?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aims
The Japanese government reimburses patients for drugs at prices specified in the Drug Price Standard (DPS) published by the National Health Insurance (NHI) scheme. It revises reimbursements for most drugs on the basis of their market prices. This study thereby identifies factors related to drugs or disease that impact market prices for drugs using the DPS list.
Materials and methods
This study first examined the 2018 DPS list to identify all listed drugs, their prices, and their stipulated reimbursements. We then excluded from this study all the drugs for which prices are set per alternate rules. We calculated the percentage divergence between market prices and DPS prices and designated it our dependent variable. We performed descriptive and a univariate analysis on each variable and constructed multivariate regression models featuring independent variables for drug characteristics that might affect market prices.
Results
We identified 1,775 drugs with prices revised only by the market. We observed higher percentage divergences between DPS and market prices for drugs with generic alternatives (p < 0.001), drugs listed in the Japanese Pharmacopoeia (p < 0.001), and drugs for which at least two new drugs entered the same therapeutic category (p < 0.001). Injectable drugs exhibited a more significant and negative correlation with percentage divergences (p = 0.009) than ingestible drugs. Drugs that treat specific organs (p < 0.001), affect metabolism (p = 0.001), and those prescribed for non-therapeutic purposes (p < 0.001) display significantly higher percentage divergence than drugs affecting the nervous system and sensory organs. Divergences are less for narcotics (p < 0.001) and drugs that counter pathologic microorganisms and parasites (p = 0.004).
Conclusions
Factors that elevate competition among pharmaceutical companies likely lower market prices for drugs, and the direction of prices under NHI in Japan is affected by the category of diseases a drug treats.
Introduction
Japan’s national health insurance (NHI) scheme reimburses patients for prescription medications that are published in the Drug Price Standard (DPS). The Japanese government sets reimbursements for each listed drug, and the reimbursement becomes the drugs’ official priceCitation1. The government revises reimbursements biennially using different criteria. With a few exceptions, revisions are based on prices wholesalers charge medical facilities and pharmaciesCitation2. As market prices generally are below listed DPS prices, biennially revised prices are often below previous listed prices. Furthermore, the reduction in the revised price will be determined depending on how much the market price is below the DPS-listed price.
The pharmaceutical industry wants to maintain prices or mitigate biennial reductions. The Federation of Pharmaceutical Manufacturers’ Associations of Japan (FPMAJ), the Pharmaceutical Research and Manufacturers of America (PhRMA), and the European Federation of Pharmaceutical Industries and Associations (EFPIA) have expressed concern about curtailing the number of drugs that qualify for a price maintenance premium (PMP)Citation3,Citation4. Given that the government limits the number of PMP-qualified drugs to reduce its outlays, market prices play an increasing role in mitigating price reductions.
Even though market prices are the only drug prices set without Japanese government intervention, few studies explore how they are determined. Only one study suggests that the number of competitors expands the gap between market prices and reimbursementsCitation5. Since off-year revisions started in FY2021, pharmaceutical companies need to understand what characteristics influence market price to predict revisions and to develop price maintenance strategies. The government needs to understand where it enjoys leeway in reducing DPS prices and reimbursements. This study identifies features of drugs that create divergences between market and DPS prices.
Methods
Selection of drugs
We consulted 2018 DPS listings and reimbursementsCitation6. To identify drug prices revised only by market prices, we excluded those subject to price maintenance (PMP, basic drugs, and minimum statutory DPS prices), additional reductions (long-listed drugs, market expansion re-pricing), price increases (recalculations for unprofitable products, premiums for orphan and pediatric drugs), and weighted average pricing (generics)Citation7. The terms of pricing rules are explained in .
Table 1. Term explanations of the rules of price revision for listed drugs.
Variables
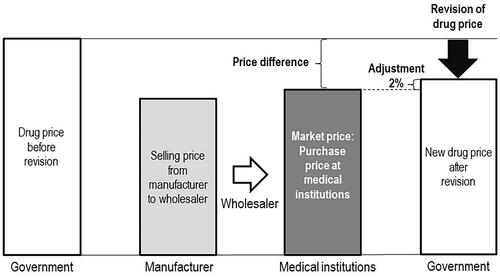
shows the association between the market price and DPS price. As a dependent variable we used the percentage difference between market and DPS prices, calculated as:
The current DPS list is dated 31 March 2018Citation8. Where prior and revised DPS prices were identical, we hypothesized a 1% divergence from market price as the midpoint of the 2% adjustment. We extracted explanatory variables from the final selection using public data for availability of generics, whether drugs are ingested or inoculated, therapeutic categories, number of new drugs in the same therapeutic category from April 2016 to March 2018, and publication in the Japanese PharmacopoeiaCitation6,Citation9–11. represents details for all parameters.
Table 2. Characteristics of drugs in the analysis.
Statistical methods
We performed descriptive analyses upon each variable and applied univariate analysis to percentage divergences for explanatory variables. We applied Mann–Whitney U tests or Kruskal–Wallis tests to categorical variables. We then constructed multivariate regression models to explore independent variables as influences on market prices, which assume linearity, independence, normality, and identically distributed errors. All explanatory variables enter the model. We estimated beta coefficients and 95% confidence intervals using STATA version 14.2.
Results
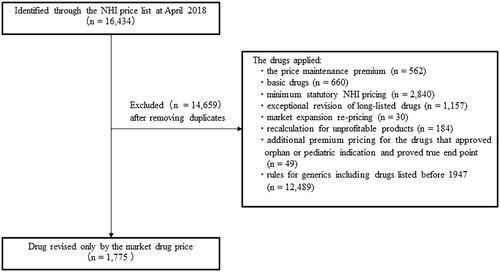
We identified 16,434 drugs and their prices from the 2018 DPS list. We excluded 14,659 duplicated drugs, drugs subject to PMP (n = 562), basic drugs (n = 660), drugs with minimum statutory DPS prices (n = 2,840), long-listed drugs eligible for exceptional revisions (n = 1,157), drugs eligible for market expansion pricing (n = 30), drugs eligible for recalculation as unprofitable (n = 184), orphan or pediatric drugs eligible for additional premium pricing (n = 49), and generic drugs, including those listed pre-1947 (n = 12,489). We identified the remaining 1,775 drugs as solely market-priced ().
Characteristics of selected drugs
summarizes their characteristics. Among the 1,775 examined drugs, 83% had no equivalent generics, 41% were used in internal medicine, and 43% were injectable. We sorted them into seven therapeutic categories: agents affecting the nervous system and sensory organs (n = 298), organ-specific agents (n = 539), agents affecting metabolism (n = 416), agents affecting cell function (n = 194), agents treating pathologic microorganisms and parasites (n = 179), agents with non-therapeutic uses (n = 113), and narcotics (n = 36). Among sampled drugs, 54% had no new drug in their therapeutic category, and 10% appeared in the Japanese Pharmacopoeia.
Univariate analysis
The mean percentage divergence is 7.15%. shows the mean percentage divergence of each drug categorized according to the Japan Standard Commodity Classification. In univariate analysis, available generics and mention in the Japanese Pharmacopoeia correlate positively and significantly with percentage divergence (p < 0.0001). Manner of administration (p = 0.0001), therapeutic category (p = 0.0001), and number of new drugs in the same therapeutic category (p = 0.0001) correlate significantly with percentage divergence ().
Table 3. Mean percentage divergence of each drug category.
Table 4. Results of the univariate analysis: association between percentage divergence and covariates.
Multivariate regression analysis
displays regression results. Availability of generics, manner of administration, therapeutic category, number of new drugs in the same therapeutic category, and mention in the Japanese Pharmacopoeia correlate significantly with percentage divergence. Percentage divergences are higher for drugs with generics (p < 0.001) or drugs mentioned in the Japanese Pharmacopoeia (p < 0.001). Injectables correlate significantly and negatively with percentage divergence (p = 0.009) compared to ingested drugs. Percentage divergence is significant (p < 0.001) for drugs with two or more new drugs in their therapeutic category. Regarding therapeutic categories, the organ-specific drugs (p < 0.001), agents that affect metabolism (p = 0.001), and that serve non-therapeutic purposes (p < 0.001) exhibit significantly higher percentage divergence than agents affecting the nervous system and sensory organs. In total, agents for pathologic microorganisms and parasites (p = 0.004) and narcotics (p < 0.001) exhibit lower divergences.
Table 5. Multivariate regression analysis.
Discussion
Factors that depress market prices are the availability of generics, appearance of new drugs in the same therapeutic category, mention in the Japanese Pharmacopoeia, drugs affecting individual organs and metabolism, and drugs with non-therapeutic purposes. Drugs associated with upwardly revised prices are injectable, treat pathologic microorganisms and parasites, or are narcotics.
Our results indicate that to remain competitive pharmaceutical companies and wholesalers must reduce prices for drugs with generic substitutes and drugs that share therapeutic categories with two or more new drugs. Also, mention in the Japanese Pharmacopoeia indicates a drug is used widely. Our results coincide with findings by Takayama and NarukawaCitation5 and suggest that injectables are more susceptible to price increases than ingested medicines.
Drugs that treat pathologic microorganisms and parasites might be more susceptible to price increases because fewer companies are developing them and escalating microbial resistance quickly renders them ineffective. It is argued that incentives are needed to encourage their developmentCitation12.
Large percentage divergences between market prices and reimbursements induce the government to reduce prices and reimbursements. Starting in 2021, moreover, the Japanese government will begin revising DPS price lists yearly. To maintain prices, pharmaceutical companies must sidestep the competitive market by developing drugs that resist large discounts in price. Meekings et al.Citation13 suggest that targeting orphan drugs is a component of successful biopharma R&D. As an alternative, companies should develop drugs that meet PMP criteria, such as those that command premiums for innovation or usefulness.
Among our study’s limitations, we assumed a percentage divergence of 1% when prior and revised DPS prices are identical. This assumption has little impact on our conclusions because percentage divergences ranged from 0–2%. We excluded drugs subject to alternate pricing rules, as they might exhibit differing percentage divergences. Finally, our basis for comparison is 2018 DPS prices. Future studies should explore longer-run trends in market prices.
Basing biennial price changes of DPS-listed drugs on market prices reflects a drug’s value. The pharmaceutical industry could maintain reimbursements by developing drugs that treat unmet needs. Government could incentivize pharmaceutical investment through price maintenance policies. As government’s drug expenditures are rising, improved pricing policy and closer relationships among market participants are needed.
Conclusion
Our results indicate that factors increasing market competitiveness will likely reduce market prices of drugs, and that the market price of a drug is affected by medical conditions it treats. It requires a strategy for the pharmaceutical company to maintain the value of drugs and for government to set reimbursements for sustainable NIH.
Transparency
Declaration of funding
No funding was received to produce this article.
Declaration of financial/other relationships
No potential conflict of interest was reported by the author.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
None reported.
Data availability statement
All necessary data used for this study are included in the manuscript.
References
- Japanese Ministry of Health Labour, and Welfare. An outline of the Japanese Medical System. [cited 2019 Aug 24]. Available from: https://www.mhlw.go.jp/english/policy/health-medical/health-insurance/index.html
- Japanese Ministry of Health Labour, and Welfare. About rule of NHI price calculation method in FY 2018 [in Japanese]. 2018.
- PhRMA and EFPIA. Consequences expected from the final drug pricing reform package. [cited 2019 Aug 24]. Available from: http://www.phrma-jp.org/wordpress/wp-content/uploads/2017/12/phrma_efpia_pressrelease20171220_e.pdf
- FPMAJ. About the drug pricing reform [in Japanese]. [cited 2019 Aug 24]. Available from: http://www.fpmaj.gr.jp/documents/2017.12.20.seimei.pdf
- Takayama A, Narukawa M. Investigation of factors affecting the degree of price gap between the national health insurance reimbursement price and the actual market price of new drugs. Regul Sci Med Prod. 2016;63:295–305.
- Japanese Ministry of Health Labour, and Welfare. Information about NHI price list and generics after April 2018. [in Japanese]. [cited 2018 Jul 7]. Available from: https://www.mhlw.go.jp/topics/2018/04/tp20180401-01.html
- Japanese Ministry of Health Labour, and Welfare. About Medical fee revision in FY 2018 [in Japanese]. [cited 2018 July 7]. Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000188411.html
- Japanese Ministry of Health Labour, and Welfare. Information about NHI price list and generics until March 31, 2018. [in Japanese]. [cited 2018 Jul 7]. Available from: https://www.mhlw.go.jp/topics/2016/04/tp20160401-01.html
- Drug Pricing Organization in Japan. Therapeutic category of drugs for selecting comparable drugs [in Japanese]. 8th ed. 2018 [cited 2018 Jul 7]. Available from: https://www.mhlw.go.jp/file/05-Shingikai-12404000-Hokenkyoku-Iryouka/0000206268.pdf
- Japan Pharmaceutical Association. New drug price listing [in Japanese]. [cited 2018 Jul 7]. Available from: https://www.nichiyaku.or.jp/drug-info/collection/index.html
- Japanese Ministry of Health Labour, and Welfare. The Japanese pharmacopoeia seventeenth edition. 2016. [cited 2018 Jul 7]. Available from: https://www.mhlw.go.jp/file/06-Seisakujouhou-11120000-Iyakushokuhinkyoku/JP17_REV_1.pdf
- Sciarretta K, Røttingen J-A, Opalska A, et al. Economic incentives for antibacterial drug development: literature review and considerations from the Transatlantic Task Force on Antimicrobial Resistance. Clin Infect Dis. 2016;63(11):1470–1474.
- Meekings KN, Williams CS, Arrowsmith JE. Orphan drug development: an economically viable strategy for biopharma R&D. Drug Discov Today. 2012;17(13–14):660–664.