 cases in the pre-PCV13 period. The increase was primarily due to increases in serotype 1 (from 337 cases [7%] to 882 cases [27%]), serotype 3 (from 115 cases [2%] to 232 cases [7%]), serotype 7 F (from 210 cases [5%] to 633 cases [19%]), and serotype 19 A (from 250 cases [5%] to 625 cases [19%]). The number of cases attributable to serotype 6 A decreased in the pre-PCV13 period compared with the pre-PCV7 period, while the number attributable to serotype 5 remained relatively constant.
The number of cases associated with serotypes 22 F and 33 F combined was 106 (2%) in the pre-PCV7 period, 137 (4%) in the pre-PCV13 period, and 184 (19%) in the post-PCV13 period.
The numbers of cases in each country are shown in Supplementary Table S1.
Mortality due to IPD
The number of estimated IPD deaths associated with V114 serotypes was 178 in the pre-PCV7 period, 121 in the pre-PCV13 period, and 34 in the post-PCV13 period (). Most of these deaths were attributable to PCV7 serotypes (129 deaths; 72%) in the pre-PCV7 period. The number of IPD deaths associated with the six additional serotypes in PCV13 increased from 45 (25%) in the pre-PCV7 period to 97 (80%) in the pre-PCV13 period. The number of IPD deaths associated with serotypes 22 F and 33 F combined was four (2%) in the pre-PCV7 period, six (5%) in the pre-PCV13 period and eight (23%) in the post-PCV13 period. IPD deaths in each country are shown in Supplementary Table S2.
Table 5. IPD deaths attributable to V114 serotypes in the pre-PCV7, pre-PCV13, and post-PCV13 periods.
Economic impact by serotype
Total discounted costs (medical costs and indirect costs) due to V114 serotypes were estimated to be €109.1 million in the pre-PCV7 period, €65.7 million in the pre-PCV13 period, and €18.7 million in the post-PCV13 period (). Of these costs, €84.1 million (77%) in the pre-PCV7 period, €50.7 million (77%) in the pre-PCV13 period, and €14.1 million (76%) in the post-PCV13 period were attributable to indirect costs, of which the majority was comprised of productivity losses due to premature death.
Table 6. Discounted direct medical costs and indirect costs (including productivity losses due to premature death) attributed to IPD in the pre-PCV7, pre-PCV13, and post-PCV13 periods (2018 Euros).
IPD associated with PCV7 serotypes accounted for the largest proportion of costs (€79.4 million; 73% of the total costs) in the pre-PCV7 period. The cost of the six additional serotypes in PCV13 but not in PCV7 increased from €27.3 million (25%) in the pre-PCV7 period to €51.5 million (78%) in the pre-PCV13 period. This was primarily due to increases in costs attributable to serotype 1 from €8.4 million (8%) to €16.1 million (25%), serotype 3 from €3.2 million (3%) to €5.1 million (8%), serotype 7 F from €5.1 million (5%) to €14.0 million (21%), and serotype 19 A from €5.6 million (5%) to €12.8 million (20%). Costs attributable to serotype 6 A decreased in the pre-PCV13 period compared with the pre-PCV7 period, and costs due to serotype 5 were similar in both periods.
Total costs associated with serotypes 22 F and 33 F together were €2.5 million (2%) in the pre-PCV7 period, €3.8 million (6%) in the pre-PCV13 period, and €4.8 million (26%) in the post-PCV13 period.
Costs by country are shown in Supplementary Table S3.
Sensitivity analysis
The discounted total cost was sensitive to uncertainties around all key parameters, as demonstrated by the one-way sensitivity analysis, but most affected by the discount rate. Total costs associated with IPD increased by 231–238% for 0% discount rates and decreased by 42–43% for 5% discount rates (). A 20% increase and decrease in total costs was observed when varying IPD incidence by 20%. Varying the CFR for meningitis and bacteremia by 20% led to a 15% increase and decrease in the total cost. When the direct medical cost of an IPD episode varied by 20%, total costs increased by 4–5% and decreased by 5%. Sensitivity analyses for each country are shown in Supplementary Table S4.
Table 7. One-way sensitivity analyses results (2018 Euros).
Discussion
This modeling analysis, that used a hypothetical unvaccinated birth cohort from eight European countries over 20 years, demonstrated that a substantial number of IPD cases and deaths were attributable to the 15 pneumococcal serotypes included in V114 during the pre-PCV7, pre-PCV13, and post-PCV13 periods. Across all three time periods, high total discounted costs were observed for V114 serotypes. The overall number of IPD cases and costs decreased over time, yet an increase in the proportion associated with the six additional serotypes in PCV13 was observed in the pre-PCV13 period compared with the pre-PCV7 period, driven by serotypes 1, 3, 7 F, and 19 A. Compared with the pre-PCV7 period, in the pre- and post-PCV13 periods, there was an increase in the proportion of cases and costs linked to 22 F and 33 F, the two unique serotypes in V114.
The findings of our analysis are consistent with the literature. Prior to the introduction of PCV7, 67–92% of all IPD cases were caused by the seven vaccine serotypes in children aged < 5 years in the eight European countries included in this analysisCitation12,Citation14,Citation20,Citation21,Citation52,Citation53,Citation55. Following the introduction of PCV7, there was a significant increase in IPD incidence associated with several non-PCV7 serotypes due to serotype replacement, in particular serotypes 1, 3, 7 F, and 19 ACitation24,Citation91,Citation92. Similarly, due to serotype replacement following PCV13 introduction, disease caused by non-PCV13 serotypes now accounts for 72% of IPD in European childrenCitation23,Citation24. Several studies have also highlighted the persistence of select vaccine serotypes after PCV13 introduction, particularly serotype 3Citation14,Citation93.
The next generation of pediatric pneumococcal vaccines must therefore continue to protect against serotypes that were highly prevalent and invasive prior to the introduction of PCV7 and PCV13, as well as extending coverage to non-vaccine serotypes that have since emerged as important causes of disease following successive PCV7 and PCV13 introductions in children. The importance of this strategy is confirmed by this modeling analysis, which estimated cases and deaths attributable to vaccine-type and non-vaccine-type serotypes (22 F and 33 F) in hypothetical birth cohorts in eight European countries in the pre-PCV7, pre-PCV13, and post-PCV13 periods. The impact of next generation PCVs on nasopharyngeal carriage and transmission of pneumococcal infections, while important, was not evaluated in the modeling analysis.
In the pre-PCV7 period, 3,374 PCV7-type IPD cases were estimated to occur in the eight birth cohorts, leading to over €100 million in direct and indirect costs. The disease burden associated with the six additional serotypes in PCV13 increased from 1,170 cases in the pre-PCV7 period to 2,544 cases in the pre-PCV13 period, at a total cost of €51.1 million. Substantial clinical and economic burden continues to be prevented by serotypes targeted by PCVs, particularly those included in PCV7. Excluding these serotypes from next-generation pediatric pneumococcal vaccines could have significant public health implications for both pediatric and adult populationsCitation94,Citation95.
There is also evidence that certain vaccine-type serotypes continue to persist in European countries that have introduced PCV13 into their routine immunization schedules for infantsCitation15,Citation96. Serotype 3 has been associated with vaccine failuresCitation97,Citation98, suggesting a need for vaccines with greater effectiveness against this serotype. In this simulation, serotype 3 caused 185 IPD cases and €4.1 million in direct and indirect costs in the post-PCV13 period. Furthermore, despite substantial disease reduction following the introduction of PCVs, disease caused by non-vaccine serotypes has emerged. In Europe, non-vaccine serotypes accounted for 72% of IPD in children in the post-PCV13 periodCitation23,Citation24, with serotypes 22 F and 33 F causing a large proportionCitation11,Citation14,Citation30–34. In this simulation, serotypes 22 F and 33 F were associated with 184 IPD cases and €4.8 million in direct and indirect costs in the post-PCV13 period.
This analysis has several limitations. First, post-meningitis sequelae, prevention of non-bacteremic pneumococcal pneumonia, and prevention of acute otitis media were not considered. Additionally, indirect costs associated with productivity loss for caregivers were estimated using conservative values for earnings, and the analysis did not include direct non-medical costs borne by families or caregivers, such as transportation and accommodation. As the model used was a Markov model that did not account for transmission dynamics, it ignored the impact of PCVs on nasopharyngeal carriage, the transmission of pneumococcal infections from person to person, and the indirect protective effect of PCVs on unvaccinated groups. Finally, pneumococcal disease in adults was not considered in this analysis since the time horizon of the model was only 20 years. Inclusion of any of these factors in the model would likely have affected overall disease and economic burden estimates.
V114 is currently in clinical development; thus, the effectiveness of this vaccine against the 15 serotypes included, and therefore the extent to which it may address the health and economic burden described here, cannot be determined based on this analysis. Additionally, the potential impact of V114 on emergence of new non-vaccine serotypes, and the subsequent increase in health and economic burden, is also unknown. It should also be noted that this analysis was not designed to evaluate the added value of V114 over existing infant vaccination programs.
Conclusions
The present analysis simulated IPD cases, deaths, and direct and indirect costs attributable to V114 serotypes across eight European countries. Three time periods were considered: pre-PCV7, pre-PCV13, and post-PCV13. Results suggested that in these countries, in the pre-PCV7 period, the majority of IPD-related morbidity, mortality, and associated costs in children were attributable to PCV7 serotypes. Four of the six additional serotypes included in PCV13 but not in PCV7 (1, 3, 7 F, and 19 A) were associated with substantially greater morbidity, mortality, and costs after the introduction of PCV7 and prior to the introduction of PCV13. The unique V114 serotypes 22 F and 33 F were associated with additional morbidity and costs of a magnitude comparable to that of serotype 3 in all three periods. The full value of next-generation pediatric pneumococcal vaccines should account for serotypes in the previously licensed vaccines, as well as the additional non-vaccine type serotypes.
Transparency
Declaration of funding
This analysis was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Declaration of financial/other interests
All authors are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, who may own stock and/or hold stock options in Merck & Co., Inc., Kenilworth, NJ.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
TP, TH, TW, and KOE designed the study; TP, GB, and TH analyzed the study data; and TP, GB, TH, and TW interpreted the study data. All authors critically reviewed the manuscript and approved the final version for submission.
Previous presentations
These data were presented as a poster at the 38th Annual Meeting of the European Society for Paediatric Infectious Diseases (ESPID), May 6–11, 2019, Ljubljana, Slovenia.
Supplemental Material
Download PDF (321.1 KB)Acknowledgements
Medical writing assistance and editorial support, under the direction of the authors, was provided by Rachel Wright, PhD, and Gauri Saal, MA Economics, of Scion (London, UK) funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, according to Good Publication Practice guidelines (https://www.acpjournals.org/doi/10.7326/M15-0288).
Notes
i SYNFLORIX is a trademark of GlaxoSmithKline Biologicals, s.a., Rixensart, Belgium.
ii PREVNAR13 is a trademark of Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer, Inc., Philadelphia, PA, USA.
iii VAXNEUVANCE is a trademark of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
References
- Wahl B, O’Brien KL, Greenbaum A, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000-15. Lancet Glob Health. 2018;6(7):e744–e757.
- G. B. D. Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–1210.
- Georgalis L, Mozalevskis A, Martinez de Aragon MV, et al. Changes in the pneumococcal disease-related hospitalisations in Spain after the replacement of 7-valent by 13-valent conjugate vaccine. Eur J Clin Microbiol Infect Dis. 2017;36(3):575–583.
- European Centre for Disease Prevention and Control. Invasive pneumococcal disease – annual epidemiological report for 2017 2019; [cited 2019 Oct 4]. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2017-invasive-pneumococcal-disease.pdf.
- Castiglia P, Pradelli L, Castagna S, et al. Overall effectiveness of pneumococcal conjugate vaccines: an economic analysis of PHiD-CV and PCV-13 in the immunization of infants in Italy. Hum Vaccin Immunother. 2017;13(10):2307–2315.
- Makwana A, Sheppard C, Borrow R, et al. Characteristics of children with invasive pneumococcal disease after the introduction of the 13-valent pneumococcal conjugate vaccine in England and Wales, 2010-2016. Pediatr Infect Dis J. 2018;37(7):697–703.
- Stockmann C, Ampofo K, Byington CL, et al. Pneumococcal meningitis in children: epidemiology, serotypes, and outcomes from 1997 to 2010 in Utah. Pediatrics. 2013;132(3):421–428.
- European Centre for Disease Prevention and Control. Disease factsheet about pneumococcal disease 2019; [cited 2019 Apr 3]. Available from: https://ecdc.europa.eu/en/pneumococcal-disease/facts.
- Ceyhan M, Ozsurekci Y, Aykac K, et al. Economic burden of pneumococcal infections in children under 5 years of age. Hum Vaccin Immunother. 2018;14(1):106–110.
- Zhang S, Sammon PM, King I, et al. Cost of management of severe pneumonia in young children: systematic analysis. J Glob Health. 2016;6(1):010408.
- Ladhani SN, Collins S, Djennad A, et al. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: a prospective national observational cohort study. Lancet Infect Dis. 2018;18(4):441–451.
- Miller E, Andrews NJ, Waight PA, et al. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;11(10):760–768.
- Ouldali N, Levy C, Varon E, et al. Incidence of paediatric pneumococcal meningitis and emergence of new serotypes: a time-series analysis of a 16-year French national survey. Lancet Infect Dis. 2018;18(9):983–991.
- van der Linden M, Falkenhorst G, Perniciaro S, et al. Effects of infant pneumococcal conjugate vaccination on serotype distribution in invasive pneumococcal disease among children and adults in Germany. PLoS One. 2015;10(7):e0131494.
- van der Linden M, Falkenhorst G, Perniciaro S, et al. Effectiveness of pneumococcal conjugate vaccines (PCV7 and PCV13) against invasive pneumococcal disease among children under two years of age in Germany. PLoS One. 2016;11(8):e0161257.
- Camilli R, D’Ambrosio F, Del Grosso M, et al. Impact of pneumococcal conjugate vaccine (PCV7 and PCV13) on pneumococcal invasive diseases in Italian children and insight into evolution of pneumococcal population structure. Vaccine. 2017;35(35):4587–4593.
- Giorgi-Rossi P, Merito M, Borgia P. Cost-effectiveness of introducing the conjugated pneumococcal vaccine to routine free immunizations for infants in Lazio, Italy. Health Policy. 2009;89(2):225–238.
- Fenoll A, Granizo JJ, Gimenez MJ, et al. Secular trends (1990–2013) in serotypes and associated non-susceptibility of S. pneumoniae isolates causing invasive disease in the pre-/post-era of pneumococcal conjugate vaccines in Spanish regions without universal paediatric pneumococcal vaccination. Vaccine. 2015;33(42):5691–5699.
- Harboe ZB, Dalby T, Weinberger DM, et al. Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumococcal disease incidence and mortality. Clin Infect Dis. 2014;59(8):1066–1073.
- Harboe ZB, Valentiner-Branth P, Benfield TL, et al. Estimated effect of pneumococcal conjugate vaccination on invasive pneumococcal disease and associated mortality, Denmark 2000-2005. Vaccine. 2008;26(29–30):3765–3771.
- Steens A, Bergsaker MA, Aaberge IS, et al. Prompt effect of replacing the 7-valent pneumococcal conjugate vaccine with the 13-valent vaccine on the epidemiology of invasive pneumococcal disease in Norway. Vaccine. 2013;31(52):6232–6238.
- Hauser C, Kronenberg A, Allemann A, et al. Serotype/serogroup-specific antibiotic non-susceptibility of invasive and non-invasive Streptococcus pneumoniae, Switzerland, 2004 to 2014. Euro Surveill. 2016;21(21):239.
- Savulescu C, Krizova P, Lepoutre A, et al. Effect of high-valency pneumococcal conjugate vaccines on invasive pneumococcal disease in children in SpIDnet countries: an observational multicentre study. Lancet Respir Med. 2017;5(8):648–656.
- Balsells E, Guillot L, Nair H, et al. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: a systematic review and Meta-analysis. PLoS One. 2017;12(5):e0177113.
- Klugman KP, Rodgers GL. Time for a third-generation pneumococcal conjugate vaccine. Lancet Infect Dis. 2021;21(1):14–16.
- Food and Drug Administration. VAXNEUVANCE™ (Pneumococcal 15-valent Conjugate Vaccine) Prescribing Information 2021 [cited 2021 21 July 2021. Available from: https://www.fda.gov/media/150819/download.
- Food and Drug Administration. Prevnar 20 Package insert 2021; [cited 2021 Aug 9]. Available from: https://www.fda.gov/media/149987/download.
- Rupp R, Hurley D, Grayson S, et al. A dose ranging study of 2 different formulations of 15-valent pneumococcal conjugate vaccine (PCV15) in healthy infants. Hum Vaccin Immunother. 2019;15(3):549–559.
- Greenberg D, Hoover PA, Vesikari T, et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine (PCV15) in healthy infants. Vaccine. 2018;36(45):6883–6891.
- Ciruela P, Izquierdo C, Broner S, et al. The changing epidemiology of invasive pneumococcal disease after PCV13 vaccination in a country with intermediate vaccination coverage. Vaccine. 2018;36(50):7744–7752.
- Slotved HC, Dalby T, Hoffmann S. The effect of pneumococcal conjugate vaccines on the incidence of invasive pneumococcal disease caused by ten non-vaccine serotypes in Denmark. Vaccine. 2016;34(6):769–774.
- European Centre for Disease Prevention and Control. Surveillance atlas of infectious diseases 2018; [cited 2019 Oct 29]. Available from: https://ecdc.europa.eu/en/surveillance-atlas-infectious-diseases.
- Istituto Superiore Di Sanita. Sorveglianza delle malattie batteriche invasive in Italia 2018; [cited 2019 Oct 29]. Available from: http://old.iss.it/binary/mabi/cont/Report2017.pdf.
- Centre National de Référence des Pneumocoques. Rapport d’activité 2016 2016; [cited 2020 Jul 17]. Available from: https://cnr-pneumo.com/docman/rapports/20-cnrp2016/file.
- Balsells E, Dagan R, Yildirim I, et al. The relative invasive disease potential of Streptococcus pneumoniae among children after PCV introduction: a systematic review and meta-analysis. J Infect. 2018;77(5):368–378.
- Ultsch B, Damm O, Beutels P, et al. Methods for health economic evaluation of vaccines and immunization decision frameworks: a consensus framework from a european vaccine economics community. Pharmacoeconomics. 2016;34(3):227–244.
- World Health Orgainzation. WHO Guide on Standardization of Economic Evaluations of Immunization Programmes 2019; [cited 2021 May 13]. Available from: https://www.who.int/immunization/documents/who_ivb_19.10/en/.
- Rubin JL, McGarry LJ, Strutton DR, et al. Public health and economic impact of the 13-valent pneumococcal conjugate vaccine (PCV13) in the United States. Vaccine. 2010;28(48):7634–7643.
- Office of National Statistics. Birth characteristics in England and Wales: 2018 2019; [cited 2020 July 07]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/bulletins/birthsummarytablesenglandandwales/2018.
- Institut National de la Statistique et des Etudes Economiques. 2019 demographic report 2020; [cited 2020 July 07]. Available from: https://www.insee.fr/en/statistiques/2382601.
- DESTATIS Statistisches Bundesamt. Births: population movement 2019; [cited 2019 Oct 22]. Available from: https://www.destatis.de/EN/Themes/Society-Environment/Population/Births/Tables/birth-deaths.html.
- Istituto Nazionale di Statistic. Birth and fertility of the resident population: Year 2018 2019; [cited 2020 July 07]. Available from: https://www.istat.it/en/archivio/237688.
- Statista. Number of births in Spain from 2006. to 2018 2020; [cited 2020 Jul 7]. Available from: https://www.statista.com/statistics/449295/number-of-births-in-spain/.
- Statista. Number of live births in Denmark from 2009 to 2019 2020; [cited 2020 July 07]. Available from: https://www.statista.com/statistics/573245/number-of-live-births-in-denmark/.
- Statistick Sentralbyra Norway. Births 2020; [cited 2020 July 07]. Available from: https://www.ssb.no/en/fodte.
- Federal Statistical Office. Births and deaths 2020; [cited 2020 July 07]. Available from: https://www.bfs.admin.ch/bfs/en/home/statistics/population/births-deaths.html.
- Weinberger R, von Kries R, van der Linden M, et al. Invasive pneumococcal disease in children under 16 years of age: Incomplete rebound in incidence after the maximum effect of PCV13 in 2012/13 in Germany. Vaccine. 2018;36(4):572–577.
- Marchetti M, Colombo GL. Cost-effectiveness of universal pneumococcal vaccination for infants in Italy. Vaccine. 2005;23(37):4565–4576.
- D’Ancona F, Caporali MG, Del Manso M, et al. Invasive pneumococcal disease in children and adults in seven Italian regions after the introduction of the conjugate vaccine. Epidemiol Prev. 2015;39(4 Suppl 1):134–138.
- Munoz-Almagro C, Jordan I, Gene A, et al. Emergence of invasive pneumococcal disease caused by nonvaccine serotypes in the era of 7-valent conjugate vaccine. Clin Infect Dis. 2008;46(2):174–182.
- Santé Publique France. Bulletin EPIBAC n°6 du réseau de surveillance des infections invasives bactériennes 2016; [cited 2019 Oct 22]. Available from: https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/infections-a-pneumocoque/documents/bulletin-national/bulletin-epibac-n-6-du-reseau-de-surveillance-des-infections-invasives-bacteriennes.
- Salleras L, Dominguez A, Ciruela P, et al. Changes in serotypes causing invasive pneumococcal disease (2005–2007 vs. 1997–1999) in children under 2 years of age in a population with intermediate coverage of the 7-valent pneumococcal conjugated vaccine. Clin Microbiol Infect. 2009;15(11):997–1001.
- Istituto Superiore Di Sanita. Dati del sistema di sorveglianza delle meningiti batteriche in Italia 1994-2006 2012 [October 29, 2019. Available from: http://old.iss.it/binary/mabi/cont/Report_meningiti_1994_2006.pdf.
- Istituto Superiore Di Sanita. Dati di sorveglianza delle malattie batteriche invasive 2007–2010 2013; [cited 2019 Oct 29]. Available from: http://old.iss.it/binary/mabi/cont/Report_MBI2_2007_2010.pdf.
- Centre National de Référence des Pneumocoques. Rapport d’activité 2003 2003; [cited 2019 Oct 29. 12/07/2018]. Available from: https://cnr-pneumo.com/docman/rapports/7-cnrp2003/file.
- Centre National de Référence des Pneumocoques. Rapport d’activité 2010 2010 [cited 2020 July 17]. Available from: https://cnr-pneumo.com/docman/rapports/14-cnrp2010/file.
- Statens Serum Institut. Overvågning i tal, grafer og kort: pneumokok meningitis, Individuelle anmeldelsespligtige sygdomme; [cited 2020 July 7]. Available from: https://statistik.ssi.dk/sygdomsdata#!/?sygdomskode=PNEU&aldersgruppe=1&xaxis=Aar&yaxis=Total&show=Table&aar=2008%7C2010&datatype=Individual.
- Ingels H, Rasmussen J, Andersen PH, et al. Impact of pneumococcal vaccination in Denmark during the first 3 years after PCV introduction in the childhood immunization programme. Vaccine. 2012;30(26):3944–3950.
- Robberstad B, Frostad CR, Akselsen PE, et al. Economic evaluation of second generation pneumococcal conjugate vaccines in Norway. Vaccine. 2011;29(47):8564–8574.
- Venetz I, Schopfer K, Muhlemann K. Paediatric, invasive pneumococcal disease in Switzerland, 1985-1994. Swiss Pneumococcal Study Group. Int J Epidemiol. 1998;27(6):1101–1104.
- Blank PR, Szucs TD. Cost-effectiveness of 13-valent pneumococcal conjugate vaccine in Switzerland. Vaccine. 2012;30(28):4267–4275.
- Kuhlmann A, von der Schulenburg JG. Modeling the cost-effectiveness of infant vaccination with pneumococcal conjugate vaccines in Germany. Eur J Health Econ. 2017;18(3):273–292.
- Klok RM, Lindkvist RM, Ekelund M, et al. Cost-effectiveness of a 10- versus 13-valent pneumococcal conjugate vaccine in Denmark and Sweden. Clin Ther. 2013;35(2):119–134.
- Ess SM, Schaad UB, Gervaix A, et al. Cost-effectiveness of a pneumococcal conjugate immunisation program for infants in Switzerland. Vaccine. 2003;21(23):3273–3281.
- Agyeman PKA, Schlapbach LJ, Giannoni E, et al. Epidemiology of blood culture-proven bacterial sepsis in children in Switzerland: a population-based cohort study. Lancet Child Adolesc Health. 2017;1(2):124–133.
- Delgleize E, Leeuwenkamp O, Theodorou E, et al. Cost-effectiveness analysis of routine pneumococcal vaccination in the UK: a comparison of the PHiD-CV vaccine and the PCV-13 vaccine using a markov model. BMJ Open. 2016;6(11):e010776.
- PMSI. Les diagnostics médicaux codés en MCO en 2017 2018; [cited 2019 Oct 29]. Available from: https://www.lespmsi.com/les-diagnostics-medicaux-codes-en-mco-en-2017/.
- PMSI. Tarifs des GHS en 2018 (DGF et OQN) 2018; [cited 2019 Oct 29]. Available from: https://www.lespmsi.com/tarifs-des-ghs-en-2018-dgf-et-oqn/.
- Trading Economics. France labor force participation rate 2019; [cited 2020 Jan 6]. Available from: https://tradingeconomics.com/france/labor-force-participation-rate.
- Journal du Net (JDN). Average salary in France 2020: net, gross, by sex, by CSP 2019; [cited 2020 Jan 6]. Available from: https://www.journaldunet.fr/patrimoine/guide-des-finances-personnelles/1166094-salaire-moyen/#salaire-brut-mensuel-moyen.html.
- Trading Economics. Italy labor force participation rate 2019; [cited 2020 Jan 06 ]. Available from: https://tradingeconomics.com/italy/labor-force-participation-rate.
- Trading Economics. Italy average nominal monthly wages 2019; [cited 2020 Jan 06 ]. Available from: https://tradingeconomics.com/italy/wages.
- Instituto Nacional de Estadistica (INE). Annual average earnings per worker – Year 2017 2019; [cited 2020 Jan 06 ]. Available from: https://www.ine.es/dyngs/INEbase/en/operacion.htm?c=Estadistica_C&cid=1254736177025&menu=ultiDatos&idp=1254735976596.
- Instituto Nacional de Estadistica (INE). Economically active population survey 2019; [cited 2020 Jan 06 ]. Available from: https://www.ine.es/dyngs/INEbase/en/operacion.htm?c=Estadistica_C&cid=1254736176918&menu=ultiDatos&idp=1254735976595.
- Diez-Domingo J, Ridao-Lopez M, Gutierrez-Gimeno MV, et al. Pharmacoeconomic assessment of implementing a universal PCV-13 vaccination programme in the valencian public health system (Spain). Vaccine. 2011;29(52):9640–9648.
- Statistics Denmark. Income 2019; [cited 2020 Jan 06 ]. Available from: https://www.dst.dk/en/Statistik/emner/arbejde-indkomst-og-formue/indkomster.
- The World Bank. Labor force participation rate, total 2019; [cited 2020 Jan 06 ]. Available from: https://data.worldbank.org/indicator/SL.TLF.CACT.ZS.
- Statistick Sentralbyra Norway. Earnings 2020; [cited July 2020 07]. Available from: https://www.ssb.no/en/lonnansatt.
- Swiss Federal Office for Statistics. Important labor market indicators, development 2018; [cited 2020 Jan 06 ]. Available from: https://www.bfs.admin.ch/bfs/de/home/statistiken/arbeit-erwerb/erwerbstaetigkeit-arbeitszeit.assetdetail.6526328.html.
- Swiss Federal Office for Statistics. Monthly gross wages by age and gender 2018; [cited 2020 Jan 06 ]. Available from: https://www.bfs.admin.ch/bfs/de/home/statistiken/arbeit-erwerb/loehne-erwerbseinkommen-arbeitskosten/lohnniveau-schweiz/personenbezogene-merkmale.assetdetail.5226961.html.
- Office of National Statistics (ONS). CPI INDEX 06: HEALTH 2015 = 100 2019; [cited 2020 Jan 06]. Available from: https://www.ons.gov.uk/economy/inflationandpriceindices/timeseries/d7bz/mm23.
- Federal Reserve Bank of St Louis (FRED). Harmonized Index of Consumer Prices: Health for Germany 2019; [cited 2019 Jan 06]. Available from: https://fred.stlouisfed.org/series/CP0600DEM086NEST.
- Federal Reserve Bank of St Louis (FRED). Harmonized Index of Consumer Prices: Health for Italy 2019; [cited 2019 Jan 06 ]. Available from: https://fred.stlouisfed.org/series/CP0600ITM086NEST.
- Federal Reserve Bank of St Louis (FRED). Harmonized Index of Consumer Prices: Health for Spain 2019; [cited 2019 Jan 06]. Available from: https://fred.stlouisfed.org/series/CP0600ESM086NEST.
- Statistics Denmark. Consumer price index 2019; [cited 2020 Jan 06 ]. Available from: https://www.dst.dk/en/Statistik/emner/priser-og-forbrug/forbrugerpriser/forbrugerprisindeks.
- Statistick Sentralbyra Norway. Consumer price index 2020; [cited 2020 July 07]. Available from: https://www.ssb.no/en/statbank/table/03014/.
- Federal Reserve Bank of St Louis (FRED). Consumer price index: all items for Switzerland 2020; [cited 2020 July 07]. Available from: https://fred.stlouisfed.org/series/CHECPIALLAINMEI.
- Federal Reserve Bank of St Louis (FRED). Harmonized Index of Consumer Prices: Health for France 2019; [cited 2019 Jan 06 ]. Available from: https://fred.stlouisfed.org/series/CP0600FRM086NEST.
- Nyman JA. Cost recommendations in the second edition of cost-effectiveness in health and medicine: a review. MDM Policy Pract. 2018;3(1):2381468318765162.
- Dasbach EJ, Elbasha EH. Verification of decision-analytic models for health economic evaluations: an overview. Pharmacoeconomics. 2017;35(7):673–683.
- Isaacman DJ, McIntosh ED, Reinert RR. Burden of invasive pneumococcal disease and serotype distribution among Streptococcus pneumoniae isolates in young children in Europe: impact of the 7-valent pneumococcal conjugate vaccine and considerations for future conjugate vaccines. Int J Infect Dis. 2010;14(3):e197–e209.
- Tin Tin Htar M, Christopoulou D, Schmitt HJ. Pneumococcal serotype evolution in Western Europe. BMC Infect Dis. 2015;15(1):419.
- Kandasamy R, Voysey M, Collins S, et al. Persistent circulation of vaccine serotypes and serotype replacement after 5 years of infant immunization with 13-valent pneumococcal conjugate vaccine in the United Kingdom. J Infect Dis. 2020;221(8):1361–1370.
- Klugman KP. Herd protection induced by pneumococcal conjugate vaccine. Lancet Glob Health. 2014;2(7):e365–e366.
- Hammitt LL, Akech DO, Morpeth SC, et al. Population effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and non-typeable Haemophilus influenzae in kilifi, Kenya: findings from cross-sectional carriage studies. Lancet Glob Health. 2014;2(7):e397–e405.
- Andrews N, Kent A, Amin-Chowdhury Z, et al. Effectiveness of the seven-valent and thirteen-valent pneumococcal conjugate vaccines in England: the indirect cohort design, 2006–2018. Vaccine. 2019;37(32):4491–4498.
- Oligbu G, Collins S, Andrews N, et al. Characteristics and serotype distribution of childhood cases of invasive pneumococcal disease following pneumococcal conjugate vaccination in England and Wales, 2006–2014. Clin Infect Dis. 2017;65(7):1191–1198.
- Silva-Costa C, Brito MJ, Pinho MD, et al. Pediatric complicated pneumonia caused by Streptococcus pneumoniae serotype 3 in 13-valent pneumococcal conjugate vaccinees, Portugal, 2010–2015. Emerg Infect Dis. 2018;24(7):1307–1314.