Abstract
Aims
Obstructive hypertrophic cardiomyopathy (oHCM) is a disease of the cardiomyocyte in which dynamic left ventricular outflow track obstruction may lead to heart failure, valvular disease, and sudden death. Little is known about healthcare resource utilization (HRU) and costs associated with oHCM. This study investigated the clinical and economic burden of oHCM in patients with or without symptoms associated with oHCM.
Methods
We used the US IBM MarketScan Commercial and Medicare Supplemental database to identify patients with oHCM (January 2009–March 2019). Control patients without cardiomyopathy were matched to each patient with oHCM based on age, sex, region, and index year (3:1 ratio). One-year HRU and cost data were compared between all oHCM, symptomatic oHCM, and asymptomatic oHCM subgroups, and their respective controls.
Results
Among 11,401 eligible patients with oHCM (mean age 57 years, 42% female), 5,667 (50%) were symptomatic (23% chest pain, 57% dyspnea, 29% fatigue, 17% syncope). oHCM was associated with significant increases in all-cause hospitalizations, hospital days, outpatient visits, and total healthcare costs (mean ± standard deviation: $26,929 ± 77,720 vs. $6,808 ± 25,712, p<.001) compared with matched controls. These differences were driven mainly by the clinical and economic burden of symptomatic oHCM, which was associated with significant increases in 1-year hospitalization rates (38.0 vs. 6.9%), hospital days (3.7 ± 9.9 vs. 0.4 ± 3.0), and total healthcare costs ($43,586 ± 103,756 vs. $6,768 ± 27,618; all p<.001). Adjustment for comorbidities had minimal impact on these differences.
Limitations
The use of claims data relies on International Classification of Diseases (ICD-9 and ICD-10) diagnosis codes, which might be inaccurate. Only commercially insured patients were included.
Conclusion
In a real-world population, oHCM was associated with substantial increases in HRU and incremental costs of ∼$20,000/year when compared with matched controls—a difference that increased to ∼$35,000/year among symptomatic patients. Further studies are warranted to understand the potential impact of specific therapies on HRU and the economic burden of oHCM.
PLAIN LANGUAGE SUMMARY
Obstructive hypertrophic cardiomyopathy (oHCM) is a medical condition in which the heart muscle becomes abnormally thick and can cause partial blockage of blood flow out of the heart. Some patients experience symptoms (such as shortness of breath, chest pain, and fatigue) from this condition while others do not. Little is known about the healthcare resource utilization (HRU) and costs associated with oHCM, and if there are any differences between patients with oHCM who experience symptoms versus those who are asymptomatic. Therefore, we performed a study to investigate the clinical and economic burden of oHCM in patients with or without symptoms associated with oHCM. Based on insurance claims data, ∼50% of all patients with diagnosed oHCM are symptomatic. Symptomatic patients experience nearly 8 times as many hospitalizations and cost the healthcare system >$35,000 per year more than matched controls. In contrast, asymptomatic patients with oHCM have a much smaller difference in HRU and costs (∼$3,600/year) compared with matched controls. The results of this study suggest that effective therapies for oHCM may provide economic value, even if the impact of therapy is limited solely to the relief of symptoms.
Introduction
Hypertrophic cardiomyopathy (HCM) is a cardiac disorder affecting approximately 0.2–0.5% of the adult populationCitation1–6. Although HCM is often caused by genetic mutations that lead to cardiac sarcomere dysfunction, it is generally diagnosed based on echocardiography demonstrating increased left ventricular wall thickness that is not explained by abnormal loading conditions or infiltrative diseaseCitation2. Obstructive HCM (oHCM)—defined as HCM with left ventricular outflow tract obstruction causing a peak left ventricular outflow gradient of at least 30 mm Hg—is observed in approximately 70% of patients with HCM and frequently leads to symptoms including dyspnea, fatigue, chest pain, or syncopeCitation7–9. Current therapeutic options for patients with oHCM are limited, do not target the underlying pathophysiology, and generally lead to only partial symptom reliefCitation1,Citation2. However, several investigational compounds that target the underlying pathophysiology of HCM have been developed recently and are currently undergoing clinical investigation with a focus on exercise tolerance, symptoms, and disease-specific health statusCitation10–13.
In addition to studying the impact of these novel therapies on patient-centered outcomes, there is an interest in understanding their costs and potential economic impact. An important first step in this line of investigation is to understand the clinical and economic burden of oHCM on both patients and the healthcare system. Although there have been several small studies of healthcare resource utilization (HRU) and costs in patients with HCM, these have generally focused on individuals undergoing invasive therapies, and there have been no longitudinal studies among unselected patientsCitation14–16. Moreover, there have been no studies quantifying the clinical and economic burden of patients with obstructive HCM, or the subgroups of patients with asymptomatic or symptomatic oHCM. To address these gaps in knowledge, we used data from an insurance claims database to examine patterns of HRU and costs associated with oHCM in the United States and to determine whether these costs differ according to patients’ symptom burden.
Methods
Study design and data source
We performed a retrospective observational cohort study using private sector healthcare claims data from the US IBM MarketScan Commercial and Medicare Supplemental database (January 2009 to March 2019). This database includes medical and pharmacy claims of insured employees covered by the health benefits programs of larger employers and their dependents across the United States, along with Medicare-eligible retirees with employer-provided Medicare supplemental plansCitation17. Available data include demographics, comorbidities, medical claims, and costs (assessed from the payer’s perspective). MarketScan data are de-identified and comply with the Health Insurance Portability and Accountability Act. As the study did not involve the collection, use, or transmittal of individual identifiable data, institutional review board approval was not required.
Sample selection
Patients with HCM were identified according to the following criteria: 1) two or more medical claims with an International Classification of Diseases (ICD-9 or ICD-10) diagnosis code for HCM (425.1, I42.1, or I42.2) that were at least 30 days apart or 2) one HCM diagnosis claim and a claim for septal reduction therapy—a procedure that is used specifically to treat oHCM—on separate dates. For the purposes of this study, septal reduction therapy was defined as either alcohol septal ablation or septal myectomy (Current Procedural Terminology codes: 93583 and 33416, ICD-9-PCS: 35.42, 37.34, 37.33, ICD-10-PCS: 02BM3ZZ, 02TM3ZZ, 025M3ZZ, 02CM3ZZ, 02CM4ZZ, 02BM0ZZ, 025M0ZZ, 02TM0ZZ, 02CM0ZZ; Supplementary Table 1) that were on distinct dates. Obstructive HCM cases were further defined as any patients who met the above criteria while also having at least one medical claim with an ICD-9-CM or ICD-10-CM diagnosis code for oHCM (425.11 or I42.1) or a medical claim for septal reduction therapy at any time in the observed claims history. To avoid including patients with diseases that mimic HCM, patients were excluded if they had a claim with a diagnosis code for Fabry disease or amyloidosis at any time.
For each patient with oHCM, an index date was randomly selected between the time the individual met inclusion criteria and the end of data availability. Patients were included in the study only if they were at least 18 years of age on the index date and had at least 12 months of continuous eligibility after the index date. These patients were further subclassified as symptomatic if they had a medical claim for fatigue, chest pain, syncope, dyspnea, heart failure, pacemaker without bradyarrhythmia, or septal reduction therapy (see Supplementary Table 1 for codes) during the 1-year follow-up period after the index date. All other oHCM patients were classified as asymptomatic.
Potential control patients were identified if they did not have any claims for cardiomyopathy (including HCM), heart failure, amyloidosis, or septal reduction therapy during the study period. For each potential control patient, a random calendar date during their claims history was selected as the index date. Potential control patients were required to be at least 18 years old as of the index date and to have 12 months of continuous eligibility after the index date. From the population of potential control patients, we randomly selected three control patients for each oHCM patient using exact matching for age, sex, geographic region, and index year.
Patient characteristics and study outcomes
Patient characteristics and comorbidities, including all components of the Charlson Comorbidity IndexCitation18,Citation19, were assessed from the claims data during the year following the index date. Diagnosis codes used to identify clinical conditions are listed in Supplementary Table 1. Twelve-month HRU and healthcare costs were assessed for all patients based on the claims data. Specific categories of HRU included all-cause inpatient hospitalizations, outpatient visits (including physician office visits, laboratory tests, diagnostic imaging, and other outpatient services), emergency room visits, and prescription drugs. For each type of visit, the proportion of patients with any utilization and the number of visits were measured. In addition to assessing counts of resource utilization, medical care costs were tabulated using similar categories. Costs were based on reimbursements paid by health plans for services and did not include copays or other cost-sharing arrangements.
Statistical analysis
Continuous variables are described as mean ± standard deviation, while categorical variables are described as proportions. The primary analysis compared patients with oHCM with their matched controls, while secondary analyses compared symptomatic oHCM patients and asymptomatic oHCM patients with their respective matched controls. Generalized estimating equations were used for all comparisons and to estimate summary statisticsCitation20. Binary outcomes were compared using models with a binomial distribution and a logit link; count outcomes were compared using models with a negative binomial distribution and a log link; and cost outcomes were compared using models with a Tweedie distribution and a log link. Model results are summarized as odds ratios (ORs) for binary outcomes and incidence rate ratios (IRRs) for count outcomes. For healthcare costs, results are reported as absolute differences along with their corresponding 95% confidence intervals (CIs). Confidence intervals for the cost differences were estimated by bootstrapping because of the non-identity link function in the models.
Although the control population of patients without oHCM was selected at random, there were important differences in patient characteristics between the cases and their matched controls. We therefore performed adjusted comparisons to provide more conservative estimates of the association between oHCM and both resource utilization and costs. For these adjusted analyses, we used the same modeling approaches as in the unadjusted analyses but also included selected components of the Charlson Comorbidity Index as covariates (AIDS/HIV, any malignancy including leukemia and lymphoma, dementia, diabetes, hemiplegia or paraplegia, metastatic solid tumor, liver disease, peptic ulcer disease, peripheral vascular disease, renal disease, and rheumatologic disease). Of note, we did not adjust for components of the Charlson Comorbidity Index that could be sequelae of oHCM (i.e. congestive heart failure, cerebrovascular disease, or myocardial infarction). We also did not adjust for conditions with symptoms similar to HCM (i.e. chronic pulmonary disease), because their inclusion in the analysis could lead to ‘overadjustment’. These adjusted analyses were performed for the overall analytic cohort as well as for the subsets of symptomatic and asymptomatic patients with their corresponding controls.
Results
Patient population
Between January 2009 and March 2019, a total of 11,401 eligible patients with oHCM were identified in the IBM MarketScan data (). Of these, 5,667 (49.7%) were identified as symptomatic based on claims codes and matched to 17,001 controls; the remaining 5,734 patients (50.3%) were identified as asymptomatic and matched to 17,202 controls.
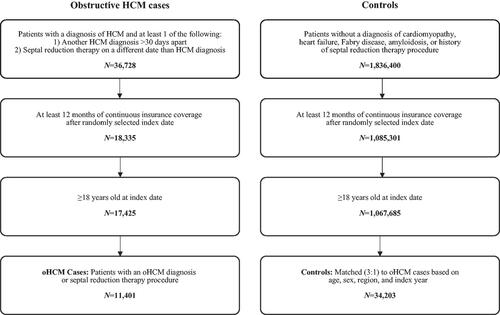
Figure 1. Study design and sample selection. The study population was drawn from the US IBM MarketScan Commercial and Medicare Supplemental Database from January 2009 to March 2019. Patients with HCM were identified based on a diagnostic code for HCM and confirmation by either a second HCM diagnostic code more than 30 days apart or a claim for septal reduction therapy on a separate date from the initial HCM diagnosis along with a randomly-selected index date after diagnostic confirmation. After excluding patients aged <18 years, those who did not have ≥12 months of continuous coverage after the index date, and those who did not have a diagnostic code for obstructive HCM, the analytic cohort comprised 11,401 patients with obstructive HCM. Potential controls without HCM were selected if they did not have a diagnosis of cardiomyopathy, heart failure, Fabry disease, amyloidosis, or a history of a septal reduction therapy procedure, and assigned a randomly selected index date. After excluding those without continuous insurance coverage for 12 months after the index date, control patients were matched to each oHCM patient in a 3:1 fashion based on age, sex, geographic region, and index year. Abbreviations. HCM, hypertrophic cardiomyopathy; oHCM, obstructive hypertrophic cardiomyopathy.
Demographic characteristics and comorbidities of all oHCM patients and their matched controls are summarized in . The mean age of the oHCM group and their matched controls was 57 ± 14 years, and 42% were female. Compared with their matched controls, patients with oHCM were more likely to have hypertension, atrial fibrillation/flutter, bradyarrhythmia, ventricular tachycardia, stroke, a history of cardiac arrest, and a number of non-cardiovascular comorbidities. The mean Charlson Comorbidity Index was significantly higher in the oHCM group compared with controls (1.6 ± 0.7 vs. 0.4 ± 1.0; p<.001). Patient characteristics, stratified by symptom status, are summarized in Supplementary Table 2. Among symptomatic oHCM patients, dyspnea was the most common symptom of the four evaluated in this study (affecting 56.6% of patients), followed by fatigue (28.5%), chest pain (22.8%), and syncope (17.0%). In the symptomatic subgroup, 87% of patients were taking at least one medication recommended for oHCM (e.g. beta blockers, non-dihydropyridine calcium channel blockers, or disopyramide).
Table 1. Patient characteristics.
Resource utilization and costs associated with oHCM
HRU and costs for patients with oHCM and their respective controls are summarized in . Compared with control patients without HCM, over the 1-year follow-up period, patients with oHCM were more likely to have an inpatient admission (22.7 vs. 6.9%, OR 3.97 [95% CI 3.74–4.22]; p<.001) and experienced more inpatient admissions (0.3 ± 0.8 vs. 0.1 ± 0.3, IRR 4.08 [95% CI 3.83–4.34]; p<.001) and hospital days/patient (2.0 ± 7.3 vs. 0.4 ± 2.9 days, IRR 4.65 [95% CI 4.21–5.11]; p<.001). Among oHCM patients with at least one hospitalization, mean length of stay was 5.5 ± 6.0 days/admission and 8.9 ± 13.3 days/year. Patients with oHCM were also more likely to have emergency department and outpatient visits than those without oHCM. As a result, patients with oHCM incurred substantially higher mean 1-year total healthcare costs than patients without oHCM ($26,929 ± 77,720 vs. $6,808 ± 25,712, difference $20,121 [95% CI $18,762–$21,446]; p<.001), driven primarily by greater inpatient and outpatient costs. After adjustment for differences in comorbidities, the cost difference decreased by approximately 15% to $17,082 (95% CI for difference $16,155–$18,542; p < 0.001) but remained highly statistically significant (Supplementary Table 3).
Table 2. Healthcare resource utilization and cost—overall oHCM population.
Resource utilization and costs associated with symptomatic oHCM
Among patients with symptomatic oHCM, differences in resource utilization and costs compared with the control population were substantially greater than those for the overall oHCM cohort (, ). Patients with symptomatic oHCM were approximately 8 times more likely to require inpatient hospitalization (38.0 vs. 6.9%, OR 8.20 [95% CI 7.58–8.88]; p<.001) and approximately 4 times as likely to seek emergency care (39.3 vs. 14.9%, OR 3.70 [95% CI 3.46–3.96]; p<.001) as their matched controls. Among symptomatic oHCM patients with at least one hospitalization, length of stay was 5.8 ± 6.3 days/admission and 9.7 ± 14.2 hospital days/year. Over the 1-year follow-up period, mean/patient healthcare costs were $43,586 in patients with symptomatic oHCM—$36,818 more than in matched controls (95% CI for difference $34,137 to $39,406; p<.001)—driven predominantly by inpatient costs. This difference remained more than $30,000/patient after adjusting for differences in comorbidities between the two groups (Supplementary Table 4).
Figure 2. Mean 1-year healthcare cost for oHCM, symptomatic oHCM, and asymptomatic oHCM versus matched controls. Total annual costs and the estimated difference in costs between oHCM patients and matched controls are shown along with subgroup analyses for patients with symptomatic oHCM and asymptomatic oHCM. Total annual cost/patient includes inpatient, emergency department, outpatient, and pharmacy costs. Abbreviation. oHCM, obstructive hypertrophic cardiomyopathy.
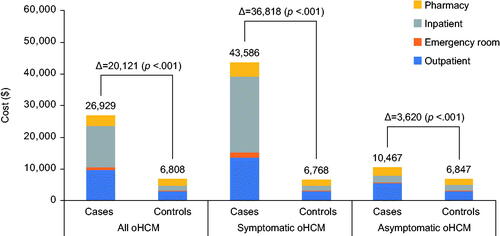
Table 3. Healthcare resource utilization and cost—symptomatic oHCM population.
Resource utilization and costs associated with asymptomatic oHCM
When the analysis was restricted to patients with asymptomatic oHCM, incremental HRU and costs compared with controls were much less striking (Supplementary Table 5). Mean 1-year total healthcare costs for patients with asymptomatic oHCM were $10,467, while the difference in 12-month healthcare-related costs for the asymptomatic oHCM group compared with patients without oHCM was approximately 90% less than that for the symptomatic oHCM group (mean difference $3,620/patient [95% CI for difference $2,827–$4,406]; p<.001). Adjustment for comorbidities had little impact on these results (Supplementary Table 6).
Discussion
This is the first large, population-based study to assess HRU and costs associated with oHCM in the United States. Using claims data from a broad range of health insurance plans, we found that patients with oHCM were nearly 4 times as likely to experience any inpatient admission and cost the healthcare system approximately $20,000 more/year than age- and sex-matched controls. These differences were driven largely by the subgroup with symptomatic oHCM (defined based on diagnostic claims), which accounted for half of the oHCM patients in this study and was associated with an 8-fold increase in hospitalizations and cost approximately $35,000/year more than matched controls. In contrast, asymptomatic oHCM was associated with no significant difference in hospitalizations and only a $3,600/patient difference in 1-year healthcare costs.
Comparison with previous studies
Although several previous studies have examined costs for specific episodes of care, to our knowledge, this is the first study to examine the longitudinal costs of unselected patients with oHCM. In a previous population-based study of patients with oHCM, Jan and colleagues used data from the National Inpatient Sample to assess inpatient costs for 2,605 patients who were hospitalized with a principal diagnosis of oHCM in 2013Citation16. They found a mean length of stay of 4.9 days and mean cost/admission of $25,433—results for length of stay that were similar to those for patients who were hospitalized in our study (5.5 days). Tripathi and colleagues also used the National Inpatient Sample to examine the cost of hospitalization for patients with HCM and found that patients with arrhythmia had hospital costs that were approximately $5,000/patient higher than those without arrhythmia ($20,522 vs. $15,636)Citation21. Our study extends these findings substantially by examining a broader range of costs (inpatient, outpatient, emergency, and pharmacy) and by extending the observation period to a 1-year time frame.
Although there are no previous studies of longitudinal costs for patients with oHCM, several previous studies have examined the economic burden of common cardiac conditions in the United States. In a recent systematic review of medical costs associated with heart failure in the United States from 2014 to 2020, Urbich and colleagues found that median annual medical care costs for heart failure care were $24,383/patient—similar to the annual cost of oHCM (symptomatic or asymptomatic) in our studyCitation22. In an analysis of administrative claims from 2004 to 2006, Kim and colleagues found the annual medical care cost of atrial fibrillation to be $20,670, compared with $11,965 in the control groupCitation23. Finally, similar to our observation that patients with symptomatic oHCM have much higher HRU and costs than patients with asymptomatic oHCM, Kempf and colleagues found that among patients with coronary artery disease (CAD), angina was the primary driver of HRU and cost. Using a claims dataset, they found that annual costs for patients with CAD and angina were $28,590 compared with $14,334 for patients who had CAD without anginaCitation24.
Implications
Our study provides several important insights into the clinical and economic burden of oHCM. Based on claims data, approximately 50% of all patients with diagnosed oHCM had one or more symptoms that may be related to the condition—a combination that was associated with particularly high HRU and incremental costs of approximately $35,000/year compared with controls. In contrast, asymptomatic patients with oHCM had a much smaller difference in HRU and costs compared with matched controls. These findings suggest that effective therapies for this condition could provide clinical and economic value, even if the impact of therapy were limited to relief of symptoms. If the pathophysiology of oHCM were impacted as well, even larger cost savings might accrue. With novel therapeutics for oHCM on the horizonCitation10–13, it will be important to conduct further studies to better understand the impact of such therapies on HRU and to determine both the cost-effectiveness of these agents and their optimal utilization.
Limitations
These results should be interpreted within the context of certain limitations. First, the use of claims data relies on ICD-9 and ICD-10 diagnosis codes, which may not be reliable in the absence of anatomic or genetic confirmation. We attempted to mitigate this limitation by requiring at least two claims for the diagnosis code of HCM at least 30 days apart or one diagnosis claim plus a claim for septal reduction therapy on distinct dates. In addition, because primary echocardiographic data were not available in the MarketScan data, we relied on claims to identify patients with obstructive HCM. Second, the sensitivity and specificity of claims data for identifying patients with symptomatic oHCM is unknown. If patients with mild symptoms were more likely to be coded as asymptomatic, this could have led to an overestimate of the costs of symptomatic oHCM. Third, our study population included only commercially insured patients. As such, our findings may not be generalizable to other patient populations such as those with fee-for-service Medicare, Medicaid, or who are uninsured.
Fourth, given the observational nature of our study, our estimates of incremental HRU and cost may be confounded despite the fact that control patients were selected at random after matching based on age, sex, index year, and region. By selecting the matched controls at random, the rates of comorbidities in the oHCM and control groups reflect the background rate of these comorbidities in the two populations, and the unadjusted differences in healthcare resource utilization and costs reflect the comparison of interest. However, to account for the possibility of residual confounding, we performed a sensitivity analysis in which we adjusted for observed differences in baseline comorbidities except for those that could reflect symptoms of oHCM, such as congestive heart failure, cerebrovascular disease, or myocardial infarction. It is reassuring that the results of these adjusted analyses were only slightly different from our primary results. Finally, in our comparisons of patients with symptomatic oHCM vs. matched controls, it is possible that some of the symptoms attributed to oHCM were related to underlying comorbidities such as chronic lung disease. In practice, it may be challenging to distinguish the root cause of symptoms in such individuals as well, thus leading to similar misclassification.
Conclusions
In this study of unselected, privately insured US patients, we found that oHCM was associated with substantial HRU, predominantly due to increased all-cause hospitalizations, and incremental costs of approximately $20,000/year—which increased to approximately $35,000/year among symptomatic patients. Further studies are needed to understand the long-term clinical and economic impact of various therapies for these groups of patients.
Transparency
Declaration of funding
Funding for this study was provided by MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb.
Declaration of financial/other interests
This study was sponsored by MyoKardia Inc., a wholly owned subsidiary of Bristol Myers Squibb and developers of mavacamten, one of the potential new drugs for treatment of obstructive hypertrophic cardiomyopathy. The analyses were designed collaboratively by the academic authors (SSJ, JAS, and DJC) in conjunction with the sponsor and performed by The Analysis Group under the direction of SSJ and DJC. The manuscript was written by SSJ and DJC.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
SSL: employee of MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb; stockholder of MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb at the time of project conception.
JX: employee of Analysis Group, Inc., which received consultancy fees from MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb for the conduct of this research.
MBS: employee of MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb; stockholder of MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb at the time of project conception.
JTF: employee of MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb; stockholder of MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb at the time of project conception.
JME: employee of MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb; stockholder of MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb at the time of project conception.
WG: employee of Analysis Group, Inc., which received consultancy fees from MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb for the conduct of this research.
JAS: received consulting fees from MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb to assist in the analysis and interpretation of PROs.
DJC: received consulting fees from MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb to assist in study design and analysis.
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Author contributions
SSJ, SSL, JX, MS, JF, JE, WG, JS, and DC participated in the study concept and design. All the authors were involved in the acquisition, analysis and interpretation of data. All the authors participated in preparing the manuscript.
Previous presentations
This work was presented virtually at the American College of Cardiology (ACC) 70th Annual Scientific Session & Expo, May 15–17, 2021.
Supplemental Material
Download MS Word (52.6 KB)Acknowledgement
None reported.
Data availability statement
The datasets generated during and analysed for the current study are not publicly available as they were used under license from IBM Watson Health.
References
- Ommen SR, Mital S, Burke MA, et al. AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J Am Coll Cardiol. 2020;76(25):e159–e240.
- Elliott PM, Anastasakis A, Borger MA, et al. ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society Of Cardiology (ESC). Eur Heart J. 2014;35(39):2733–2779.
- Gersh BJ, Society of Thoracic Surgeons, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;124(24):2761–2796.
- Ammirati E, Contri R, Coppini R, et al. Pharmacological treatment of hypertrophic cardiomyopathy: current practice and novel perspectives. Eur J Heart Fail. 2016;18(9):1106–1118.
- Maron BJ, Gardin JM, Flack JM, et al. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA study. Coronary artery risk development in (young) adults. Circulation. 1995;92(4):785–789.
- Semsarian C, Ingles J, Maron MS, et al. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249–1254.
- Heitner SB, Jacoby D, Lester SJ, et al. Mavacamten treatment for obstructive hypertrophic cardiomyopathy: a clinical trial. Ann Intern Med. 2019;170(11):741–748.
- Maron BJ. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med. 2018;379(7):655–668.
- Ho CY, For the SHaRe Investigators, Day SM, Ashley EA, et al. Genotype and lifetime burden of disease in hypertrophic cardiomyopathy: insights from the sarcomeric human cardiomyopathy registry (SHaRe). Circulation. 2018;138(14):1387–1398.
- Olivotto I, Oreziak A, Barriales-Villa R, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;396(10253):759–769.
- Adhikari AS, Trivedi DV, Sarkar SS, et al. β-Cardiac myosin hypertrophic cardiomyopathy mutations release sequestered heads and increase enzymatic activity. Nat Commun. 2019;10(1):2685.
- Cytokinetics. REDWOOD-HCM: randomized evaluation of dosing with CK-3773274 in obstructive outflow disease in HCM (REDWOOD-HCM) [updated 2021 May 7; cited 2021 Jun 1]. Available from: https://clinicaltrials.gov/ct2/show/NCT04219826.
- Spertus JA, Fine JT, Elliott P, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): health status analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397(10293):2467–2475.
- Yandrapalli S, Harikrishnan P, Andries G, et al. TCT-831 differences in Short-Term outcomes and cost between alcohol septal ablation and septal myectomy for hypertrophic cardiomyopathy in the United States. J Am Coll Cardiol. 2018;72(13):B331.
- Gul MH, Htun ZM, Aung TK, et al. Abstract 18228: Placement of implantable cardioverter defibrillator in hypertrophic obstructive cardiomyopathy with paroxysmal ventricular tachycardia and their outcomes. Circulation. 2017;136(Suppl_1):A18228.
- Jan AS, Rehman S, Rungatscher A, et al. Hypertrophic obstructive cardiomyopathy and the cost of treatment. EJCM. 2016;4(2):27–32.
- IBM Watson Health. IBM MarketScan research databases for health services researchers [updated 2019; cited 2021 Jun 1]. Available from: https://www.ibm.com/downloads/cas/6KNYVVQ2.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- Hubbard AE, Ahern J, Fleischer NL, et al. To GEE or not to GEE: comparing population average and mixed models for estimating the associations between neighborhood risk factors and health. Epidemiology. 2010;21(4):467–474.
- Tripathi B, Khan S, Arora S, et al. Burden and trends of arrhythmias in hypertrophic cardiomyopathy and its impact of mortality and resource utilization. J Arrhythmia. 2019;35(4):612–625.
- Urbich M, Globe G, Pantiri K, et al. A. A systematic review of medical costs associated with heart failure in the USA (2014–2020). Pharmacoeconomics. 2020;38(11):1219–1236.
- Kim MH, Johnston SS, Chu BC, et al. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4(3):313–320.
- Kempf J, Buysman E, Brixner D. Health resource utilization and direct costs associated with angina for patients with coronary artery disease in a US managed care setting. Am Health Drug Benefits. 2011;4(6):353–361.
