Abstract
Background
Extremely preterm (EP) infants have high rates of respiratory morbidity and correspondingly high healthcare resource utilization.
Objectives
Data from the PHARMO Perinatal Research Network were analyzed to quantify the burden of EP birth in the Netherlands.
Methods
A retrospective analysis included infants <28 weeks gestational age with a birth record in the Perinatal Registry (1999–2015) and data in the PHARMO Database Network. Outcomes of interest included select comorbidities, hospital readmissions, and costs of hospitalization and medication up to 1- and 2-years corrected age. Outcomes were stratified by birth period (1999–2005, 2000–2009, 2010–2015) and by diagnosis of bronchopulmonary dysplasia (BPD) and chronic lung disease (CLD).
Results
The cohort included 168 EP infants (37 born 1999–2005, 51 born 2006–2009, 80 born 2010–2015). Median (Q1–Q3) birth weights decreased by birth period from 970 (840–1,035) g in 1999–2005 to 853 (695–983) g in 2010–2015. Overall, BPD and CLD were reported during the birth hospitalization in 40% and 29% of infants, respectively; rates of BPD increased and rates of CLD decreased by birth period. Eighty-four percent of EP infants had an additional comorbidity. Mean (standard deviation) costs of birth hospitalization were €110,600 (€73,000) for 1999–2005, €119,350 (€60,650) for 2006–2009, and €138,800 (€130,100) for 2010–2015. Birth hospitalization and total costs for up to 1- and 2-years corrected age were higher for infants with BPD and/or CLD than for those without either complication.
Conclusion
Healthcare resource utilization and costs for EP infants, especially for those with respiratory morbidities, increased between 1999 and 2015. Future cost-effectiveness analyses are essential to determine the economic impact of this change and underscore the need for new therapeutic interventions to decrease clinical sequelae in this vulnerable population.
Introduction
Infants born before 28 weeks gestational age (GA), termed extremely preterm (EP), have high rates of morbidity and mortalityCitation1–4. Survival to discharge of EP infants has increased in developed countries as a result of advances in neonatal and maternal care practices, such as antenatal corticosteroid use, less aggressive ventilation, and strict infection control practicesCitation5–8.
The incidence of comorbidities of preterm birth has also improved, except for bronchopulmonary dysplasia (BPD), which has either remained steady or increased over timeCitation5,Citation9–11. Greater use of active resuscitation and improvements in intensive care have led to increased survival and therefore persistence of BPDCitation5,Citation12. Because BPD is the most common complication of preterm birthCitation13 and is associated with prolonged hospitalization for pulmonary complications and development of chronic lung diseases (CLDs)Citation14–16, increased survival has resulted in increased healthcare resource utilization (HCRU) and costs.
In 2010, Dutch guidelines lowered the recommend active care of neonates from 25 weeks GA to 24 weeks GA, with comfort care recommended for live births below this threshold. Two studies characterized the consequence of this change: Geurtzen et al.Citation17 surveyed physician’s preferences on treatment decisions, and van Beek et al.Citation12 evaluated the impact of guideline implementation on neonatal survival, mortality, and timing of death.
From the physician survey, the widest variation in individually preferred treatment options was around care at 24 and 25 weeks GA. Views differed on the lowest GA limit for interventions such as cesarean section and neonatologist presence at birth. Factors for restricting active treatment included congenital malformations, sub-normal GA size, and incomplete course of steroids, with a median of optional comfort care at parental requestCitation17.
As expected, implementation of the guidelines on neonates led to an increase in NICU admissions at 24 weeks GA (27% for the 2007–2009 reference group to 69% for the 2011–2017 post-guideline change group, p < 0.001) resulting in increased survival to discharge from 13% (reference group) to 34% (post-guideline change group, p < 0.001). A downstream effect of the guideline change was a decrease in deaths attributed to respiratory insufficiency (respiratory distress syndrome [RDS] and BPD combined) from 34% for 2011–2014 to 23% for 2015–2017 (p = 0.006). Overall (2011–2017), the cause of death for 29.9% of the non-surviving infants was due to respiratory issues (19.1% RDS, 10.8% BPD)Citation12.
Characterizing the burden of BPD is critical for providing parents and healthcare providers with decision-making information and for developing strategies to improve outcomes. Because HCRU data are compiled based on local standards, developing data representative of an entire country is challenging. The PHARMO Perinatal Research Network (PPRN)Citation18, established by linkage of the Netherlands Perinatal Registry (Perined)Citation19 and the PHARMO Database Network (PHARMO)Citation20, uniquely captures birth and treatment information, including longitudinal pharmacy usage and hospital discharge records. This study was undertaken to broaden understanding of the burden of EP birth, using the Netherlands as a model. The main objective was to assess respiratory morbidities, hospital length of stay (LOS), HCRU, and costs for infants born <28 weeks GA. To assess the impact of respiratory complications, data were stratified by the presence of BPD and CLD and by birth period.
Methods
Study design and conduct
This retrospective analysis of PPRN dataCitation21 included infants with a birth record in Perined and data in PHARMO. The latter is a population-based, patient-level network of healthcare databases (including inpatient and outpatient pharmacies, hospitals, and general practices). The longitudinal nature of PHARMO enables retrospective studies of >4 million residents (25%) of a well-defined population in the Netherlands for an average of 10 yearsCitation22,Citation23. For this study, the PHARMO Out-patient Pharmacy Database and Hospitalization Database were used. Perined contains validated data from ∼95% of pregnancies in the NetherlandsCitation24. Data acquisition complied with rules governing the use of patient-level healthcare data in the Netherlands.
Analysis population
Data in the PPRN for all infants born preterm (24–37 weeks GA) from 1 January 1999 to 31 December 2015 were selected. To eliminate the effect of congenital malformations on the burden of EP birth, infants with one or more major congenital malformations, such as central nervous system malformations, diaphragmatic hernia, or gastroschisis, were excluded from the analysis. Infants were followed from birth until transfer out of the database, death, or the end of the study period (31 December 2016), whichever occurred first. Eligible infants were divided into four subgroups: EP, <28 weeks GA; very preterm, 28–<32 weeks GA; moderately preterm, 32–<34 weeks GA; and late preterm, 34–<37 weeks GA. This analysis focused on the subgroup of EP infants.
Outcome measures
BPD was defined as requiring supplemental oxygen for >28 days from the day of birth or still requiring supplemental oxygen at 36 postmenstrual weeks; this group also included infants identified through the ICD-10 hospitalization discharge code for BPD (P27.1). This definition was based on the literature and expert opinion (Supplementary Table S1). The severity of BPD could not be differentiated. CLD comprised several respiratory diagnoses (Supplementary Table S2) and was also identified by recorded use of pulmonary medications (i.e. furosemide, hydrochlorothiazide, inhaled steroids, systemic corticosteroids, inhaled bronchodilators, or inhaled mucolytics). The definition of CLD used in this study was based on the literature and expert opinion (Supplementary Table S1). We assessed CLD that occurred up to 1- and 2-years corrected age (CA; chronological age minus the number of weeks the infant was preterm).
LOS was determined for the birth hospitalization and was categorized as short or long based on the median LOS. The number, rate, and LOS of all-cause hospital readmissions and pulmonary-related hospital readmissions were assessed at 1- and 2-years CA. Costs (in Euros [€]) are presented for the birth hospitalization, all hospitalizations (excluding the birth hospitalization), all outpatient dispensed medication, and total costs (hospitalizations and medication) up to and including 1- and 2-years CA per person-year (PY).
Hospitalization costs were based on standard tariffs for nursing days in 2014 as prescribed by the Dutch Healthcare AuthorityCitation25, indexed to the year of hospital admission according to annual changes in the Netherlands Consumer Price Index (CPI)Citation26. The annual change in CPI is measured as the increase in the CPI compared to the corresponding period in the previous year. Costs of outpatient dispensed medication were based on claim amounts recorded in the PHARMO Out-patient Pharmacy DatabaseCitation20.
Statistical analysis
Outcomes were stratified by birth period (1999–2005; 2006–2009; 2010–2015). Additional comorbidities (intraventricular hemorrhage, necrotizing enterocolitis, periodic limb movement, RDS, retinopathy of prematurity, and sepsis) were also summarized by birth period. No imputations were performed for missing data. Predictors of long versus short LOS of birth hospitalization were assessed using a multivariable Poisson regression model to yield relative risk. Potential predictors included BPD during birth hospitalization, CLD during birth hospitalization, birth weight, 5-min Apgar score, birth year, type of birth, mode of delivery, age and ethnicity of mother, parity, and the presence of at least one pregnancy risk factor. Significant predictors were identified from the sex- and GA-adjusted model when the lower bound of the 95% CI for the relative risk exceeded unity. Because of the descriptive nature of this study based on real-world data, no formal power analysis was performed, and no a priori hypotheses were tested.
Ethics approval and consent to participate
This article reports on a retrospective analysis of anonymized healthcare data and does not contain any studies with human participants or animals performed by any of the authors.
Results
Study population and available data
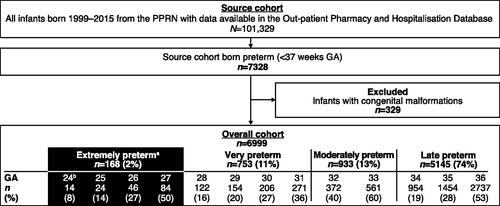
Of 101,329 infants born between 1999 and 2015, 7,328 (7%) were born preterm (<37 weeks GA). After exclusion of infants with congenital malformations (329/7,328 [4%]), the analysis cohort comprised 168 EP infants (very, moderate, and late preterm total sample sizes are shown in ). Based on Dutch perinatal guidelines, infants born <24 weeks GA were not identified. Of the 168 EP infants, 37 were born in 1999–2005, 51 in 2006–2009, and 80 in 2010–2015. Median (interquartile range, Q1–Q3) birth weights decreased over time: 970 (840–1,035) g for 1999–2005, 915 (750–1,090) g for 2006–2009, and 853 (695–983) g for 2010–2015.
Figure 1. Flow diagram showing the study population identified in the PPRN. aInfants born <28 weeks GA are the primary focus of the analyses reported in this article. bGiven the restrictive treatment policy of infants born at ≤24 weeks GA in the Netherlands, the study cohort only included pregnancies from 24 weeks GA onwards. Abbreviations. GA, gestational age; PPRN, PHARMO Perinatal Research Network.
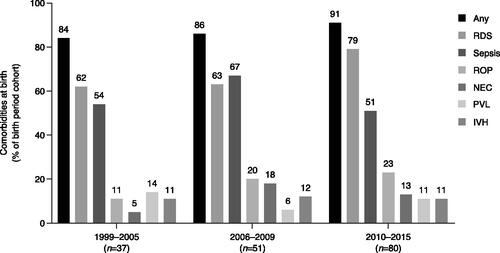
Birth hospitalization data were available for 76% of EP infants. Overall, 90% and 80% of EP infants had follow-up data available from birth until ≥1-year CA and ≥2-years CA, respectively (median = 5.0 years; interquartile range = 2.6–8.1). Total PYs of follow-up were 203 and 346 at 1- and 2-years CA, respectively. Infant and maternal characteristics of the population are summarized in . In this cohort, BPD and CLD were reported during the birth hospitalization in 40% and 29% of EP infants, respectively. The incidence of BPD increased with later birth periods (24% in 1999–2005, 41% in 2006–2009, 46% in 2010-2015) and the prevalence of CLD decreased (46% in 1999–2005, 33% in 2006–2009, 19% in 2010–2015). Birth weight for infants with BPD (alone or with CLD) was lower than the median for the overall cohort. While additional comorbidities (IVH, ROP, NEC, sepsis, PVL, or RDS) were reported in 83% (n = 65) of infants without BPD or CLD, this rate rose to >90% for infants with BPD and/or CLD (). The rates of any additional comorbidities, and particularly RDS and ROP, increased over successive birth periods, whereas the rates of other comorbidities remained mainly consistent across birth periods ().
Table 1. Selected infant and maternal characteristics by morbidity status during birth hospitalization of infants in the PHARMO Perinatal Research Network born EP (<28 weeks GA) from 1999 to 2015.
Pulmonary complications
Of 168 EP infants, 43% (rate/PY = 0.51) and 47% (rate/PY = 0.33) were diagnosed with other pulmonary complications during 1- and 2-years CA, respectively. Commonly recorded pulmonary complications included apnea (1- and 2-years CA = 18%), hypoxemia/hypoxia (1- and 2-years CA = 18%), respiratory infection (1-year CA = 13%; 2-years CA = 16%), and acute respiratory distress (1- and 2-years CA = 12%).
During the 1- and 2-years CA periods, 50% (rate/PY = 0.41) and 59% (rate/PY = 0.29) of EP infants, respectively, received medication for pulmonary complications at least once. Inhaled bronchodilators (1-year CA = 36%; 2-years CA = 46%) were the most frequently recorded medications dispensed for pulmonary complications (1-year CA rate/PY = 0.30 and 2-years CA rate/PY = 0.22), followed by diuretics (1- and 2-years CA = 20%) and inhaled corticosteroids (1-year CA = 18%; 2-years CA = 24%).
Birth hospitalization and readmissions
Median LOS of birth hospitalization for EP infants was 81 days. Mean (standard deviation [SD]) of short and long birth hospitalization LOS was 57 (25) and 107 (19) days, respectively. In the adjusted model, CLD during birth hospitalization, GA at birth, and maternal pregnancy risk factors were significantly predictive of long LOS ().
Table 2. Adjusted model predicting long LOS of birth hospitalizationa among EP infants (<28 weeks GA) in the PHARMO Perinatal Research Network born from 1999 to 2015.
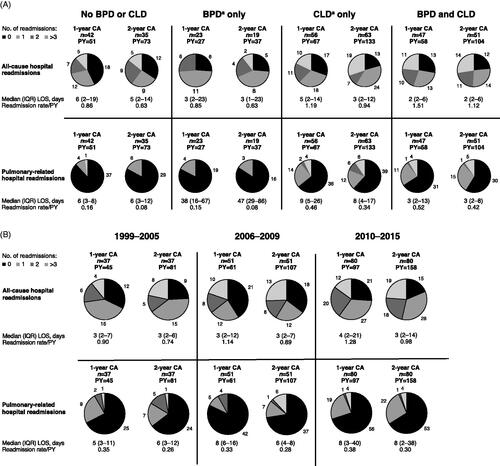
Among the infants born EP, 61% (rate/PY = 0.51) and 68% (rate/PY = 0.33) developed CLD in the periods up to 1- and 2-years CA, respectively. For infants with CLD up to 1-year CA, 47 (46%) were also diagnosed with BPD. Rates of all-cause and pulmonary-related hospital readmissions were highest in the subgroup with both BPD and CLD (). Compared with infants without BPD or CLD, all-cause and pulmonary readmission rates were higher for infants with CLD alone but not for infants with BPD alone. The rates of all-cause and pulmonary-related hospital readmissions showed a trend of increase by birth period ().
Figure 3. Frequency of hospital readmissions at 1- and 2-years CA after birth among EP (<28 weeks gestational age) infants in the PHARMO Perinatal Research Network. (a) Infants with a birth record from 1999 to 2015 by pulmonary morbidity cohort. aWithin the time periods of outcome assessment (note: cohort sizes can therefore be larger than those presented at birth). (b) Frequency of hospital readmissions at 1- and 2-years CA after birth among EP infants in the PHARMO Perinatal Research Network with a birth record from 1999 to 2005, 2006 to 2009, and 2010 to 2015. Abbreviations. BPD, bronchopulmonary dysplasia; CA, corrected age; CLD, chronic lung disease; EP, extremely preterm; IQR, interquartile range; LOS, length of stay; PY, person-year.
Costs of healthcare resource utilization
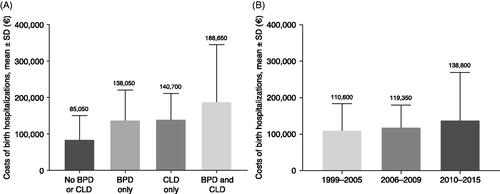
The mean (SD) costs of birth hospitalization for the overall cohort of EP infants was €126,350 (€101,900) and increased by birth period (€110,600 [€73,000] in 1999–2005; €119,350 [€60,650] in 2006–2009; €138,800 [€130,100] in 2010–2015 []). Costs for birth hospitalization were higher for infants with BPD or CLD (or both) than for those without either respiratory complication (); for EP infants with neither BPD nor CLD, mean (SD) birth hospitalization costs were €85,050 (€65,600), compared with €188,650 (€156,300) for infants with BPD and CLD.
Figure 4. Mean costs of birth hospitalization for EP infants (<28 weeks gestational age). (a) By pulmonary morbidity; (b) by birth period. Abbreviations. BPD, bronchopulmonary dysplasia; CLD, chronic lung disease; EP, extremely preterm; SD, standard deviation.
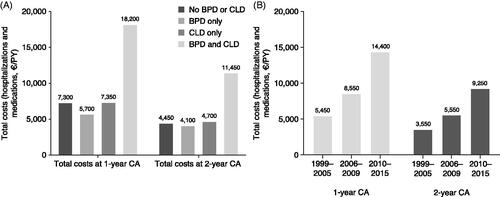
In the periods up to 1- and 2-years CA, total costs (including non-birth hospitalizations and medication) for all causes were higher for EP infants with both BPD and CLD than without BPD or CLD, BPD alone, or CLD alone (). At 1-year CA, hospitalization costs per PY (excluding birth hospitalization) were €3,800, €3,650, €3,450, and €12,950 for infants with neither BPD nor CLD, BPD alone, CLD alone, and both BPD and CLD, respectively. At 2-years CA, hospitalization costs were €2,350, €2,650, €2,500, and €8,300, respectively. In the periods up to 1- and 2-years CA, total costs increased by birth period ().
Figure 5. Costs of health care resource utilization among EP infants (<28 weeks gestational age). (a) By pulmonary morbidity; (b) by birth period. Costs are for infants with data available on birth hospitalization. Abbreviations. BPD, bronchopulmonary dysplasia; CA, corrected age; CLD, chronic lung disease; EP, extremely preterm; PY, person-year.
Discussion
Results of this population-based study using data from the PPRN provide insight into the pulmonary outcomes, resource needs, and cost impact associated with preterm birth, adding to previous evidence that greater burdens are associated with pulmonary complicationsCitation12. Taken as an example, these data from the Netherlands also provide support for institutional estimates of HCRU in Europe within the context of EP birth with and without BPD or CLD.
This study quantified the respiratory burden and HCRU among EP infants born in the Netherlands between 1999 and 2015. It demonstrates the high prevalence of BPD and CLD among EP infants (40% and 29%, respectively, during birth hospitalization), and high hospital readmission rates particularly among infants with CLD alone and infants with both BPD and CLD. Significant predictors of a long LOS for the birth hospitalization were CLD during birth hospitalization, GA at birth, and pregnancy risk factors. Analysis of these data by birth period suggested lower mean birth weights, higher rates of BPD, lower rates of CLD, and an increase in hospitalization and medication costs with more recent birth periods. On the other hand, rates of individual comorbidities that are often observed in preterm infants, such as IVH and NEC, did not seem to change over time.
A lower incidence of BPD was estimated by de Waal et al.Citation27, who performed a prospective, population-based cohort study of preterm infants born in the Netherlands in 2007. In a cohort of 215 infants who survived long enough to receive neonatal care, 33.3%, 29.1%, and 20.9% of infants born at 24, 25, and 26 weeks GA, respectively, were diagnosed with BPD. BPD based on “oxygen dependency at 36 post-menstrual weeks” was identified in the study using 2001 guidelines, but the use of International Classification of Diseases, 10th Revision (ICD-10) codesCitation28 was not specified in the publication. The present study identified BPD as needing supplemental oxygen for either >28 days or at 36 postmenstrual weeks (based on Perined records) and also included infants identified through the ICD-10 hospitalization discharge code for BPD, which may explain the higher incidence of BPD in our study compared with the incidence of BPD in the earlier study in the NetherlandsCitation24.
Data from Europe on outcomes of preterm birth are limited. A retrospective evaluation performed in the Netherlands that compiled postpartum (up to 1 year) neonatal HCRU data from a prospective cohort study and three randomized clinical trials showed higher neonatal care costs were associated with lower GACitation29. Mean costs of €60,783–€88,052 for singleton infants born at 24–28 weeks GA were primarily driven by LOS in a neonatology ward, particularly in intensive and medium care units. Maier et al.Citation30 observed LOS of birth hospitalization for preterm infants ranging from 52.4 to 76.5 days across 10 regions in nine European countries. The median LOS was 55 days in 2011–2012 for infants born between 22 + 0 and 31 + 6 weeks GA. For infants born 25–29 weeks GA, the adjusted mean LOS was 61.6 days. Our analyses support that a main driver of increased LOS is respiratory complications.
The analyses by birth period, carried out in an effort to highlight where evolving treatment strategies were having an effect, were limited somewhat by the small numbers of infants following stratification. Not surprisingly, though, the change in Dutch guidelines in 2010 to provide active care as early as 24 weeks GA resulted in an increase in the number of hospital readmissions within the first 2 years CA, and higher overall costs, especially for infants with both BPD and CLD.
Our study generates a unique set of longitudinal data combining multiple settings of care by linkage of Perined to the PHARMO Database Network for analysis of preterm birth complications and real-world HCRU. Our definitions of BPD and CLD may be useful for future researchers in determining cost drivers of preterm birth. We used standard tariffs for nursing days for the calculation of costs of hospitalizations. In the Netherlands, these tariffs are based on average costs of diagnostics, associated specialist fees, and inpatient medicationCitation25. As our data indicate, the individual costs may vary significantly depending on the presence and number of comorbidities being treated.
Another limitation of this study is that our data set did not include complete birth hospitalization data for all infants; transfers from the birth hospital to a separate site with a neonatal intensive care unit were recorded but such transfers may have been recorded differently at different institutions. If transfers were recorded as readmissions rather than a continuation of the birth hospitalization, this would have confounded estimated readmissions and/or LOS. Additionally, infants who died shortly after birth (i.e. the most severe cases of preterm birth) are under-represented in the study cohort because they were less likely to be linked from Perined to the PHARMO Database Network. Under-representation of these infants could have resulted in an overestimate of the overall costs but an underestimate of BPD as well as CLD. Lastly, pulmonary outcomes may have been under-recorded. The Hospitalization Database requires only one primary discharge diagnosis, whereas secondary diagnoses are optional. Under-recording of events along with misclassification of infants with BPD or CLD in the “no BPD or CLD” group could have resulted in underestimation of HCRU and cost differences.
Conclusion
These data highlight and quantify over time the healthcare burden associated with EP birth and the associated increase in pulmonary complications. Our findings of high prevalence of BPD and CLD among EP infants, along with accompanying high HCRU which has increased over time, underscore the need for new interventions in this vulnerable population and provide a basis for future cost-effectiveness analyses.
Transparency
Declaration of funding
This study was funded by Shire, a Takeda company. Takeda provided funding to Excel Medical Affairs for support in writing and editing this manuscript.
Declaration of financial/other interests
EH, JO, and FP-vB are employees of the PHARMO Institute for Drug Outcomes Research. This independent research institute performs financially supported studies for government and related healthcare authorities and several pharmaceutical companies. CS and VN are employees of Takeda and own stocks/options in Takeda. SPS was an employee of Takeda at the time of the study.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
EH, JO, FP-vB, CS, and SPS contributed to the study conception and design. Data collection, analysis, and interpretation were performed by EH, JO, and FP-vB. CS, VN, and SPS participated in data interpretation. All authors participated in the drafting and revision of this manuscript, and each gave their final approval prior to its submission.
Supplemental Material
Download MS Word (45.6 KB)Acknowledgements
The authors thank all healthcare providers contributing information to the PHARMO Database Network as well as all contributors from the Netherlands Perinatal Registry (Perined) for their assistance and for granting access to Perined. Mark Poirier and Shirley Louise-May of Excel Medical Affairs (Fairfield, CT, USA) provided writing assistance for this manuscript.
References
- Howson CP, Kinney MV, McDougall L, et al. Born too soon: preterm birth matters. Reprod Health. 2013;10(Suppl 1):S1.
- March of Dimes, PMNCH, Save the Children, et al. Born too soon: the global action report on preterm birth. [cited 2021 Jan 25]. Available from: https://www.who.int/maternal_child_adolescent/documents/born_too_soon/en/.
- Blencowe H, Cousens S, Chou D, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10(Suppl 1):S2.
- Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–2172.
- Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. 2015;314(10):1039–1051.
- EURO-PERISTAT. European perinatal health report: health and care of pregnant women and babies in Europe in 2010. [cited 2018 Aug 12]. Available from: http://www.europeristat.com/reports/european-perinatal-health-report-2010.html.
- Glass HC, Costarino AT, Stayer SA, et al. Outcomes for extremely premature infants. Anesth Analg. 2015;120(6):1337–1351.
- Lawn JE, Davidge R, Paul VK, et al. Born too soon: care for the preterm baby. Reprod Health. 2013;10(Suppl 1):S5.
- Costeloe KL, Hennessy EM, Haider S, et al. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ. 2012;345(dec04 3):e7976.
- Mowitz ME, Mangili A, Han L, et al. Prevalence of chronic respiratory morbidity, length of stay, inpatient readmissions, and costs among extremely preterm infants with bronchopulmonary dysplasia. Expert Rev Pharmacoecon Outcomes Res. 2021;21(5):1117–1125.
- Mowitz ME, Ayyagari R, Gao W, et al. Health care burden of bronchopulmonary dysplasia among extremely preterm infants. Front Pediatr. 2019;7:510.
- van Beek PE, Groenendaal F, Broeders L, et al. Survival and causes of death in extremely preterm infants in The Netherlands. Arch Dis Child Fetal Neonatal Ed. 2021;106(3):251–257.
- Jobe AH. Mechanisms of lung injury and bronchopulmonary dysplasia. Am J Perinatol. 2016;33(11):1076–1078.
- Klinger G, Sirota L, Lusky A, et al. Bronchopulmonary dysplasia in very low birth weight infants is associated with prolonged hospital stay. J Perinatol. 2006;26(10):640–644.
- Álvarez-Fuente M, Arruza L, Muro M, et al. The economic impact of prematurity and bronchopulmonary dysplasia. Eur J Pediatr. 2017;176(12):1587–1593.
- Davidson LM, Berkelhamer SK. Bronchopulmonary dysplasia: chronic lung disease of infancy and long-term pulmonary outcomes. J Clin Med. 2017;6(1):4.
- Geurtzen R, Draaisma J, Hermens R, et al. Perinatal practice in extreme premature delivery: variation in Dutch physicians’ preferences despite guideline. Eur J Pediatr. 2016;175(8):1039–1046.
- Houben E, Broeders L, Steegers EAP, et al. Cohort profile: the PHARMO Perinatal Research Network (PPRN) in The Netherlands: a population-based mother-child linked cohort. BMJ Open. 2020;10(9):e037837.
- Perined. [cited 2021 Apr 1]. Available from: www.perined.nl.
- Kuiper JG, Bakker M, Penning-van Beest FJ, et al. Existing data sources for clinical epidemiology: the PHARMO database network. Clin Epidemiol. 2020;12:415–422.
- Houben E, Broeders L, Steegers EAP, et al. Cohort profile: the PHARMO Perinatal Research Network (PPRN) in the Netherlands: a population-based mother-child linked cohort. BMJ Open. 2020;10(9):e037837.
- Herings R, Pedersen L. Pharmacy-based medical record linkage systems. In: Strom BL, Kimmel SE, Hennessy S, editors. Pharmacoepidemiology. 5th ed. Chichester (UK): Wiley-Blackwell; 2012. p. 270–286.
- van Herk-Sukel MPP, van de Poll-Franse LV, Lemmens VEPP, et al. New opportunities for drug outcomes research in cancer patients: the linkage of the Eindhoven Cancer Registry and the PHARMO Record Linkage System. Eur J Cancer. 2010;46(2):395–404.
- Stichting Perinatale Registratie Nederland. 10 jaar Perinatale Registratie Nederland, de grote lijnen. [cited 2019 Feb 5]. Available from: https://assets.perined.nl/docs/6bffb57e-f3e7-437c-8c04-873ade4f8171.PDF
- Nederlandse Zorgautoriteit. Tarieven en prestaties ziekenhuiszorg. [cited 2017 Sept 27]. Available from: https://www.nza.nl/regelgeving/tarieven/tarieven-en-prestaties-ziekenhuiszorg/
- Centraal Bureau voor de Statistiek. Jaarmutatie consumentenprijsindex; vanaf 1963. [cited 2021 Jan 25]. Available from: https://opendata.cbs.nl/statline/#/CBS/nl/dataset/70936NED/table
- de Waal CG, Weisglas-Kuperus N, van Goudoever JB, et al. Mortality, neonatal morbidity and two year follow-up of extremely preterm infants born in The Netherlands in 2007. PLoS One. 2012;7(7):e41302.
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–1729.
- van Baaren GJ, Peelen MJCS, Schuit E, et al. Preterm birth in singleton and multiple pregnancies: evaluation of costs and perinatal outcomes. Eur J Obstet Gynecol Reprod Biol. 2015;186:34–41.
- Maier RF, Blondel B, Piedvache A, et al. MOSAIC and EPICE research groups. Duration and time trends in hospital stay for very preterm infants differ across European regions. Pediatr Crit Care Med. 2018;19(12):1153–1161.