Abstract
Aims
Present cost-effectiveness analysis of nivolumab monotherapy vs. commonly prescribed third-line (3 L+) treatment in small cell lung cancer (SCLC).
Materials and methods
A three health states partitioned survival model (progression-free, progressed disease, and death; US payer perspective) was developed. The systematic literature review identified no randomized controlled or single-arm trials with separate outcomes for 3 L + SCLC patients. Topotecan was chosen as a comparator because it is frequently prescribed in real-world practice for 3 L SCLC. Clinical inputs for topotecan were derived from the Flatiron database with inclusion/exclusion criteria matched to patients treated with 3 L + nivolumab in CheckMate 032. Intravenous (IV) and oral topotecan clinical efficacy were assumed equivalent. Base-case analysis used a 20-year lifetime horizon. An annual discount rate of 3.0% for costs and outcomes was applied. Uncertainty was assessed using sensitivity analyses adjusted for key parameters.
Results
Incremental cost per quality-adjusted life-year (QALY) gained with nivolumab was US$153,312 vs. IV topotecan and US$123,003 vs. oral topotecan, respectively. When results were disaggregated, nivolumab-related costs were mainly driven by drug acquisition costs, and topotecan-related costs were primarily due to adverse event treatment. Mean overall survival (OS) was 21.69 months with nivolumab and 5.80 months with IV or oral topotecan. More favorable outcomes were found by the landmark response analyses. Deterministic sensitivity analyses showed that changes to the discount rate for costs and outcomes and body weight had the greatest impacts on results.
Limitations
Included use of real-world data for OS outcomes associated with 3 L topotecan, use of second-line topotecan data for progression-free survival, and no indirect costs.
Conclusions
Based on the literature on willingness-to-pay for a QALY in metastatic cancer, nivolumab monotherapy might represent a cost-effective option for 3 L + treatment of SCLC compared with IV and oral topotecan. Sensitivity analysis using response-based methods yielded further favorable cost-effectiveness estimates.
Introduction
Lung cancer remains the most common cancer worldwide; it is also the leading cause of cancer-related deaths, accounting for 18.4% of such deaths in 2018 [Citation1]. Although small cell lung cancer (SCLC) represents only 15% of all lung cancers [Citation2], with the majority being associated with tobacco exposure [Citation3], it is extremely aggressive and is usually widely metastatic at the time of diagnosis [Citation3]. Unfortunately, the recurrence rate of SCLC is high, with a 5-year survival of 25% or lower [Citation4–6]. There are also limited treatment options for patients with recurrent disease.
Nivolumab is a fully human immunoglobulin G4 programmed death-1 immune checkpoint inhibitor antibody that was the only US Food & Drug Administration (FDA)–approved agent for the third-line (3 L+) treatment of metastatic SCLC at the time of this study initiation [Citation7]. The efficacy of nivolumab for the treatment of recurrent SCLC was demonstrated in the phase 1/2, multicenter, open-label CheckMate 032 trial (NCT01928394) [Citation8]. Nivolumab monotherapy (3 mg/kg administered by infusion every 2 weeks until disease progression or unacceptable toxicity) was effective in the 3 L + population (i.e. 109 patients with limited- or extensive-stage SCLC after two or more chemotherapy regimens) in the CheckMate 032 trial [Citation9]. At a median follow-up of 28.3 months (from the first dose to database lock), the blinded independent central reviewer–the confirmed response rate was 11.9% (95% confidence interval [CI] = 6.5–19.5) with a median duration of response of 17.9 months (range 3.0–42.1). At 6 months, 17.2% of patients were progression-free. The 12- and 18-month overall survival (OS) rates were 28.3% and 20.0%, respectively. The safety profile of nivolumab monotherapy in CheckMate 032 was consistent with the established safety profile of nivolumab across multiple indications; 11.9% of patients had grade 3/4 treatment-related adverse events (AEs) and 2.8% of patients discontinued because of treatment-related AEs [Citation9].
Data for other (unapproved) 3 L + treatments are limited. Until recently [Citation10], the National Comprehensive Cancer Network guidelines lacked specific recommendations for the 3 L + treatment of SCLC [Citation11]. Published data based on retrospective series are mostly from single institutions or single-arm analyses. Available cytotoxic therapies provide minimal efficacy with a low overall response rate and short duration of progression-free survival (PFS) or OS benefit in the range of weeks.
The aim of this study was to explore different methodologies to enhance the rigor of a cost-effectiveness analysis of nivolumab monotherapy vs. commonly prescribed 3 L + treatment in patients with SCLC
Methods
Cost-effectiveness analysis for nivolumab monotherapy as 3 L + treatment in SCLC
Model structure
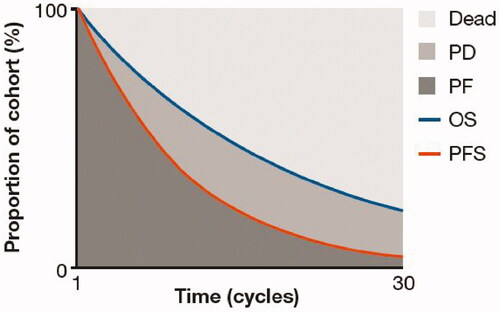
In line with previous economic evaluations and technology appraisals of treatments for cancer, including advanced SCLC [Citation12–14], a partitioned survival model was developed to evaluate the incremental cost-effectiveness of nivolumab monotherapy vs. routine treatment options in patients receiving 3 L + therapy for SCLC. The model consisted of three mutually exclusive health states: progression-free (PF), progressed disease (PD), and death (). All patients entered the model in the PF health state and transitioned through the model in weekly cycles. Patients could move to the PD or death health states using the definition of disease progression in the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [Citation15], but could not return to the PF state. Health state occupancy is estimated from the area under the PFS and OS curves. The model was developed using a maximum lifetime horizon of 20 years. The base-case analysis adopted a US payer perspective with a full reimbursement, and an annual discount rate of 3.0% for costs and outcomes was applied. The costs and effectiveness of treatments were calculated by combining the estimated time spent in the PF and PD states with the costs and health utilities assigned to those states. The primary outcomes of the analysis were incremental cost per life-year gained (LYG) and incremental cost per quality-adjusted life-year (QALY) gained. Survival models were fitted using the flexsurv package in R and modeled using the flexsurvreg function [Citation16].
Model inputs
Clinical data for nivolumab monotherapy as 3 L + therapy in patients (n = 109) with limited- or extensive-stage SCLC were derived from the single-armed CheckMate 032 trial, which was initiated in 2013 (January 2018 database lock) [Citation17]. Overall, 71.6% of patients received two prior systemic treatment regimens, 22.9% received three regimens, and 5.5% received four or more regimens. Prior platinum-based therapies included carboplatin (67.0%) and cisplatin (56.9%). Most patients (65.1%) had platinum-sensitive SCLC [Citation9].
No other agents were approved for the 3 L + treatment of SCLC in the United States at the time of this analysis. A systematic literature review (SLR) identified no randomized controlled or single-arm trials that reported outcomes separately for 3 L + patients for any treatment. However, an analysis of real-world data from the Flatiron health electronic records database (Flatiron) identified patients with SCLC who were receiving 3 L treatment between January 2011 and September 2017; these patients have been described previously [Citation18,Citation19]. Topotecan was the predominant 3 L treatment in Flatiron. Other 3 L treatments included single-agent chemotherapy (e.g. paclitaxel, gemcitabine, irinotecan, docetaxel), carboplatin-based doublets (e.g. carboplatin + etoposide, carboplatin + paclitaxel, carboplatin + irinotecan), as well as triplet combination chemotherapy regimens (e.g. cyclophosphamide + doxorubicin + vincristine, etoposide + paclitaxel + topotecan). Oral and intravenous (IV) topotecan were therefore selected as the comparators for this analysis, with OS data from Flatiron used to derive clinical inputs for these arms. The incidence of grade ≥3 treatment-emergent AEs occurring in ≥1% of patients in CheckMate 032 [Citation17] and in ≥5% of patients in a phase 3 trial of topotecan [Citation20] was also included in the analysis (Supplementary Table S1).
US-specific unit costs (in 2018 US$; i.e. disease management; drug acquisition, administration, and monitoring costs; subsequent treatments; and cost per treatment-related AE) used in the analysis are summarized in Supplementary Table S2 and Supplementary Table S3. Disease-management costs represented the resource used to provide care to patients with SCLC regardless of treatment [Citation21]; these amounted to US$670.47 (PF health state) and US$1937.53 (PD health state) per 4 weeks, respectively. An end-of-life/terminal care cost of US$11,387 was included and applied as a one-off fixed cost to patients entering the death health state [Citation22]. Following failure with 3 L nivolumab monotherapy or comparator therapy, a proportion of patients (26.61% in each arm based on CheckMate 032 [Citation17]) was assumed to go on to subsequent treatment consisting of systemic anti-cancer therapies in the fourth line for the calculation of subsequent treatment costs. The average time on subsequent treatment was assumed to be 4.9 months in all patients; this treatment duration was taken from the Flatiron real-world data [Citation19]. Cost and disutilities of AEs were applied as a one-off cost/decrement in the first weekly cycle. That is, the total treatment cost and disutility per episode of each AE were multiplied by the proportion of each AE listed in Supplementary Table S1 and included in week 1. It was assumed that both treatment cost per episode and disutility per episode accounted for the duration of the specific AE.
Utility values were derived from patient-level EuroQoL-5D questionnaire data from CheckMate 032 [Citation17]. The mean (standard error) [95% CI] was 0.791 (0.019) [0.753–0.830] for the PF health state and 0.786 (0.036) [0.702–0.870] for the PD health state, respectively. Death was assumed to have a utility value of zero.
Model transitions
To estimate transitions between health states in the partitioned survival model, efficacy outcomes for the nivolumab arm were extrapolated over a lifetime horizon using parametric curves fitted to OS and PFS data from the CheckMate 032 trial [Citation17]. The process for fitting parametric curves to patient-level data was based on guidance from the National Institute for Health and Care Excellence (NICE) [Citation23] and is detailed in the Supplementary Methods and Supplementary Figure S1.
Survival analysis for nivolumab monotherapy
To estimate cumulative PFS and OS over the 20-year time horizon, standard and spline-based parametric survival curves were fitted to patient-level data from CheckMate 032 [Citation17]. These curves were used to extrapolate survival beyond the trial time horizon, as CheckMate 032 had a median follow-up of 28.3 months [Citation8]. The survival analysis was conducted in line with published guidance from the NICE decision support unit.
Best-fitting survival models were identified by visual inspection of the plots and by application of Akaike information criterion (AIC)/Bayesian information criterion (BIC) statistics, which provide an indicator of goodness-of-fit [Citation23]. As well as assessing the ability of each parametric distribution to fit the observed trial data, it is important to validate the long-term survival estimates predicted by the extrapolated part of the curve. Mean and median survival estimated by each distribution were also assessed.
The generalized gamma curve was chosen from the best-fitting curves to model OS for nivolumab monotherapy in the base-case analysis as it provided the best fit according to the validation criteria, which included goodness of fit to the observed data measured by AIC and BIC (Supplementary Table S4), clinical validity (OS and PFS curves did not cross), and validation of the extrapolation using external data sources. CheckMate 003 provided survival data for squamous NSCLC patients who received nivolumab up to 5 years [Citation24]. In the absence of long-term survival for SCLC patients, the conditional survival estimates from CheckMate 003 were used to validate the extrapolation for SCLC patients up to 5 years based on clinician recommendations of clinical similarities between the squamous NSCLC and SCLC patient groups. The generalized gamma curve is shown in Supplementary Figure S2. At 5 years, generalized gamma estimated a 9% OS rate relative to the 11% conditional OS rate for CheckMate 003, which suggests that 5-year survival estimated by the generalized gamma curve is a conservative prediction compared with estimates for squamous NSCLC patients in CheckMate 003. The 2-knot odds spline fitted curve was chosen as the base-case distribution to model PFS as it provided a good fit to both PFS and duration of treatment data. This allowed for the distributions to be kept consistent between the two endpoints. The validation criteria are summarized in Supplementary Table S5.
Survival analysis adjusting for IV and oral topotecan
CheckMate 032 does not include a comparator arm of standard chemotherapy and the SLR identified zero trials suitable for a network meta-analysis. We, therefore, used the individual patient-level data from CheckMate 032 and real-world electronic patient records from a US database to estimate relative efficacy adjusting for differences between patient characteristics.
A population was derived from the Flatiron SCLC cohort (n = 218) that matched the inclusion/exclusion criteria of CheckMate 032. This population included patients receiving 3 L therapy with no immuno-oncology agents in any line (n = 78) as patients with prior treatment with immuno-oncology agents were not permitted in the CheckMate 032 trial [Citation18]. Parametric curves were fitted to an OS Kaplan–Meier curve using Flatiron real-world data [Citation19], and extrapolated over a lifetime horizon [Citation23]. The Kaplan–Meier curve was digitized and individual patient data were generated using the Guyot method [Citation25]. A number of parametric curves were fitted to the patient data using the flexsurv package in R. The exponential function was chosen to model OS for topotecan as it provided the best fit to the data according to AIC and BIC statistics (Supplementary Table S6).
As well as fitting parametric curves directly to the Flatiron data, an indirect treatment comparison analysis using a matching-adjusted indirect comparisons (MAIC) approach was conducted to assess the comparative effectiveness of nivolumab vs. the treatments that make up standard-of-care (SOC) in the Flatiron cohort [Citation18]. Three different methods were used to derive hazard ratios for nivolumab vs. SOC, adjusted to account for population differences (regression, inverse probability weighting and doubly robust). The naïve, unadjusted comparison showed nivolumab monotherapy to be more efficacious compared with SOC (hazard ratio [HR] = 0.63 [95% CI = 0.44–0.90]).
Results from the inverse probability weighting (IPW) and doubly robust methods (DRM) analyses were comparable and increased the HR estimates slightly, but both methods still showed nivolumab to be superior. The IPW analysis produced an adjusted HR of 0.70 (95% CI = 0.54–0.89) and the DRM analysis produced an adjusted HR of 0.68 (95% CI = 0.52–0.90). These HRs were applied to the nivolumab monotherapy arm as sensitivity analyses. The use of these HRs was not determined to be appropriate for the base case as visual inspection of the Flatiron and nivolumab monotherapy OS curves indicated that the proportional hazards assumption was violated. Applying an HR to the nivolumab monotherapy arm provides a clinically unrealistic OS curve for topotecan with a long tail and a small percentage of patients alive at 5 and even 10 years. This is not consistent with the OS curves observed in Flatiron or clinical trials for topotecan for 2 L SCLC such as the ACT-1 trial where all patients had died before 3 years [Citation26]. This analysis includes absolute adjustment from the MAIC, but assumes that the proportional hazards assumption holds between nivolumab and topotecan, which creates a very strong survival plateau for the topotecan patients. As there is unlikely to be a survival plateau for SCLC patients who receive topotecan in clinical practice, this analysis is conservative.
PFS data were not available for patients who received 3 L topotecan from Flatiron. Instead, the HR between the OS and PFS arms from the ACT-1 trial for topotecan in second-line (2 L) SCLC [Citation26] was used to adjust the OS curve from the Flatiron data to model PFS for topotecan. ACT-1 was a phase 3 trial in which 637 patients with SCLC were randomized to amrubicin 40 mg/m2 IV on days 1–3 or topotecan 1.5 mg/m2 IV on days 1–5 as 2 L treatment. The median OS (primary endpoint) was 7.8 months with topotecan and the median PFS was 3.5 months. OS and PFS Kaplan–Meier curves from the ACT-1 clinical trial for topotecan for 2 L patients [Citation26] were digitized to reconstruct the patient-level data (Supplementary Figure S2). A cox proportional hazards model was used to derive an HR for OS vs. PFS (HR = 2.9).
Sensitivity analyses
Landmark response analysis
The landmark response analysis is an alternative approach to the standard partitioned survival model that allows for the modeling of long-term survival by response status [Citation27,Citation28]. A landmark response analysis was conducted for the nivolumab monotherapy data from CheckMate 032 [Citation17]. Response-based analyses previously conducted for nivolumab in NSCLC demonstrated that a landmark time point of 6 months was appropriate, as it allowed enough time for most responses to occur, while still providing sufficient follow-up beyond the landmark point to model survival [Citation29]. A shorter time period of 4 months was deemed clinically appropriate for the landmark time point in the SCLC model due to the more aggressive nature of this disease.
Using CheckMate 032 data, a number of standard parametric models were fitted to the survival curves for complete responders, partial responders, PD, and stable disease; these categories were based on RECIST 1.1 criteria [Citation15]. The exponential distribution provided the overall best fit as it was the best fitting model for the PD group and the second-best fitting for the complete/partial responders and stable disease groups according to AIC/BIC criteria. The generalized gamma curve was used to model OS for the entire cohort for the first 4 months to maintain consistency with the partitioned survival model.
Data on response status was not available from Flatiron, so it was not possible to model survival by response status for IV and oral topotecan. For the structural sensitivity analysis, the landmark response analysis was used to model costs and outcomes in the nivolumab arm whilst the partitioned survival model from the base case analysis was used to model costs and outcomes from the IV and oral topotecan arm.
The OS curves are shown in Supplementary Figure S3. These results were similar to the OS extrapolation in the partitioned survival model (see Supplementary Figure S4), thus providing good validation for the base case model.
Treatment stopping rules
Scenario analyses were conducted to explore the impact of including different treatment stopping rules. Although the protocol for CheckMate 032 specified that patients should receive treatment until progression, or toxicity [Citation17], other clinical trials for nivolumab and other immuno-oncology agents have specified or evaluated up to maximum treatment duration. For example, CheckMate 003 implemented a 96-week treatment-stopping rule [Citation24]. This approach has been accepted in health technology appraisals for NSCLC in the UK [Citation30–36]. For this scenario analysis, treatment costs (i.e. drug acquisition, administration, and monitoring costs) were included up to a time point (1, 2, or 5 years). Beyond this time point, zero cost was assumed.
Deterministic and probabilistic sensitivity analyses
A deterministic sensitivity analysis (DSA) and probabilistic sensitivity analysis (PSA) were conducted to assess the impact of uncertainty in model inputs on the outcomes for the base-case analysis of nivolumab monotherapy vs. IV and oral topotecan. One-way DSA was undertaken by varying key parameters by their standard error, 95% CI or ±20% of the expected values (base case) based on data availability. The key parameters included in the DSA were: discount rate (varied from 0% to 6%); body weight and body surface area (varied by ±20%); costs (disease management, drug acquisition, drug administration, and drug monitoring, varied by ±20%); and utilities (PF and PD health states, varied by 95% CI). Key parameters included in the PSA were clinical data (survival distribution parameters for PFS, OS, and duration of treatment [sampled by the multivariate normal distribution, with the correlation between shape and scale parameter]); costs (disease management, administration, monitoring, and other costs [sampled by gamma distribution]); and utilities (utility weights assigned to PF and PD heath states, and AE disutilities [sampled by beta and gamma distribution]).
Results
Cost-effectiveness analysis for nivolumab monotherapy as 3L treatment in SCLC
The incremental cost per QALY gained with nivolumab monotherapy was US$153,312 vs. IV topotecan and US$123,003 vs. oral topotecan (). Nivolumab monotherapy had a larger QALY gain than IV and oral topotecan (1.23 vs. 0.37) (). Nivolumab monotherapy was also associated with more total life-years gained than IV or oral topotecan (1.56 vs. 0.48). The mean OS was 21.69 months with nivolumab monotherapy and 5.80 months with IV or oral topotecan. The mean PFS was 12.58 months with nivolumab monotherapy and 1.94 months with IV or oral topotecan. Nivolumab monotherapy had a higher total cost (US$187,728) compared with IV topotecan (US$55,329) and oral topotecan (US$81,565), which was primarily due to the drug acquisition costs. The cost of treating adverse events was a key driver for the total costs associated with topotecan IV and topotecan oral ().
Table 1. Base-case incremental results of nivolumab monotherapy vs. topotecan.
Table 2. Total and disaggregated QALYs by treatment.
Table 3. Total and disaggregated costs by treatment.
Alternative survival analysis adjusting for single-arm related issues
The impact of applying the HRs is shown in . All scenarios shown resulted in higher ICERs than in the base case analysis. This is due to the assumption that the OS curves for nivolumab monotherapy and topotecan would have the same shape leading to long tails and increased OS estimates for the topotecan arms. When applying the IPW HR of 0.70, the model estimates 6% of patients are still alive at 3 years, 3% at 5 years, and 1% at 10 years. This is a very optimistic survival profile for topotecan patients when compared to the overall survival curves from Flatiron and from 2 L SCLC trials such as ACT-1, where all patients had died by 3 years [Citation26].
Table 4. The impact of applying OS HRs for nivolumab monotherapy vs. topotecan.
Landmark response analysis
The OS generated by the landmark response analysis was very similar to the OS estimated by the generalized gamma curve in the partitioned survival model. However, the PFS estimated using the landmark response analysis produced more pessimistic extrapolations compared to the partitioned survival model, resulting in lower QALYs. The incremental cost per QALY gained with nivolumab monotherapy was US$111,054 vs. IV topotecan and US$73,110 vs. oral topotecan ().
Table 5. Landmark response analysis results for nivolumab monotherapy vs. topotecan.
Treatment stopping rules
The impact of treatment stopping rules on the cost-effectiveness analysis results is shown in . If treatment was stopped at 2 years, the incremental cost per QALY gained with nivolumab monotherapy was US$54,129 vs. IV topotecan and US$23,762 vs. oral topotecan, and the incremental cost per LYG with nivolumab monotherapy was US$42,980 vs. IV topotecan and US$18,857 vs. oral topotecan.
Table 6. The impact of treatment stopping rules for nivolumab monotherapy vs. topotecan.
Deterministic and probabilistic sensitivity analyses
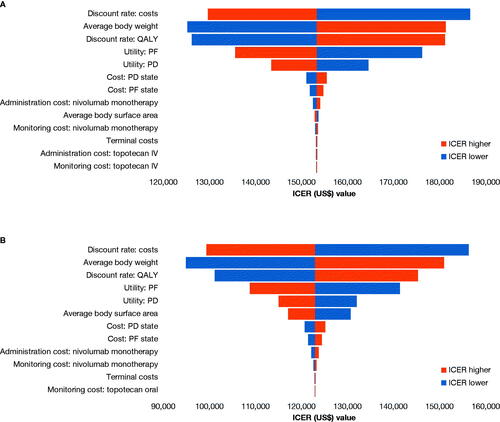
Tornado diagrams showing the DSA results for IV and oral topotecan are presented in . The discount rate for costs and outcomes and body weight had the largest impact on the results for both IV and oral topotecan.
Figure 2. Tornado diagram for the deterministic sensitivity analysis results for nivolumab monotherapy vs. IV (a) and oral (b) topotecan. Abbreviations. ICER, incremental cost-effectiveness ratio; IV, intravenous; PD, progressed disease; PF, progression-free; QALY, quality-adjusted life-year.
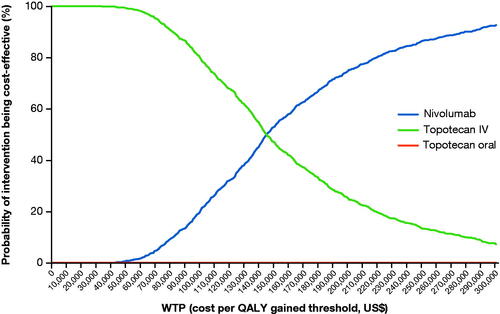
A total of 1000 probabilistic iterations were performed (Supplementary Figure S5) and the resultant cost-effectiveness acceptability curve (CEAC) is shown in . The multi-way CEAC curve showed that nivolumab monotherapy had a 22%, 74%, and 86% probability of being cost-effective vs. IV topotecan and oral topotecan at a willingness-to-pay (WTP) threshold of US$100,000, US$200,000, and US$250,000 per QALY gained, respectively.
Discussion
The cost-effectiveness of nivolumab monotherapy as a 3 L + treatment for SCLC was determined in a three-health state, partitioned survival model from a US perspective. The analysis demonstrated that the incremental cost per QALY gained was US$153,312 vs. IV topotecan and US$123,003 vs. oral topotecan, and the incremental cost per LYG was US$121,736 vs. IV topotecan and US$97,613 vs. oral topotecan. The DSA indicated that the analysis was sensitive to the discount rates applied and the dose/cost was sensitive to changes in patient body weight. The PSA results demonstrated that nivolumab was cost-effective (vs. IV topotecan) across a range of WTP thresholds. In addition, scenario analyses that explored the impact of different treatment stopping rules indicated that the incremental cost per QALY gained and incremental cost per LYG may increase with a longer treatment duration with nivolumab monotherapy.
Based on a targeted review of the literature [Citation37–40], it is anticipated that the WTP threshold for a QALY gained is at least US$250,000 in the United States, potentially with an even higher threshold of US$300,000 for interventions within end-of-life settings such as SCLC. Nivolumab monotherapy would, therefore, be considered a cost-effective 3 L + treatment option for SCLC compared with IV and oral topotecan. If treatment was stopped at 2 years, the incremental cost per QALY gained with nivolumab monotherapy would decrease to US$54,129 vs. IV topotecan and US$23,762 vs. oral topotecan, respectively.
From a clinical perspective, it is important to note that these results need to be considered in relation to the high unmet medical need for improved survival within SCLC and its significant health burden. In contrast with other cancers, survival in lung cancer has not improved in recent years, and the long-term prognosis of SCLC remains poor [Citation4–6,Citation41].
Partitioned survival models are a well-established approach in economic evaluations of immuno-oncology agents, and the base case used in this paper was validated by a number of sensitivity analyses. An earlier landmark response analysis of nivolumab data from CheckMate 032 (November 2017 database lock) showed that response at the 4-month landmark time point was associated with significantly longer OS [Citation27]. The findings at this time point were also consistent with sensitivity analyses with alternative 2- and 6-month landmarks. Although the sample size was limited, this earlier model supported the appropriateness of the landmark response analysis and 4-month time point in this setting.
However, there are some limitations associated with this cost-effectiveness analysis. The analysis aimed to compare nivolumab with the most commonly used 3 L treatments in SCLC. Topotecan was chosen as it was most frequently prescribed in Flatiron. The survival outcomes estimated in the model were not taken from a randomized head-to-head trial and unadjusted real-world data from a registry were used to the model OS for IV and oral topotecan. In the absence of data for PFS, the analysis used an HR between OS and PFS from another clinical trial to adjust the OS arm for IV and oral topotecan. This requires the assumption that the relationship between OS and PFS for IV and oral topotecan is proportional and remains constant across populations. The absence of response data from Flatiron to inform the landmark response analysis was a limitation of the structural sensitivity analysis. However, as the OS data from Flatiron are very mature, there is little uncertainty around the long-term survival outcomes for IV and oral topotecan predicted by the partitioned survival model. It is also well established that the durability of chemotherapy is very short. Thus, changes to the model structure for this arm are likely to have minimal impact on the results. Another limitation of the landmark response analysis is that it requires splitting the cohort into smaller samples to analyze and extrapolate survival. For these reasons, the landmark analysis was not deemed appropriate as a structure for use in the base case analysis. Furthermore, the model did not account for indirect costs such as hospitalizations, emergency room visits, and outpatient care, which have been identified as significant costs associated with managing SCLC patients [Citation42]. The model did not look at the cost impact of SCLC on caregivers or lost productivity due to premature death. In addition, with two programmed death-ligand 1 (PD-L1) inhibitors now showing positive trial results when added into first-line chemotherapy for SCLC and knowing that the data from Flatiron and the CheckMate 032 trial were derived from patients who were PD-L1 inhibitor–naïve, the relevance of our analyses to patient populations in countries where first- and second-line programmed death-1 or PD-L1 inhibitor use becomes commonplace will be unknown.
The results of the CheckMate 032 trial for patients receiving 3 L + treatment for SCLC presented a compelling case that nivolumab represents a “step change” in disease management where few effective treatment options have been available [Citation17], and led to an accelerated FDA approval in 2018. However, subsequent phase 3 confirmatory studies in different SCLC treatment settings, CheckMate 451 and CheckMate 331, did not meet their primary endpoints of OS. As a result, it was decided to voluntarily withdraw this indication in the US at the end of 2020. This action was taken in accordance with the FDA’s standard procedures for evaluating accelerated approvals that have not met their post-marketing requirements. In the absence of any approved 3L treatment, nivolumab remains as a category 3 A recommendation in the National Comprehensive Cancer Network guidelines. However, the SCLC treatment landscape has evolved; two PD-L1 inhibitors, durvalumab and atezolizumab, have both shown benefits when added into first-line platinum-etoposide chemotherapy for advanced SCLC [Citation14,Citation43]. Therefore, in the future, it is less likely that patients with SCLC will be immuno-oncology-naïve at 3L treatment like the patients enrolled in CheckMate 032, making the setting described here less relevant. Nevertheless, these analyses provide a useful example of how a robust cost-effectiveness analysis can still be constructed from a single-armed trial combined with auxiliary data on patients treated with competitor therapies using advanced statistical methods.
Conclusions
Within this challenging disease landscape, nivolumab monotherapy had the potential to bring significant health benefits to PD-L1 inhibitor–naïve patients with 3 L + SCLC in comparison with existing treatment options. The findings from this analysis indicate that nivolumab monotherapy would be a cost-effective option for 3 L + treatment of SCLC compared with IV and oral topotecan at a WTP threshold of US$250,000 based on varying methodologies and model assumptions
Transparency
Declaration of funding
This analysis was funded by Bristol Myers Squibb.
Declaration of financial/other interests
DRC declares no conflicts of interest. AJG, PA, JRP, and YY are employees of Bristol Myers Squibb. KD is a former employee of Parexel. CS is a former employee of Parexel. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors contributed to the study concept and design, and data analysis and interpretation. CS and KD were contracted by Bristol Myers Squibb to develop the economic model. All authors contributed to manuscript preparation and approved the final version.
Supplemental Material
Download EPS Image (1.5 MB)Supplemental Material
Download EPS Image (1.5 MB)Supplemental Material
Download EPS Image (1.5 MB)Supplemental Material
Download EPS Image (1.5 MB)Supplemental Material
Download MS Word (717.7 KB)Acknowledgements
The authors would like to thank Nancy Schoenherr (former employee of Bristol Myers Squibb) for analysis and input. Writing support was provided by Parexel and was funded by Bristol Myers Squibb.
Data availability statement
BMS policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
References
- GLOBOCAN. New global cancer data: GLOBOCAN 2018; 2018. [updated 12 Sept 2018; cited 11 August 2020]; Available from: https://www.uicc.org/new-global-cancer-data-globocan-2018
- Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer. 2017;17(12):725–737.
- Lally BE, Urbanic JJ, Blackstock AW, et al. Small cell lung cancer: have we made any progress over the last 25 years? Oncologist. 2007;12(9):1096–1104.
- Amini A, Byers LA, Welsh JW, et al. Progress in the management of limited-stage small cell lung cancer. Cancer. 2014;120(6):790–798.
- Chute JP, Chen T, Feigal E, et al. Twenty years of phase III trials for patients with extensive-stage small-cell lung cancer: perceptible progress. J Clin Oncol. 1999;17(6):1794–1801.
- Fruh M, De Ruysscher D, Popat S, et al. Small-cell lung cancer (SCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi99–105.
- US Food and Drug Administration. FDA grants nivolumab accelerated approval for third-line treatment of metastatic small cell lung cancer 2018. [updated August 16, 2018; cited 11 August 2020]. Available from: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm617370.htm
- Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(7):883–895.
- Ready N, Farago AF, de Braud F, et al. Third-line nivolumab monotherapy in recurrent SCLC: CheckMate 032. J Thorac Oncol. 2019;14(2):237–244.
- National Comprehensive Cancer Network (NCCN), Small cell lung cancer. Version 2; 2021.
- Kalemkerian GP, Loo BW, Akerley W, et al. NCCN guidelines insights: small cell lung cancer, version 2.2018. J Natl Compr Canc Netw. 2018;16(10):1171–1182.
- Smare C, Lakhdari K, Doan J, et al. Evaluating partitioned survival and Markov decision-analytic modeling approaches for use in cost-effectiveness analysis: estimating and comparing survival outcomes. Pharmacoeconomics. 2020;38(1):97–108.
- Bullement A, Cranmer HL, Shields GE. A review of recent decision-analytic models used to evaluate the economic value of cancer treatments. Appl Health Econ Health Policy. 2019;17(6):771–780.
- Zhang L, Hang Y, Liu M, et al. First-line durvalumab plus platinum-etoposide versus platinum-etoposide for extensive-stage small-cell lung cancer: a cost-effectiveness analysis. Front Oncol. 2020;10:602185.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247.
- Jackson CH. Flexsurv: a platform for parametric survival modeling in R. J Stat Softw. 2016;70:i08.
- CheckMate 032 clinical trial report (data on file). Princeton (NJ): Bristol Myers Squibb; 2018.
- Keeping ST, Cope S, Chan K, et al. Comparative effectiveness of nivolumab versus standard of care for third-line patients with small-cell lung cancer. J Comp Eff Res. 2020;9(18):1275–1284.
- Flatiron Heath, Flatiron health records (data on file). Princeton (NJ): Bristol Myers Squibb; 2018.
- Eckardt JR, von Pawel J, Pujol J-L, et al. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol. 2007;25(15):2086–2092.
- Centers for Medicare and Medicaid Services. 2018. CMS physician fee schedule 2018 [cited 11 August 2020]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/
- Bremner KE, Krahn MD, Warren JL, et al. An international comparison of costs of end-of-life care for advanced lung cancer patients using health administrative data. Palliat Med. 2015;29(10):918–928.
- Latimer NR. NICE DSU technical support document 14: survival analysis for economic evaluations alongside clinical trials – extrapolation with patient-level data. Report by the Decision Support Unit; 2013. [cited 2020 August 11]. Available from: http://nicedsu.org.uk/wp-content/uploads/2016/03/NICE-DSU-TSD-Survival-analysis.updated-March-2013.v2.pdf
- CheckMate 003 clinical trial report (data on file). Princeton (NJ): Bristol Myers Squibb; 2018.
- Guyot P, Ades AE, Ouwens MJNM, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9.
- von Pawel J, Jotte R, Spigel DR, et al. Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J Clin Oncol. 2014;32(35):4012–4019.
- Santi I, Smare C, Juarez-Garcia A, et al. The impact of landmark response on overall survival: implications for the economic evaluation of immune-therapy (I-O) treatment in small cell lung cancer (SCLC). Barcelona, Spain: PRM157 ISPOR Europe.
- Ouwens MJNM, Mukhopadhyay P, Zhang Y, et al. Estimating lifetime benefits associated with immuno-oncology therapies: challenges and approaches for overall survival extrapolations. Pharmacoeconomics. 2019;37(9):1129–1138.
- Santi I, Johal S, Yuan Y, et al. The impact of response at a landmark on overall survival: implications for the economic evaluation of the value of immune-oncology (I-O) treatment in non-small cell lung cancer (NSCLC). Value Health. 2018:21(suppl1):S211.
- National Institute for Health and Care Excellence. Nivolumab for previously treated squamous non-small-cell lung cancer. Technology appraisal guidance [TA483]; 2017. [cited 2020 August 11]. Available from: https://www.nice.org.uk/guidance/ta483/chapter/4-Committee-discussion
- National Institute for Health and Care Excellence. Pembrolizumab for treating PD-L1-positive non-small-cell lung cancer after chemotherapy. Technology approasial guidance [TA428]. 2017. [updated 2017 September 12; cited 2020 August 11]. Available from: https://www.nice.org.uk/guidance/ta428.
- National Institute for Health and Care Excellence. Atezolizumab in combination for treating metastatic non-squamous non-small-cell lung cancer. Technology appraisal guidance [TA584]. 2019. [updated 2019 5 June; cited March 2021]. Available from: https://www.nice.org.uk/guidance/ta584/chapter/1-Recommendations
- National Institute for Health and Care Excellence. Atezolizumab for treating locally advanced or metastatic non-small-cell lung cancer after chemotherapy. Technology appraisal guidance [TA520]. 2018. [updated 16 May 2018; cited March 2021]. Available from: https://www.nice.org.uk/guidance/ta520/chapter/1-Recommendations.
- National Institute for Health and Care Excellence. Pembrolizumab for untreated PD-L1-positive metastatic non-small-cell lung cancer. Technology appraisal guidance [TA531]. 2018. [updated 18 July 2018; cited March 2021]. Available from: https://www.nice.org.uk/guidance/ta531/chapter/1-Recommendation.
- National Institute for Health and Care Excellence. Pembrolizumab with carboplatin and paclitaxel for untreated metastatic squamous non-small-cell lung cancer. Technology appraisal guidance [TA600]. 2019. [updated 11 September 2019; cited March 2021]. Available from: https://www.nice.org.uk/guidance/ta600/chapter/1-Recommendations.
- National Institute for Health and Care Excellence. Pembrolizumab with pemetrexed and platinum chemotherapy for untreated, metastatic, non-squamous non-small-cell lung cancer. Technology appraisal guidance [TA683]. 2021. [updated 10 March 2021; cited March 2021]. Available from: https://www.nice.org.uk/guidance/ta683/chapter/1-Recommendations.
- Becker G, Murphy K, Philipson T. National Bureau of Economic Research Working Paper 13333: the value of life near its end and terminal care. 2007. [cited 11 August 2020]. Available from: http://www.nber.org/papers/w13333.pdf.
- Nadler E, Eckert B, Neumann PJ. Do oncologists believe new cancer drugs offer good value? Oncologist. 2006;11(2):90–95.
- Seabury SA, Goldman DP, Maclean JR, et al. Patients value metastatic cancer therapy more highly than is typically shown through traditional estimates. Health Aff. 2012;31(4):691–699.
- Young KC, Kelly AG, Holloway RG. Reading a cost-effectiveness or decision analysis study: five things to consider. Neurol Clin Pract. 2013;3(5):413–420.
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) cancer statistics review 1975-2012. [cited 2020 August 11]. Available from: https://seer.cancer.gov/archive/csr/1975_2012/results_merged/topic_historical_mort_trends.pdf
- Enstone A, Greaney M, Povsic M, et al. The economic burden of small cell lung cancer: a systematic review of the literature. Pharmacoecon Open. 2018;2(2):125–139.
- Horn L, Mansfield AS, Szczęsna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229.