Abstract
Aims
High dose trivalent influenza vaccine (HD TIV) and adjuvant TIV (aTIV) have been developed specifically for adults aged 65 and older (65+) who are at high risk of life-threatening complications. However, there is a scarcity of evidence comparing the clinical and cost-effectiveness of HD TIV and aTIV. The aim of this study was to determine the cost-effectiveness of HD TIV versus aTIV in the England and Wales 65+ population.
Methods
A cost-utility analysis was conducted using a decision tree with two influenza related outcomes: Laboratory confirmed cases that could result in GP consultation, and hospitalizations that may result in premature mortality. Due to a lack of comparative evidence, the effectiveness of HD TIV versus aTIV was calculated indirectly, based on relative effectiveness estimates for each vaccine versus a common comparator, standard dose (SD) TIV. The primary analysis included hospitalizations explicitly due to influenza/pneumonia. Cost-effectiveness was established for three scenarios applying differing relative effectiveness estimates for aTIV versus SD TIV. Uncertainty was analysed in one-way deterministic sensitivity analyses. A secondary analysis included hospitalizations due to any respiratory illness.
Results
The minimum population impact of vaccination with HD TIV rather than aTIV was 13,092 fewer influenza cases, 1,109 fewer influenza related deaths, 4,673 fewer hospitalizations, and 3,245 fewer GP appointments. HD TIV was cost-effective versus aTIV for all three effectiveness scenarios, with incremental cost-effectiveness ratios (ICER) equal to £1,932, £4,181, and £8,767 per quality adjusted life year. Results were consistent across the secondary analysis and deterministic sensitivity analyses.
Limitations
The analysis was limited by a lack of robust and consistent effectiveness data for aTIV.
Conclusion
HD TIV is cost-effective versus aTIV in people aged 65+ in England and Wales. Use of HD TIV over aTIV could increase clinical benefits and reduce the public health and economic burden of influenza.
Introduction
Influenza vaccination programmes are a public health priority, particularly for older adult populations who are at higher risk of complications including lower respiratory tract infection, hospitalization, and deathCitation1. Seasonal flu epidemics are typically caused by influenza type A (subtypes H1N1 and H3N2) and different type B virusesCitation2. Consequently, the World Health Organization (WHO) currently recommend vaccines containing multiple type A and B antigens, for example inactivated trivalent (TIV) and quadrivalent influenza vaccines (QIV)Citation3. The standard dose influenza vaccination typically contains 15 µg of antigen per strain. In a meta-analysis of 10 randomized controlled trials (RCTs), standard dose trivalent vaccines (SD TIV, Fluzone®, Sanofi Pasteur) had an effectiveness in healthy adults of around 59%, which varied substantially across influenza seasons and subtypesCitation4.
The effectiveness of standard dose vaccines is diminished in older populations likely due to immune system functionality and antibody response which declines naturally with advancing ageCitation5. To address issues of reduced effectiveness, specific vaccine formulations have been developed in adults 65 years of age and older, herein referred to as 65+. A high dose trivalent influenza vaccine (HD TIV, Fluzone® High-Dose, Sanofi Pasteur), which contains 60 µg of haemagglutinin (HA) antigen per strain, generates higher antibody responses and greater effectiveness than SD influenza vaccinesCitation6,Citation7: Evidence from a phase IIIb–IV, multi-centre, double blinded RCT demonstrated that HD TIV is more efficacious versus SD TIV in adults 65+Citation8, a finding which has been consistently supported by multiple observational studiesCitation9–18 and a meta-analysis covering over 12 million HD TIV recipients across nine influenza seasonsCitation6,Citation19. Furthermore, standard dose vaccines are not recommended by the Joint Committee on Vaccination and Immunization (JCVI). Instead, high dose and adjuvanted vaccines are recommended for adults 65+Citation20.
Adjuvant TIV (aTIV, Flaud, Seqirus) contains 15 µg HA antigen per strain with the addition of MF59 oil-in-water emulsion of squalene oilCitation21. There is a scarcity of data comparing aTIV with active vaccines in RCTs and moreover there is inconsistency in observational study findings published in the literature. Four out of six observational studies included in a systematic review did not identify statistically significant differences between aTIV and other SD influenza vaccinesCitation22; and a recent case control study in the UK did not identify significant benefits for aTIV versus non-adjuvant vaccines for the 2018/19 influenza seasonCitation23. Furthermore, a systematic literature review by the European Centre for Disease Prevention and Control found no statistical difference in relative effectiveness between aTIV and non-adjuvanted TIV and QIV vaccines in adult populationsCitation24. Therefore, the in vivo benefits of aTIV remain uncertain. Furthermore, a recent randomized clinical trial found that aQIVs failed to demonstrate superiority versus a non-influenza vaccine in older adults during seasons with high amounts of vaccine strain mismatchCitation25.
The respective difference in the evidence profiles for HD TIV and aTIV is reflected in assessments made by the National Advisory Committee on Immunization in Canada (NACI). In a literature review, NACI states that there is good quality, “grade A”, evidence indicating superiority for high dose vaccines versus SD TIV, whereas the effectiveness evidence for adjuvant vaccines versus unadjuvanted vaccines is “insufficient”Citation26. A retrospective cohort study on more than 12 million fee-for-service US Medicare beneficiaries found high-dose egg-based vaccines were not significantly different to adjuvanted egg-based vaccines, which was 7.5% more effective than standard (i.e. nonadjuvanted) vaccinesCitation12. A second retrospective cohort study of over 2 million people aged 65+ in the USA indicates HD TIV may be up to 12% more effective than aTIV in preventing respiratory related hospitalizationsCitation27.
If HD TIV is effective over aTIV, its preferential use in the 65+ population could reduce the impact of influenza and may have public health implications, particularly by reducing hospitalizations and early mortality which are key contributors to the overall economic burden of influenzaCitation28.
Aims and objectives
The main objective of this analysis was to determine the cost-effectiveness of HD TIV versus aTIV for adults 65+ in England and Wales from a UK healthcare perspective by utilizing the best available evidence to date. A further objective was to explore cost-effectiveness results across several deterministic scenarios, thus accounting for uncertainty in the effectiveness evidence for aTIV. In addition, we established the cost-effectiveness of HD TIV versus aTIV for (i) a primary analysis which defined hospitalizations explicitly due to influenza/pneumonia and (ii) a secondary analysis which included all hospitalizations due to any lower respiratory infection.
Methods
Model perspective and structure
This was a cost-utility analysis, adopting a UK healthcare perspective including costs incurred by the NHS and Prescribed Specialized Services (PSS) and health benefits as quality adjusted life years (QALYs). The analysis estimated the incremental cost-effectiveness ratio (ICER) of HD TIV versus aTIV, assuming a cost-effectiveness threshold (CE threshold) between £20,000 and £30,000 per QALY, as stipulated in the NICE reference caseCitation29.
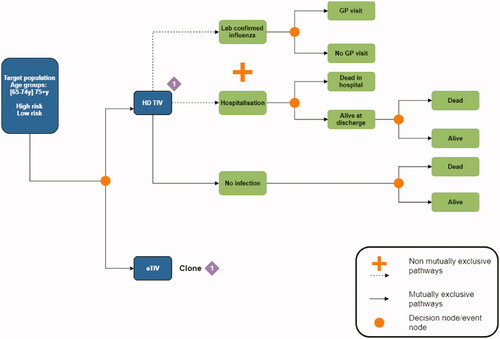
We constructed a decision tree to compare the cost-effectiveness of HD TIV with aTIV over a single influenza season. The decision tree included two disease pathways following vaccination: (i) Laboratory confirmed influenza (LCI) cases that could result in a general practitioner (GP) visit and (ii) hospital stays which could lead to premature death (). The primary analysis defined hospitalizations specifically due to influenza or pneumonia which was expanded to include all respiratory related hospitalizations in a secondary analysis. The two disease pathways were not mutually exclusive,Footnotei meaning populations could enter one, both, or neither following vaccination. The model was conducted over a single average influenza season. A lifetime horizon for QALYs was adopted by establishing life expectancies for populations who were discharged from hospital and to a third non-disease pathway who were neither LCI cases nor hospital attendees. To avoid double counting we included influenza related mortality as an outcome on the hospitalization, but not the LCI, disease pathway.
Target population
The target population included adults 65+ in England and Wales stratified into three groups according to their age and risk of influenza-related complications: (i) People aged 65–74 with chronic respiratory disease or chronic heart disease, who are defined as “high risk” as their underlying condition predisposes them to influenza related complications; (ii) people aged 65–74 without underlying medical conditions who are at relatively lower risk of complications; and (iii) all people aged 75 and over including both low and high-risk groups. Age groups were analysed separately due to differences in vaccination coverage, vaccination effectiveness, and the likelihood of GP or hospital attendance if infected. Due to limitations in available data it was only possible to differentiate the age 65–74 risk groups by likelihood of GP attendance. Mean costs and QALYs were calculated independently for each of the three populations. Cost-effectiveness results were pooled across the full population of over 65s by calculating a weighted average across the stratified age and risk groups. We also report cost-effectiveness results separately for the 65–74 (pooled across high and low risk) and 75+ age groups. Population parameters by age and risk group are reported in .
Table 1. Model input parameters by age and risk group.
Model parameters
Vaccination coverage
The model included both vaccinated and unvaccinated populations. The probability of vaccination was estimated for each age and risk group separately. To establish the percentage of the population receiving the HD TIV and aTIV vaccination we applied typical coverage rates as described in Public Health England Annual Flu reportsCitation30, equal to 62.7% in people aged 65–74 (for both low and high and risk groups), and 80% in the 75+ age group.
Probability of lab confirmed influenza
To calculate the probability of LCI in the unvaccinated cohort, we applied a baseline influenza attack rate of 7.50%, this being the mid-point of global influenza attack rates (between 5% and 10%) reported by the World Health Organization for all adult populations, that is, not specific for populations aged 65+Citation31. Probabilities for the vaccinated cohort were calculated by multiplying the baseline influenza attack rate for the unvaccinated cohort by the vaccine effectiveness (VE) of HD TIV and aTIV versus no vaccination.
Probability of hospitalization
The probability of hospitalization was calculated using age stratified rates for pneumonia and influenza related hospitalizations, defined as ICD10 codes J09-J18, from a longitudinal studyCitation32. We applied a single baseline probability for hospitalization across both the vaccinated and unvaccinated cohorts as the original studyCitation32 utilized Clinical Practice Research Database (CPRD) and Hospital Episode Statistics (HES) data, therefore establishing rates in a UK population where SD TIV was available. Probabilities for the vaccinated cohort were then adjusted based on VE for HD TIV and aTIV versus no vaccination.
Vaccination effectiveness
The key inputs into the economic model were: (i) VE, which measures the proportion reduction in cases among vaccinated versus unvaccinated populations; and (ii) relative vaccine effectiveness (rVE), which measures the proportion reduction in cases for populations vaccinated with one vaccine relative to another vaccine. Due to restrictions in data availability, VE for both HD TIV and aTIV (versus no vaccination) were estimated indirectly by rearranging rVE estimates for HD TIV and aTIV versus SD TIV and VE estimates for SD TIV (versus no vaccination).Footnoteii
We calculated the VE for SD TIV on LCI cases as a weighted average across influenza A, matched B, and mismatched B. The VE for influenza type A and matched B were both equal to 46% based on a pooled estimate across both lineages in observational evidence across the 2005–2008 UK influenza seasonsCitation33. The mismatched status of influenza B was defined as a poor match between the vaccine and the circulating strain. Consequently, mismatched influenza B was assigned a reduced VE of 28% based on a published cost-effectiveness studyCitation34. To pool VE for SD TIV, we estimated the mean percentage of circulating influenza virus from annual Public Health England (2010–2017) influenza reports as equal to 78% for influenza A, 10.7% for matched influenza B, and 11.3% for mismatched influenza BCitation30. The VE for SD TIV on hospitalizations could not be disaggregated and was set equal to 28% across all strains and subtypes based on a published cost-effectiveness studyCitation34.
The rVE against lab-confirmed influenza of HD TIV versus SD TIV for the LCI pathway was sourced from a phase 3b/IV randomized efficacy trial and equal to 24.2%Citation8. Meanwhile, the rVE for HD TIV versus SD TIV on hospitalization endpoints was equal to 24.3%, this being obtained from a meta-analysis informed by two RCTs and three observational studies and covering multiple influenza seasonsCitation6. The rVE estimates indicate that for every 100 influenza cases in populations vaccinated with SD TIV, there would be roughly 76 cases in populations vaccinated with HD TIV (i.e. a 24% reduction).
Despite large observational studies being conducted to evaluate the effectiveness of influenza vaccinesCitation12, due to inconsistent results from season to seasonCitation22, randomized clinical trials (RCTs) remain the gold standard when assessing vaccine efficacy. Therefore, the economic model used values from RCTs whenever available.
The 2021 Ständige Impfkommission (STIKO) guideline systematic review and the 2020 European Centre for Disease Prevention and Control (ECDC) systematic review both identified one RCT reporting on rVE of HD-TIV compared with conventional vaccination in LCI. The rVE of aTIV in preventing LCI was compared only with unvaccinated populations or standard dose vaccines in non-randomized studiesCitation35,Citation36. No RCTs or observational studies compared aTIV and HD-TIV directlyCitation35,Citation36. Due to this scarcity of trial-based evidence and heterogeneous findings from observational studies, three different rVE scenarios were investigated for aTIV versus SD TIV. The base case (scenario 1) applied an rVE of 0% on both LCI and hospitalization endpoints reflecting NACI evidence assessments and the non-significant differences identified between aTIV and SD TIV in multiple observational studiesCitation22,Citation23,Citation26. The second scenario applied an rVE of 6% on LCI endpoints based on a non-significant effect size from a Spanish observational studyCitation37, and assumed an rVE of 10% on hospitalization endpoints.Footnoteiii Finally, scenario 3 applied the largest rVE effectiveness estimates of 12% on LCI and 20% on hospitalization endpoints: The former estimate was informed by an observational study in an Italian populationCitation38 which has been challenged for its academic quality by NACICitation26; the latter estimate mirrored an assumption in a published economic model assessing the cost-effectiveness of aTIV versus SD TIV, however this was not based on either RCT or observational dataCitation39. All VE and rVE for the base case and scenario analyses are summarized in .
Table 2. Vaccine effectiveness/relative vaccine effectiveness.
Probability of general practitioner visits
We calculated the probability of GP attendance following LCI separately for the 65–74 and 75+ age groups by dividing the total number of GP attendances due to influenza by the total number of people in the population with influenza (i.e. influenza attack rate multiplied by total population size). Data for the numerator were sourced from a published linear regression analysis estimating the total number of people in England and Wales who attended influenza related GP appointments. This was stratified by age in a database utilizing Hospital Episode Statistics (HES)Citation40.
Probability of mortality
The probability of premature mortality following hospitalization was obtained directly from published literature which used HES data to estimate the percentage of patients who died following hospitalization for influenza or pneumonia by age group, defining cases by ICD-10 code (J09-18Footnoteiv)Citation41. We obtained age-group specific absolute risks of mortality due to any cause from age- and gender-weighted life tables published by the Office for National StatisticsCitation42.
Quality adjusted life years
We calculated gender weighted life expectancies by age group using the mortality data from UK life tables to establish the expected remaining life years for subjects who survived the influenza seasonCitation42. Total quality adjusted life years (QALYs) were then calculated by aggregating health utilities across subjects’ remaining life years whilst incorporating utility reductions for any LCI cases or hospitalizations. Baseline utility values by age group are reported in and were obtained from the published literature based on self-reported UK EQ-5D scoresCitation43. We applied utility reductions per LCI case using influenza specific utility values from an HTA reportCitation44 and assuming a duration of infection equal to 6 days. The total disutility per influenza related hospitalization was obtained from a published cost-effectiveness analysis which reported summed QALY detriments for hospital patients with uncomplicated influenza like illness and pneumococcal pneumoniaCitation34. All QALYs occurring after 12-months were discounted at 3.5%.
Table 3. Unit costs (2018 GB£) and QALYs.
Unit costs
All unit costs are reported in . Vaccination costs for HD TIV and aTIV were obtained from Sanofi/Seqirus list prices and applied to the proportion of the population who received the vaccination (i.e. the vaccination coverage rate). All administration costs were excluded as this was an incremental analysis and both vaccination procedures and coverage rates were assumed to be equivalent. GP consultation costs were obtained from a published UK based cost-effectiveness analysis which reported the mean primary care staff and prescription costs (2008 GB£) per influenza episode utilizing the General Practice Research DatabaseCitation45. Costs (2008 GB£) per hospitalisation were taken from the same cost-effectiveness study, which calculated the mean length of stay for all LCI hospitalizations (ICD code J10) by daily NHS reference costs (tariff DZ11B for lobar, atypical or viral pneumonia without complications)Citation45. All unit costs from published literature were inflated to 2018 values using the Hospital and Community Services Pay and Prices indexCitation46. Discounting was not required for costs as these occurred across a single influenza season, within a year of vaccination.
Uncertainty analysis
Uncertainty was investigated for the primary analysis using one-way deterministic sensitivity analyses (DSA) which individually varied input parameters and recalculated ICERs according to the altered values. The DSA was limited to the scenario with the highest efficacy rates for aTIV. A Tornado plot was generated with parameter ranges equal to 95% confidence intervals or ±15% of the mean if confidence intervals were not available. The DSA included scenarios which reduced the rVE of HD TIV versus SD TIV from (i) 24.3% to 17% for influenza related hospitalizations, and (ii) 24.2% to 9.70% for LCI cases. In addition, we investigated uncertainty around HD TIV vaccination costs by identifying the economically justifiable price (EJP). We calculated the EJP by identifying the maximum price at which HD TIV remained cost-effective, that is, achieved an ICER below the CE threshold of £20,000 and £30,000 per QALY.
A secondary analysis was also conducted which expanded the influenza and pneumonia hospitalizations outcome to include hospitalizations due to any lower respiratory infection. Whilst providing a less specific endpoint for influenza, the outcome was selected for the secondary analysis to include data from a retrospective observational study which directly compared the rVE of HD TIV and aTIVCitation27. For the secondary analysis, we estimated rates of hospitalization by age group using HES databases including all non-elective admissions with primary respiratory diagnosis (defined as ICD10 codes J00-J99) in four November–May influenza seasons between 2015 and 2018Citation47. Vaccine efficacy rates for HD TIV and aTIV (versus no vaccination) were calculated by rearranging equations for rVE of HD TIV versus aTIV and VE for SD TIV ().Footnotev
Table 4. Model inputs used in the secondary analysis (all respiratory hospitalizations).
Results
Primary analysis: pneumonia/influenza hospitalizations
High dose TIV was found to be cost-effective in adults aged 65+ in England and Wales when compared with aTIV, assuming the lower CE threshold estimate equal to £20,000 (). The results for the primary analysis were robust across all three aTIV influenza/pneumonia hospitalization effectiveness scenarios with ICERs equal to £1,932 per QALY for the base case (scenario 1), £4,181 per QALY for scenario 2, and £8,767 per QALY for scenario 3.Footnotevi
Table 5. Cost-effectiveness results for HD TIV versus aTIV (2018 GB£).
The net impact of HD TIV versus aTIV on population health and NHS costs is displayed in . Across a single influenza season, HD TIV was predicted to result in substantially fewer cases of laboratory confirmed influenza, hospitalizations, and influenza-related deaths when compared with populations vaccinated with aTIV. Across the lifetime, HD TIV achieved 11,460 (scenario 1), 8,539 (scenario 2), and 5,618 (scenario 3) additional QALYs when compared with aTIV. Populations who received HD TIV had a higher total cost burden, with incremental population costs versus aTIV equal to £22,144,118 (scenario 1), £35,700,436 (scenario 2), and £49,256,755 (scenario 3). Differences in healthcare costs were driven by higher vaccination costs for HD TIV, which exceeded cost savings attributable to reductions in primary and secondary healthcare resources required for influenza treatment ().
Table 6. Net population impact of HD TIV and aTIV for one influenza season (2018 GB£).
The EJP analysis indicated that uncertainty in the price of HD TIV was not likely to impact on the cost-effectiveness findings. The price of HD TIV could be increased from £20.00 (list price) to £47.91 (scenario 1), £38.21 (scenario 2), and £28.50 (scenario 3) whilst remaining cost-effective versus aTIV at a CE threshold of £20,000 per QALY. When applying a CE threshold of £30,000 per QALY, the EJP of HD TIV increased to £63.37 (scenario 1), £49.72 (scenario 2), and £36.08 (scenario 3).
Age group specific results (primary analysis, base case scenario)
Results for the primary analysis and base case rVE for aTIV differed when stratifying populations by age group. The ICER for HD TIV versus aTIV decreased when populations were restricted to those aged 75 years and older, therefore remaining cost-effective at CE thresholds <£20,000 for all scenarios. In contrast, the ICERs increased when populations were limited to those aged 65–74: HD TIV remained cost-effective for the rVE aTIV effectiveness assumption in scenarios 1 and 2 but was not cost-effective for scenario 3 where ICERs exceeded the upper CE threshold of £30,000. The difference in results across age groups was driven by higher rates of hospitalization for people aged over 75 resulting in substantially more hospitalizations avoided for populations who received HD TIV ().
Table 7. Cost-effectiveness results by age group: HD TIV versus aTIV (2018 GB£).
Secondary analysis (all respiratory hospitalizations)
Cost-effectiveness results were robust for the secondary analysis, which used direct rVE evidence for HD TIV versus aTIV on expanded endpoints including all respiratory hospitalizations. The ICER for HD TIV versus aTIV was equal to £2,800, with incremental population costs equal to £13,685,101 and incremental lifetime population QALYs equal to 4,888. The economic analysis estimated 21,128 respiratory related hospitalizations per influenza season would be avoided if adults 65+ were vaccinated with HD TIV rather than aTIV.
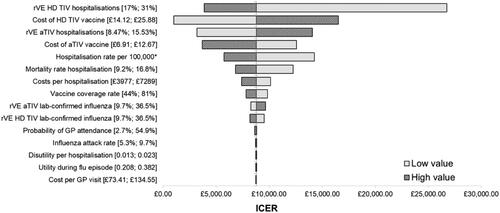
Deterministic sensitivity analysis
Deterministic sensitivity analysis was conducted for aTIV scenario 3 as this was the most uncertain scenario with an ICER closest to the CE threshold. Results were robust with HD TIV remaining cost-effective versus aTIV for all DSA scenarios when applying a £30,000 per QALY CE threshold and all but one DSA scenario when applying the £20,000 per QALY CE threshold (). The DSA identified the rVE of HD TIV on hospitalization outcomes as an important area of uncertainty as reducing parameter values from 24.2% (base case) to 17.17% (DSA low value) increased the ICER to £26,707. In contrast, the DSA which reduced the rVE of HD TIV on LCI cases from 24.3% (base case) to 9.70% (DSA low value) had a minimal impact only increasing the ICER slightly to £9,562. The DSA identified vaccination costs and hospitalization rates as drivers of the cost-effectiveness, meanwhile varying epidemiological utility had a relatively minor impact on the ICER ().
Discussion
This cost-utility analysis using the best available evidence to date identified HD TIV as cost-effective versus aTIV in populations aged 65+, when adopting a healthcare payer perspective and assuming a CE threshold of £20,000 per QALY. The additional vaccination costs associated with HD TIV were outweighed by substantial benefits achieved through preventing cases of influenza and subsequent reductions to health morbidity, mortality, and primary and secondary healthcare costs. Our results are informed by rVE and rely on a positive VE for SD TIV. The VE for SD TIV may be substantially reduced if there is a poor match between the vaccine and circulating influenza strainsCitation4. Therefore, our results are most applicable to future influenza seasons with similar levels of influenza (78% influenza A, 11% for matched influenza B, and 11% mismatched B) and VE for SD TIV (46% influenza A, 46% influenza B, 28% mismatched B).
The results of the uncertainty analysis showed the cost-effectiveness of HD TIV was robust to changes in the majority of parameter values. Due to the model assumptions, the rVE of HD TIV versus SD TIV on hospitalizations was a key driver of cost-effectiveness. However, even when using an rVE parameter value equal to the lower 95% confidence interval (as reported in the multi-season influenza meta-analysisCitation6) the resultant ICER was less than the upper CE threshold of £30,000 per QALY recommended in the NICE reference caseCitation29. Reductions in the number of hospitalizations are likely to be an important outcome for healthcare providers, particularly during busy winter seasons when demand for healthcare and hospital bed days often exceeds resource supplyCitation48.
This is the first economic analysis to compare the cost-effectiveness of HD TIV with aTIV. Findings from this analysis could be used to inform future UK vaccination recommendations which require both clinical and cost-effectiveness evidence. We estimate a substantial reduction in the number of influenza cases, influenza-related hospitalizations, and influenza-related deaths with a relatively modest increase in vaccination costs in adults 65+ who are offered HD TIV rather than aTIV.
Our model estimated around 500,000 cases of influenza occur in adults 65+ during a single influenza season in England and Wales, costing the NHS roughly £400 million and resulting in 50,000 hospitalizations and over 10,000 deaths. Roughly 70% of the cost burden and over 85% of influenza related deaths occurred in populations aged 75 and over, despite this age group only containing around 45% of the total population in the model. The increasing health burden of influenza with age explains results from the scenario analysis where ICERs for HD TIV versus aTIV were decreased (i.e. increased cost-effectiveness) when populations were restricted to the 75 years and older age group. Based on findings from the published literature we assumed rates of pneumonia/influenza related hospitalizations are over four times higher in those aged over 75s when compared with 65–74Citation32, with the older population also twice as likely to die following hospitalizationCitation41.
The population impact of influenza is slightly higher in our analysis than annual estimates from published burden of illness studies. Green et al.Citation49 estimated mortality for adults 65+ in England and Wales ranged from 4,000 to 13,000 across six influenza seasons. Matias et al.Citation41 estimated mean UK influenza deaths and hospitalizations for adults 65+ of 6,270 and 15,214, respectively. Meanwhile, Moss et al.Citation50 calculated UK 18+ hospitalizations due to influenza equal to 46,215 (2017/18) and 39,670 (2018/19). The primary driver of population outcomes in our model were the influenza attack rate and hospitalization rate. For HD TIV the DSA applying the lower attack rate reduced total influenza cases from 468,857 to 312,571 and the DSA applying the lower hospitalization rate reduced influenza deaths to 7,297 and hospitalizations to 31,439. Neither the attack rate nor hospitalization rate were key drivers of cost-effectiveness as both DSAs resulted in an ICER below £20,000 per QALY.
This analysis is subject to several limitations. Firstly, our literature review did not identify any clinical trial which directly established the rVE of HD TIV versus aTIV on influenza and pneumonia hospitalizations, the adopted primary endpoints. This gap is confirmed by the ECDC and STIKO systematic reviews that did not find head-to-head studies comparing aTIV and HD-TIVCitation35,Citation36. Furthermore, both reviews concur on the scarcity of robust trial data and inconsistency of published evidence to inform rVE estimates of aTIV versus SD TIV; hence we could not identify nor conduct a robust indirect treatment comparison or network meta-analysis. This was in-line with the STIKO and ECDC guidelines, which only reported on one RCT estimating efficacy against LCI with HD-TIV compared with SD-TIVCitation35,Citation36 that we used for model inputs. Conversely, neither of these groups used observational studies to assess the impact of HD-TIV against LCI, and the ECDC guidelines noted that “pooled analyses of effectiveness data comparing adjuvant with non-adjuvanted vaccines was restricted by limited study numbers and statistical and clinical heterogeneity”Citation35. Consequently, vaccine benefits were estimated using the best available rVE estimates for HD TIV and aTIV versus a common comparator, SD TIV. The results from the primary analysis were supported by the secondary analysis which incorporated direct evidence for HD TIV versus aTIV on all respiratory hospitalization outcomes. It should be noted that the ECDC systematic review did identify observational studies to derive rVE estimates. However, the cost-effectiveness model in this study used RCT derived inputs where possible and assessed their uncertainty through sensitivity analysis. This is a limitation of the parameter inclusion criteria used as part of this study. Future studies are required to explore the estimated cost-effectiveness of influenza vaccines if observational studies were also included to derive rVE estimates.
Secondly, quadrivalent vaccines (both HD QIV and aQIV) were not modelled due to a lack of data. However, there is no reason to assume the comparative findings of HD TIV versus aTIV should differ from HD QIV versus aQIV. Essink et al.Citation51 found that aQIV induces a similar immune response as aTIV and has a comparable reactogenicity and safety profile. Adjuvant QIV has shown superior immunogenicity over aTIV against the additional B strain. Similarly, HD QIV is comparable to HD TIV in terms of reactogenicity and safety profile, but has a superior induced immune-response to HD TIVCitation52. Given that the quadrivalent vaccine shows similar improvements over both the HD TIV and aTIV, it is likely that what applies for aTIV versus HD TIV will also apply to aQIV versus HD QIV.
Next, to account for underlying uncertainty in aTIV rVE parameter values, results were obtained for three effectiveness scenarios informed through an assumption in the published literatureCitation39 and using observational study dataCitation37,Citation38. A scenario analysis in one of the observational studies by Mannino et al.Citation38 reported a higher aTIV rVE estimate (equal to 25%) than for the one for HD TIV, issued from an RCT. Whilst it is possible that the rVE of aTIV exceeds HD TIV, concurrent appraisals suggest it unlikely as: there is direct evidence of increased effectiveness for HD TIV versus aTIV on a wider outcome including all respiratory hospitalizations; the scenario analysis by Mannino et al.Citation38 was limited to the weeks with peak circulating influenza virus which may overstate effectiveness when compared with the studies conducted over a full influenza seasonCitation6; and a systematic review found that only two out of six observational studies identified statistically significant increases in rVE for aTIV versus SD TIVCitation22; NACI and ECDC systematic reviews concluded on a lack of consistent evidence for aTIV superior to SD-TIV.
We were not able to combine GP attendance and hospitalization within a single influenza pathway due to differences in case definitions from the underlying evidence. Therefore, our decision model was structured to include non-mutually exclusive pathways where populations could contract both LCI and attend hospital due to influenza/pneumonia. Both pathways were included to avoid underestimating the cost-effectiveness of HD TIV as both GP visits and hospitalization are key contributors to the overall health and economic burden of influenzaCitation39,Citation45. We avoided double counting effects on life years by restricting mortality effects to the hospitalization pathway. Minimal double counting of influenza related disutility may have occurred if people had both LCI and a hospital attendance, however disutility was not show to impact cost-effectiveness in the DSA. Our decision model may underestimate cost-effectiveness for HD TIV as it does not model influenza population transmission. Herd effects could have been included using a dynamic transmission model, however, this type of modelling was not selected given the disparity between data requirements and data availability.
Due to a lack of available data, vaccine related adverse events (AEs) were not included in the analysis, but this is not likely to impact the findings given the strong safety profiles for both HD TIVCitation52 and aTIV. Furthermore, a recent study has shown there to be similar safety profiles between both vaccines in those aged 65 years and olderCitation53. Limited data also meant the age 65–74 risk groups were only differentiated by the likelihood of a GP visit following infection. Higher risk populations may have increased vaccine coverage, hospitalization, and mortality rates and therefore grouping parameter values may have underestimated the cost-effectiveness of HD TIV in this sub-population. Furthermore, the HD TIV cost-effectiveness may have been underestimated for all risk and age groups as influenza is likely to be linked to several cardiorespiratory complications that were not included within the influenza related hospitalization endpoint.
Additionally, the high-risk group was defined as those with respiratory and heart diseases as these individuals are likely to experience the biggest impacts of influenza. The high-risk group could be expanded in future studies to also include those with diabetes, renal diseases, immunocompromised and other chronic conditions, providing there is sufficient data available to allow for their inclusion in the analysis.
COVID-19
It should be noted that the inputs collected to feed the cost-effectiveness model were based on data prior to the COVID-19 pandemic, and therefore they may change in the forthcoming years. On one hand, it is possible that public health measures (e.g. masks, social distancing, and lockdowns) would decrease the spread of respiratory viruses and result in higher ICERs (i.e. HD TIV less cost-effective). Furthermore, by missing two influenza seasons, unpredicted mutations may result in vaccines that are less effective in general. On the other hand, due to this absence of influenza virus circulation, a strong epidemic rebound may occurCitation54 that would decrease the ICERs (i.e. HD TIV more cost-effective). Moreover, in light of the COVID-19 pandemic, influenza vaccine coverage increase (e.g. people more likely to get the influenza vaccine, and/or co-administration of influenza and COVID-19 booster) would result in further reduction of ICERs. Finally, the impact of COVID-19 on future government overall spending on immunization – and its prioritization – is currently unknown, as well as how the broader benefits of vaccination could be considered when assessing vaccines’ value for the society. Future work is required to update parameter values and reassess cost-effectiveness when relevant data becomes available regarding the impact of the COVID-19 pandemic.
Conclusion
This is the first study to establish the cost-effectiveness of HD TIV versus aTIV in a population aged over 65. In the UK, vaccination with HD TIV instead of aTIV substantially reduced influenza related GP visits, hospitalizations, and mortality. The analysis was limited by a lack of robust and consistent effectiveness data for aTIV, which should be a priority area for future research.
Transparency
Declaration of funding
This work was funded by Sanofi Pasteur. Sanofi Pasteur were involved in the design of the economic model, analysis, and interpretation of results as well as the writing of the manuscript.
Declaration of financial/other relationships
IG, NL, FPA and SC are employees of Sanofi Pasteur, which manufactures HD-TIV and may/may not hold shares in the company.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (25.8 KB)Acknowledgements
None reported.
Notes
i Further discussion of the non-mutually exclusive pathways is provided in the Limitations section.
ii See Supplementary Material A for detailed methodology.
iii The rVE estimate of 10% on hospitalization endpoints was applied as this is the mid-point between the lower and upper values applied in the base case scenario (0%) and scenario 3 (20%). The proportional increase in rVE estimates for aTIV on hospitalization endpoints is consistent with the proportional increase on LCI endpoints across each scenario (0%, 6%, 12%).
iv Mortality estimates for the scenario analysis were obtained from published study by Matias et al. (2016)41. who defined hospitalization due to any respiratory disease using ICD codes J00-99.
v See Supplementary Material B for full details.
vi See for rVE of aTIV per scenario.
References
- Mertz D, Kim TH, Johnstone J, et al. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ. 2013;347:f5061.
- Agor JK, Özaltın OY. Models for predicting the evolution of influenza to inform vaccine strain selection. Hum Vaccin Immunother. 2018;14(3):678–683.
- World Health Organization. Influenza (seasonal) fact sheet [Internet]. Geneva (Switzerland): WHO; 2016 [cited 2019 Sep 25]. Available from: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
- Osterholm MT, Kelley NS, Sommer A, et al. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(1):36–44.
- Sasaki S, Sullivan M, Narvaez CF, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest. 2011;121(8):3109–3119.
- Lee JK, Lam GK, Shin T, et al. Efficacy and effectiveness of high-dose versus standard-dose influenza vaccination for older adults: a systematic review and meta-analysis. Expert Rev Vaccines. 2018;17(5):435–443.
- Robertson CA, DiazGranados CA, Decker MD, et al. Fluzone® high-dose influenza vaccine. Expert Rev Vaccines. 2016;15(12):1495–1505.
- DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371(7):635–645.
- DiazGranados CA, Dunning AJ, Jordanov E, et al. High-dose trivalent influenza vaccine compared to standard dose vaccine in elderly adults: safety, immunogenicity and relative efficacy during the 2009-2010 season. Vaccine. 2013;31(6):861–866.
- Gravenstein S, Davidson HE, Han LF, et al. Feasibility of a cluster-randomized influenza vaccination trial in U.S. nursing homes: lessons learned. Hum Vaccin Immunother. 2018;14(3):736–743.
- Gravenstein S, Davidson HE, Taljaard M, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccination on numbers of US nursing home residents admitted to hospital: a cluster-randomised trial. Lancet Respir Med. 2017;5(9):738–746.
- Izurieta HS, Chillarige Y, Kelman J, et al. Relative effectiveness of influenza vaccines among the United States elderly, 2018-2019. J Infect Dis. 2020;222(2):278–287.
- Izurieta HS, Thadani N, Shay DK, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in US residents aged 65 years and older from 2012 to 2013 using medicare data: a retrospective cohort analysis. Lancet Infect Dis. 2015;15(3):293–300.
- Richardson DM, Medvedeva EL, Roberts CB, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccination in community-dwelling veterans. Clin Infect Dis. 2015;61(2):171–176.
- Robison SG, Thomas AR. Assessing the effectiveness of high-dose influenza vaccine in preventing hospitalization among seniors, and observations on the limitations of effectiveness study design. Vaccine. 2018;36(45):6683–6687.
- Shay D, Chillarige Y, Kelman J, et al. Comparative effectiveness of High-Dose versus Standard-Dose influenza vaccines among US medicare beneficiaries in preventing postinfluenza deaths during 2012-2013 and 2013-2014. J Infect Dis. 2017;215(4):510–517.
- Young-Xu Y, Snider JT, van Aalst R, et al. Analysis of relative effectiveness of high-dose versus standard-dose influenza vaccines using an instrumental variable method. Vaccine. 2019;37(11):1484–1490.
- Young-Xu Y, Van Aalst R, Mahmud SM, et al. Relative vaccine effectiveness of high-dose versus Standard-Dose influenza vaccines among veterans health administration patients. J Infect Dis. 2018;217(11):1718–1727.
- Lee JK, Lam GK, Shin T, et al. Efficacy and effectiveness of high-dose influenza vaccine in older adults by circulating strain and antigenic match: an updated systematic review and meta-analysis. Vaccine. 2021;39:A24–A35.
- Joint Committee on Vaccination and Immunisation. JCVI advice on influenza vaccines for 2021-2022 [Internet]. London (UK): JCVI; 2020 [cited 2021 Jul 19]. Available from: https://app.box.com/s/t5ockz9bb6xw6t2mrrzb144njplimfo0/file/737845224649)
- Haq K, McElhaney JE. Immunosenescence: influenza vaccination and the elderly. Curr Opin Immunol. 2014;29:38–42.
- Domnich A, Arata L, Amicizia D, et al. Effectiveness of MF59-adjuvanted seasonal influenza vaccine in the elderly: a systematic review and meta-analysis. Vaccine. 2017;35(4):513–520.
- Pebody RG, Whitaker H, Ellis J, et al. End of season influenza vaccine effectiveness in primary care in adults and children in the United Kingdom in 2018/19. Vaccine. 2020;38(3):489–497.
- European Centre for Disease Prevention and Control. Systematic review of the efficacy, effectiveness and safety of newer and enhanced seasonal influenza vaccines for the prevention of laboratory confirmed influenza in individuals aged 18 years and over. Stockholm (Sweden): ECDC; 2020.
- Beran J, Reynales H, Poder A, et al. Prevention of influenza during mismatched seasons in older adults with an MF59-adjuvanted quadrivalent influenza vaccine: a randomised, controlled, multicentre, phase 3 efficacy study. The Lancet Infect Dis. 2021;21(7):1027–1037.
- National Advisory Committee on Immunization. Literature review update on the efficacy and effectiveness of High-Dose (fluzone® High-Dose) and MF59-adjuvanted (fluad®) trivalent inactivated influenza vaccines in adults 65 years of age and older. Ottawa (Canada): PHAC; 2018.
- van Aalst R, Gravenstein S, Mor V, et al. Comparative effectiveness of high dose versus adjuvanted influenza vaccine: a retrospective cohort study. Vaccine. 2020;38(2):372–379.
- Trucchi C, Paganino C, Orsi A, et al. Hospital and economic burden of influenza-like illness and lower respiratory tract infection in adults ≥50 years-old. BMC Health Serv Res. 2019;19(1):585.
- National Institute for Health and Care Excellence. Guide to the methods of technology appraisal [Internet]. London (UK): NICE; 2013 [cited 2021 March 9]. Available from: https://www.nice.org.uk/process/pmg9/chapter/the-reference-case
- Public Health England. Annual flu reports [Internet]. London (UK): PHE; 2018 [cited 2019 Nov 19]. Available from: https://www.gov.uk/government/statistics/annual-flu-reports
- World Health Organization. WHO global epidemiological surveillance standards for influenza. Geneva (Switzerland); WHO.
- Millett ER, Quint JK, Smeeth L, et al. Incidence of community-acquired lower respiratory tract infections and pneumonia among older adults in the United Kingdom: a population-based study. PLoS One. 2013;8(9):e75131.
- Fleming DM, Andrews NJ, Ellis J, et al. Estimating influenza vaccine effectiveness using routinely collected laboratory data. J Epidemiol Community Health. 2010;64(12):1062–1067.
- Baguelin M, Camacho A, Flasche S, et al. Extending the elderly-and risk-group programme of vaccination against seasonal influenza in England and Wales: a cost-effectiveness study. BMC Med. 2015;13(1):236.
- European Centre for Disease Prevention and Control. Systematic review of the efficacy, effectiveness and safety of newer and enhanced seasonal influenza vaccines 2020 [Internet]. Solna (Sweden): ECDC; 2020 [cited 9 Mar 2021]. Available from: https://www.ecdc.europa.eu/en/publications-data/seasonal-influenza-systematic-review-efficacy-vaccines
- Michaelis K, Scholz S, Buda S, et al. Beschluss und wissenschaftliche begründung der ständigen impfkommission (STIKO) für die aktualisierung der Influenza-Impfempfehlung für personen im alter von ≥ 60 jahren. 2020;(1):3–25. Available from: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2021/Ausgaben/01_21.pdf?__blob=publicationFile
- Puig-Barberà J, Natividad-Sancho A, Calabuig-Pérez J, et al. MF59-adjuvanted and virosomal influenza vaccines for preventing influenza hospitalization in older people: comparative effectiveness using the Valencia health care information system. Vaccine. 2013;31(37):3995–4002.
- Mannino S, Villa M, Apolone G, et al. Effectiveness of adjuvanted influenza vaccination in elderly subjects in Northern Italy. Am J Epidemiol. 2012;176(6):527–533.
- Thorrington D, van Leeuwen E, Ramsay M, et al. Assessing optimal use of the standard dose adjuvanted trivalent seasonal influenza vaccine in the elderly. Vaccine. 2019;37(15):2051–2056.
- Pitman R, Melegaro A, Gelb D, et al. Assessing the burden of influenza and other respiratory infections in England and Wales. J Infect. 2007;54(6):530–538.
- Matias G, Taylor RJ, Haguinet F, et al. Modelling estimates of age-specific influenza-related hospitalisation and mortality in the United Kingdom. BMC Public Health. 2016;16(1):481.
- Office for National Statistics. National life tables Great Britain (2014-2016) [Internet]. Newport (UK): Office for National Statistics; 2017 [cited 2019 Nov 19]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables
- Szende A, Janssen B, Cabases J. Self-reported population health: an international perspective based on EQ-5D. Dordrecht (The Netherlands): Springer; 2014.
- Turner DA, Wailoo AJ, Nicholson KG, et al. Systematic review and economic decision modelling for the prevention and treatment of influenza A and B. Health Technol Assess. 2003;7(35):1–170.
- Pitman R, Nagy L, Sculpher MJV. Cost-effectiveness of childhood influenza vaccination in England and Wales: results from a dynamic transmission model. Vaccine. 2013;31(6):927–942.
- Curtis LA, Burns A. Unit costs of health and social care 2018. Canterbury (UK): Personal Social Services Research Unit (PSSRU); 2018.
- NHS UK. NHS reference costs 2016/17 [Internet]. London (UK): NHS; 2018 [cited 2019 Nov 19]. Available from: https://improvement.nhs.uk/resources/reference-costs/
- Appleby J. Nuffield winter insight briefing 1: winter beds pressures. J Nuffield Trust. 2016;:2017–2001. Available from: https://www.nuffieldtrust.org.uk/files/2017-01/winter-beds-pressures-final.pdf
- Green HK, Andrews N, Fleming D, et al. Mortality attributable to influenza in England and Wales prior to, during and after the 2009 pandemic. PLoS One. 2013;8(12):e79360.
- Moss JW, Davidson C, Mattock R, et al. Quantifying the direct secondary health care cost of seasonal influenza in England. BMC Public Health. 2020;20(1):1–8.
- Essink B, Fierro C, Rosen J, et al. Immunogenicity and safety of MF59-adjuvanted quadrivalent influenza vaccine versus standard and alternate B strain MF59-adjuvanted trivalent influenza vaccines in older adults. Vaccine. 2020;38(2):242–250.
- Chang L-J, Meng Y, Janosczyk H, et al. Safety and immunogenicity of high-dose quadrivalent influenza vaccine in adults ≥65 years of age: a phase 3 randomized clinical trial. Vaccine. 2019;37(39):5825–5834.
- Schmader KE, Liu CK, Harrington T, et al. Safety, reactogenicity, and Health-Related quality of life after trivalent adjuvanted vs trivalent High-Dose inactivated influenza vaccines in older adults: a randomized clinical trial. JAMA Netw Open. 2021;4(1):e2031266.
- Laurie KL, Rockman S. Which influenza viruses will emerge following the SARS‐CoV‐2 pandemic? Influenza Other Respi Viruses. 2021;15(5):573–576.
- Office for National Statistics (UK). Population estimates for UK, England and Wales, Scotland and Northern Ireland (mid 2016) [Internet]. Newport (UK): Office for National Statistics; 2017 [cited 2019 Nov 19]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland
- Public Health England. Influenza: the green book, chapter 19 [Internet]. London (UK): PHE; 2015 [cited 2019 Nov 19]. Available from: https://www.gov.uk/government/publications/influenza-the-green-book-chapter-19.
- NHS UK. HES Database - hospital admitted patient care activity, 2016-17 [Internet]. London (UK): NHS; 2017 [cited 2019 Nov 19]. Available from: https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity/2016-17