?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
With the increasing occurrence of infectious diseases in lower-and-middle-income countries (LMICs), emergency preparedness is essential for rapid response and mitigation. Economic evaluations of mitigation technologies and strategies have been recommended for inclusion in emergency preparedness plans. We aimed to perform an economic evaluation using dynamic transition modeling of ebola virus disease (EVD) vaccination in a hypothetical community of 1,000 persons in the Democratic Republic of Congo (DRC).
Method
Using a modified SEIR (Susceptible, Exposed, Infectious, Recovered, with Death added [SEIR-D]) model that accounted for death and epidemiological data from an EVD outbreak in the DRC, we modeled the transmission of EVD in a hypothetical population of 1,000. With our model, we estimated the cost-effectiveness of an EVD vaccine and an EVD vaccination intervention.
Results
The results showed vaccinating 50% of the population at risk prevented 670 cases, 538 deaths, and 22,022 disability-adjusted life years (DALYs). The vaccine was found to be cost-effective with an incremental cost-effectiveness ratio (ICER) of $95.63 per DALY averted. We also determined the minimum required vaccination coverage for cost-effectiveness to be 40%. Sensitivity analysis showed our model to be fairly robust, assuring relatively consistent results even with variations in such input parameters as cost of screening, as well as transmission, infection, incubation, and case fatality rates.
Conclusion
EVD vaccination in our hypothetical population was found to be cost-effective from the payer perspective. Our model presents an efficient and reliable approach for conducting economic evaluations of infectious disease interventions as part of an emergency preparedness plan.
PLAIN LANGUAGE SUMMARY
Infectious diseases affect lower-and-middle-income (LMIC) countries, with their limited resources, disproportionately more. This is certainly the case for ebola virus disease (EVD), a rare, severe, and mostly fatal disease. Vaccination is now available, but whether it is cost-effective remains an open question. We evaluated its cost-effectiveness by modeling the spread of EVD in a hypothetical population of 1000 persons. Using data from an EVD outbreak in the Democratic Republic of Congo (DRC), we estimated how many of persons susceptible to infection were exposed to the virus, became infected, recovered, or died. We did so for two scenarios: vaccinating versus not vaccinating enough people to achieve herd immunity. We assumed vaccinating 50% of the people and estimated how many infections, deaths, and disability-adjusted life years (DALY; the loss of the equivalent of a year of full health) would be prevented by vaccination. Our cost-effectiveness metric was the incremental cost of preventing one DALY.
The outcomes of vaccination offset the additional costs. Vaccinating 50% of the population of 1000 cost an additional $2,081,358 but prevented 670 infections, 538 deaths, and 22022 DALYs. Preventing one DALY cost $95.63. This is very cost-effective as it is less than the gross domestic product (GDP) per capita for the DRC of $556.81. Vaccination below 40% of the population was less cost-effective, was most cost-effective at the herd immunity threshold of 45.3%, beyond which there were diminishing returns. Summarized, vaccination against EVD is clinically very effective and certainly worth the additional cost.
Introduction
Despite the availability of measures to prevent them, a wide range of hazards continues to afflict public health the world over; necessitating timely and effective response and mitigationCitation1. It is in this light that emergency preparedness – “the knowledge, and capacities and organizational systems developed by governments, response and recovery organizations, communities and individuals to effectively anticipate, respond to and recover from the impacts of likely, imminent, emerging or current emergencies” – emergedCitation2. In fact, countries are requested to develop and maintain a process in which action, funding, partnerships, and commitments exist at all levels to minimize the risks posed by such emergencies and to attenuate their impactCitation1. The immense global consequences of the coronavirus disease (COVID-19), along with other outbreaks such as inter alia, ebola virus disease (EVD) and measles, spotlight the emergency prospects of infectious diseases. Emergency preparedness aimed at establishing systems and plans for early detection and effective response are, thus, critical for managing infectious disease outbreaksCitation3.
By virtue of their demographic, geographic, epidemiologic, and socio-economic characteristics, low-and-middle-income countries (LMICs) are more vulnerable to infectious disease outbreaksCitation4,Citation5. While all countries are struggling with increasing trends in various communicable and non-communicable diseases, the burden is disproportionately higher in LMICs where diseases such as HIV/AIDS, malaria, tuberculosis, EVD, among others, persist with high mortality ratesCitation4. A complicating factor in the case of infectious diseases in LMICs is that there are often multiple simultaneous outbreaks for health systems to surveille, respond to, and contain using limited resources. With several priorities competing for these constrained resources, it is important that effective preparedness plans be in place for efficient infectious disease prevention and management. Research examining emergency preparedness in low-resource settings is, however, minimalCitation6. Studies that have examined various forms of emergency preparedness in these settings have evaluated the resources available in the event of natural disaster, proximity to healthcare services in the event of a health crisis, and preparedness for infectious disease outbreaks, specifically of COVID-19 [7–9]. These studies, though diverse in scope, all conclude that there are limited resources available to locals and health professions in the occurrence of an emergency situation, which inevitably leads to poorer outcomes for those affected and places greater burdens on already strained healthcare systemsCitation6–9.
Reacting to an ongoing outbreak, rather than preparing for it, places yet another strain on the healthcare system that results in increased costs to manage the outbreak that might have been better used in preparing for it. To respond rapidly to outbreaks, several components must be incorporated into an effective plan, most of which are usually lacking in LMICs and pose threats to the development and implementation of such plans. Sustainable resources are required for developing capacities such as surveillance, diagnostics, training, equipment and supplies, human and logistic resources, etc., with all readiness actions closely tied to sufficient contingency financing 5[3]. A pertussis outbreak in Ethiopia highlighted the lack of important infrastructure to properly investigate and manage outbreaks in low-resource settingsCitation10. In addition to finances, there is the need for structured and reliable evidence to inform public health decision-making in the development of a preparedness planCitation3. Furthermore, reflections on the Ebola outbreak suggest that community engagement and relative socio-political stability, as well as the emphasis on the cultural practices that underlie disease transmission and the latent health of at-risk populations contributing to the increasing burden of the infectious disease, are essential toward ending the outbreakCitation11. An emergency preparedness plan, therefore, ought to rely on a systematic evidence-based approach that considers all contingencies for informing decision-making and efficient implementation.
Miglietta and colleagues propound that health technology assessment (HTA) provides reliable evidence and is valuable for developing emergency preparedness plansCitation3. In their perspective on emergency preparedness, they propose how HTA domains can address different components of a preparedness plan and ultimately be used for populating the plan, allowing stakeholders to put together a systematic and robust tool for timely emergency management. According to their recommendations, the costs and economic evaluation domain of HTA is instrumental for addressing resource utilization for case finding and management, as well as assessing the cost-effectiveness of different health technologies intended for use in outbreak situations. This approach offers evidence in support of choices related to prevention, diagnosis, treatment, and response optionsCitation3. Especially in the case of insufficiently understood diseases, such decisions need to be made rapidly and as best as possible to ensure maximum impact. The lack of cost-benefit evidence justifying the inclusion of EVD vaccination into routine vaccinationCitation12 speaks, particularly to this issue. Given no consensus on the most cost-effective vaccination approach for EVD, Bausch put forward a vaccination strategy that combines various existing approaches such as widespread routine vaccination of healthcare workers and those at high risk, population-based vaccination campaigns during outbreaks, targeted vaccination of sex partners of male survivors and a possible expansion of the target population after the outbreak is declared overCitation12. While such a strategy may be useful over a long period, it may be limited by time, which is critical especially for highly contagious and fatal infectious diseases. In this case, an emergency preparedness plan can facilitate a quick decision on the most appropriate choice and help distribute resources towards other priority areas.
The frequency of infectious disease outbreaks in low-resource settings and the financial and human resource burdens that these outbreaks bring present an opportunity to identify mechanisms that encourage surveillance and support preparedness efforts within the limitations that are often present in LMICs. There is a lack of literature on ways to support emergency preparedness, particularly for infectious disease outbreaks, in a way that leads to empowerment of local leadership and healthcare systems to build capacity that leads to a more informed response when an outbreak occurs. We aimed to develop a dynamic transmission model (DTM) to determine the cost-effectiveness of (a) an EVD vaccine and (b) a vaccination program at large in a hypothetical population of 1,000.
Methods
Overview
We used a dynamic compartmental model to estimate the cost-effectiveness of vaccinating a proportion of the population compared to a base scenario of no vaccination for a hypothetical population of 1,000. We used the size of 1,000 to reflect the relatively small proportion of the entire population who are most at risk of the infection during a given outbreak; while also providing a base count from which multiples can be estimated as needed for larger populations. The population size of 1,000 also recognizes that in the case of EVD, outbreaks often originate in more isolated rural areasCitation13. Our choice of the model allowed us to account for the force of infection while also incorporating the indirect benefits of vaccination in the form of herd immunity. We based our model on the epidemiological data obtained from an EVD outbreak in the Democratic Republic of Congo (DRC)Citation14. Given that the mean length of an EVD outbreak is about 10 monthsCitation15, and the uncertainty of waning vaccine effectiveness, along with the possible emergence of new outbreaks and variants, we limited the time horizon to one year.
We considered effectiveness in terms of infections, deaths, and disability-adjusted life-years averted (DALYs). We based our estimation of DALYs on the median population age of 19.2 yearsCitation16 and life expectation of 60.97 years in DRCCitation17, accounting for no variations in the ages of patients. The analysis takes a payer perspective with the objective of assisting payer organizations and funders in assessing potential investments for an emergency preparedness plan and informing donor and payer decision making. For this study, the payer is defined broadly as any organization, government or third party who purchases and pays for the EVD vaccine on behalf of the end user. This perspective is to reflect the dependence on government and donor funding in LMICs where vaccination programs are concerned as several literature have shownCitation18–20.
Intervention: Vaccination
With the approval of two vaccines against EVDCitation12, and the commencement of vaccination in DRCCitation21, we chose vaccination as our intervention of choice. The role of vaccination in our model was two-fold: first, to consider the counterfactual ideal of how vaccination would impact disease transmission in our theoretical population and second, to quantify the economic impact of vaccination by comparing the cost of disease transmission through the population with and without vaccination. Data on vaccine effectiveness was obtained from published literature, while the proportion to be vaccinated was estimated based on the herd immunity threshold using the formula:
where
is the basic reproductive number.
We assumed vaccination of the general population and given a herd immunity threshold of 45.3%, vaccination coverage proportion for this model was set at 50%.
Dynamic transmission modeling
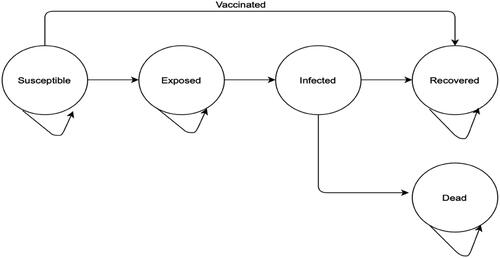
We used the SEIR-D, a modification of the standard SEIR model in which we incorporated death as a final state in our model (). Transition probabilities from one state to another were determined by epidemiological parameters obtained from published literature. We converted rates and durations into probabilities and further scaled-down all probabilities to daily cycle probabilities. Rates were obtained from durations by taking the inverse of the durations in days while rates were converted into daily probabilities using the formulaCitation22:
where r is the rate and t the duration in days.
The population at risk moves to or through one of the following possible states: susceptible, exposed, infected, recovery or death. We accounted for the infectious nature of the disease with transitions from one state to another being dependent on the number of infected persons in the model at each cycle. To keep the model tractable, we conservatively assumed that individuals moved linearly in this model with infected individuals either recovering or dying. Our model did not allow for re-infection, nor did it account for population changes that were not a direct result of the disease (i.e. births, all-cause mortality, immigration, and emigration). Given that the mean duration of the epidemic was 200 daysCitation14,Citation15, we allowed the model to run over a period of 1 year or 365 days to populate the states of the model.
Following compartmental modeling conceptsCitation14, the following modified differential equations, that account for a separate death compartment, were used for modeling:
where N is the population, S is the number of this population susceptible, E is the number exposed, I is the number infected, R is the number who recover from the infection and D is the number who die from the infection. We assumed an initial population size of 1,000 with 1 infected patient. Susceptible persons become exposed at a rate of βI/N where β is the transmission rate and I/N represents the probability of contact with an infected person. Given an incubation period of 1/ξ days in which exposed persons are assumed not infectious and asymptomatic, exposed persons move into the infected compartment at a rate of ξ per day. Infected persons either recover at a rate of δ or die at a rate of µ per day.
Cost-effectiveness analysis
We estimated the cost per infection averted, cost per death averted, and cost per disability-adjusted life year (DALY) averted. Infections and deaths averted were defined as the number of infections and deaths, respectively, that would have happened in the absence of the intervention and was calculated as the number of infections and deaths among the unvaccinated cohort minus the number of infections among the vaccinated cohort.
Using the equation for calculation of DALYsCitation23,Citation24, we estimated DALYs as the sum of years of life lost (YLL) and years of life lived with disability (YLDs). We obtained disability weights from the Global Burden of Disease Study 2015 (GDB, 2015). Formulas used for DALYs calculation are given below:
where K is the age weighting modulation factor (0 if no age weighting modulation factor is used); YLL_L is the life expectancy at age of death; s the average age at premature death due to disease minus average age at disease onset; and D the disability weight.
Costs of treatment, screening, and vaccination were obtained from published literature. The cost of vaccination comprised the price of the vaccine as well as the composite cost of administration. Given that the vaccine price is unknown, we employed estimations of the total cost of vaccination from the World Health Organization (WHO)Citation25 and the United Nations Children’s Emergency Fund (UNICEF)Citation26. All costs were converted to 2021 USD using the US Consumer Price Index for Medical CareCitation27. With the assumptions that all persons in the model, except those who die, would be screened, all those who are infected will be treated, and a proportion of the susceptible population will be vaccinated for the vaccine model, we obtained total costs of screening of EVD, treating a case of EVD, and vaccinating an individual against EVD. Since we modeled this disease over a one-year period, no discounting was applied for either costs or outcomes.
Incremental cost-effectiveness ratios (ICER) for all outcomes of interest were calculated using the formula:
Using the WHO-CHOICE recommendations for cost-effectivenessCitation28, we considered the intervention highly cost-effective if the ICER per DALY averted was less than 1*gross domestic product (GDP) of the country and cost-effective if the ICER per DALY averted was less than 3* GDP.
Sensitivity analyses
Sensitivity analyses were conducted to assess the robustness of our model. In one-way sensitivity analyses, we varied model input parameters individually from low to high values, with all other variables fixed. Parameter ranges were based on 95% CIs, in the absence of which parameters were varied by ± 10% of their point estimates. Results for these one-way analyses were presented in line plots showing how the ICER per DALY averted changes with varying parameters as well as compiled in a tornado plot.
In two-way sensitivity analyses, we assessed the effects of linearly increasing vaccination coverage in the population and the other parameters (one at a time) on the ICER per DALY averted. The results of these analyses were presented in heat maps.
We conducted probabilistic sensitivity analyses (PSA) using simultaneous random sampling from estimated probability distributions for uncertain parameters. A total of 1,000 Monte Carlo simulations were run, allowing for the quantification of combined uncertainties. The results were visually presented using cost-effectiveness planes and cost-effectiveness acceptability curves (CEAC) over a range of different willingness to pay (WTP) thresholds. Where available, ranges for the input parameters were based on 95% CIs or standard errors (or computed from standard deviations) and where these were not reported, varied by ±10% of the point estimates. We used gamma distribution for the cost parameters, beta distribution for utility and probability parameters, and lognormal distribution for the vaccination proportion parameter. The baseline values, ranges, and distributions of model parameters are presented in .
Table 1. Model input parameters – baseline values, ranges, and distributions applied for sensitivity analyses.
Results
Health outcomes
From our analysis, vaccinating 50% of the 1,000 people susceptible to EVD infections will prevent an estimated 670 infections (or 99.3% of expected infections without vaccination) and 538 (or 99.1% of expected deaths without vaccination) deaths, while also averting a total of 22,022 DALYs (or 99.2% of expected DALYs without vaccination), corresponding to 22.022 DALYs per person) ().
Table 2. Infections, deaths and DALYs averted by vaccination in a population of 1,000.
shows the dynamics of the movement of the 1,000 persons within each of the 5 SEIR-D compartments without (panel A) and with vaccination (panel B). Without vaccination, the Susceptible population is projected to decline from the initial 999 to about 300 at 200 days, at which time it reaches an asymptote. Simultaneously, the population projected to Die due to EVD increases rapidly from zero, crossing the Susceptible curve at 149 days, and reaching an asymptote at 535 Deaths at 215 days. Of the population of 1,000 subjects, only 135 are projected to have Recovered from infection at the end of the outbreak. At that time, in descending order, the population of 1,000 either have Died (54.3%), remain Susceptible (32.3%), or have recovered (13.5%).
Figure 2. Graphical representation of dynamic transmission model without (Panel A) and with vaccination (Panel B).
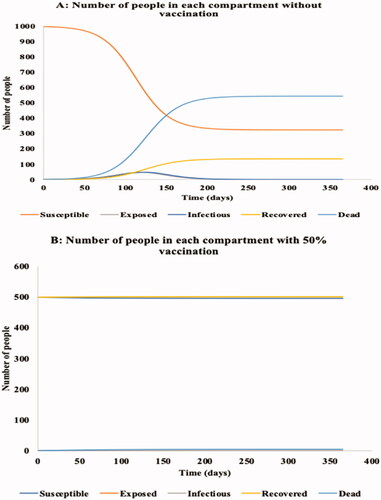
Conversely, with vaccination of 50% of the population, 499 persons are no longer susceptible. The entire population appears to reach asymptote by day 50, with 5 (0.5%) persons becoming infected over the period, 4 of whom die (0.4%).
Base case cost-effectiveness analysis
The results of the base case analysis are presented in . The vaccination program resulted in increased total costs while decreasing infections, deaths, and DALYs. While a total cost of $4,723,258.42 was expected from not vaccinating in the population, vaccinating 50% of the population cost a total of $6,804,616.21. As indicated by the ICERs, preventing each additional infection, death or DALY was estimated to cost $3,100.64, $3,869.0, and $94.51, respectively.
Table 3. Results of base case cost-effectiveness analysis.
One-way sensitivity analyses
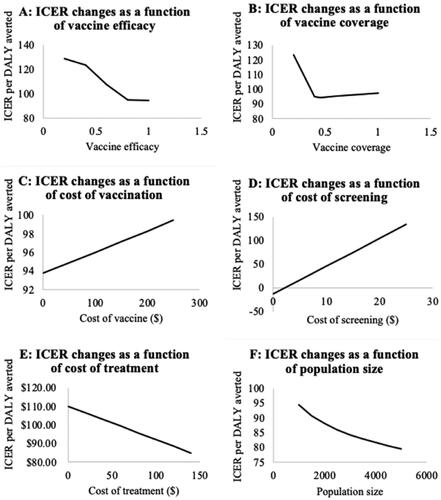
In these analyses, the ICER of DALYs averted was found to be a function of vaccine efficacy, vaccination coverage, vaccine cost, screening cost, treatment cost, and population size, (, panels A through F). The ICER per DALY averted declined steadily from vaccine efficacy of 20% to 40% and decreased sharply to 80% after which it remained constant over increasing vaccine efficacy. Similarly, ICER declined rapidly from a vaccination coverage of 20%, reaching its lowest value at a coverage rate of 45% which coincides with the herd immunity threshold, and rising marginally at higher rates of vaccine/vaccination coverage (Panel B). With increasing costs of vaccination (Panel C) and screening (Panel D), ICER per DALY averted increased linearly, while it decreased with the increasing cost of treatment (Panel E). The ICER per DALY averted also declined gradually with increasing population size (Panel F).
Figure 3. Results of one-way sensitivity analyses. Abbreviations. ICER, incremental cost effectiveness ratio; DALY, disability adjusted life year.
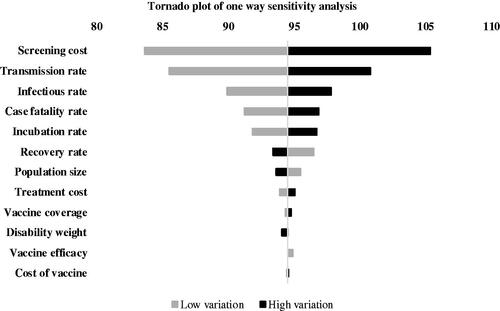
presents a tornado diagram showing the results of the one-way sensitivity analysis for the cost-effectiveness analysis in terms of DALYs averted. The centerline represents the baseline ICER of $95.62 per DALY averted. Results of our model were most sensitive to the cost of screening for EVD and the transmission rate; with the infectious and case fatality rates exerting a modest influence. The cost of vaccination, the proportion of vaccination coverage, and vaccine efficacy had a minor influence on the results of our model.
Figure 4. Tornado plot showing compiled one-way sensitivity analyses of model parameters. Middle line represents the base case incremental cost utility ratio (ICER) per disability adjusted life year (DALY) averted ($94.51). Horizonal bars represent the variations in the ICERs as a result of variations in model parameters.
Two-way sensitivity analyses
The heat maps in depict the ICER for DALYs averted as a function of vaccine coverage under various scenarios of vaccination cost, vaccine efficacy, screening cost, population size, and treatment cost. Dark green indicates the highest cost-effective scenarios, dark red the least cost-effective scenarios, and yellow modestly cost-effective scenarios, with variations in these colors’ shades reflecting areas of relative upward or downward transitions in cost-effectiveness.
Figure 5. Heat maps showing incremental cost-effectiveness ratio (ICER) per disability-adjusted life year (DALY) averted from varying vaccination coverage and other parameters. Green areas represent favorable ICERs, red areas represent least favorable ICERs.
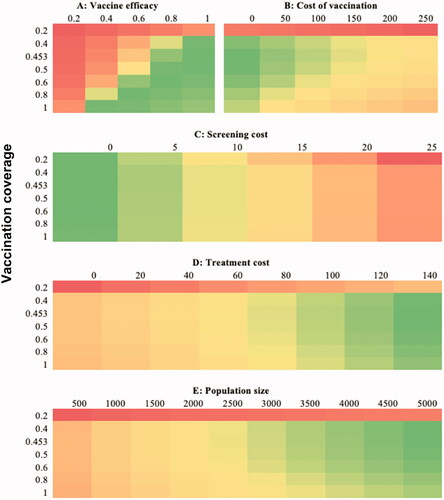
Panel A shows changes in cost-effectiveness with a simultaneous change in vaccine efficacy and vaccination coverage. Vaccinating 20% or less of the population and vaccine efficacies of 20% and below results in the least favorable ICERs per DALY averted. As both coverage and efficacy increase, ICERs increase to the highest cost-effectiveness with coverage of 100% and efficacy of 40% or coverage of at least 40% and efficacy of 100%. Vaccinating the entire population at risk using a vaccine with efficacy beyond 60%, just like vaccinating more than 60% of the population at risk with a fully efficacious vaccine results in slightly decreased cost-effectiveness. Considering the cost of vaccination (panel A), a cost of zero (unsurprisingly) yields the best ICER at vaccine coverage of 45–50%. At costs of $50 and, slightly less so, of $100 the increase in ICER is marginal. Unless the cost is zero and coverage is at least 40%, the ICER estimates are highly cost-effective though with a slight diminishing return at vaccination rates exceeding 50%. Higher vaccine costs and higher vaccination coverageCitation33 are less cost-effective. Vaccination rates of less than 20% are the least cost-effective. Similar to how ICERs vary with the cost of vaccination, costs of screening at 0 were the most cost-effective scenarios. Irrespective of the vaccination coverage, screening costs above $25 are least cost-effective while there is increasing cost-effectiveness with decreasing cost of screening (Panel C). With varied treatment costs (Panel D) and population size (Panel E), cost-effectiveness is least when coverage is below 40%. The vaccination program approaches more favorable levels of cost-effectiveness with an increasing treatment cost of about $80 and population size of 2,500 and increases to most favorable as these increase.
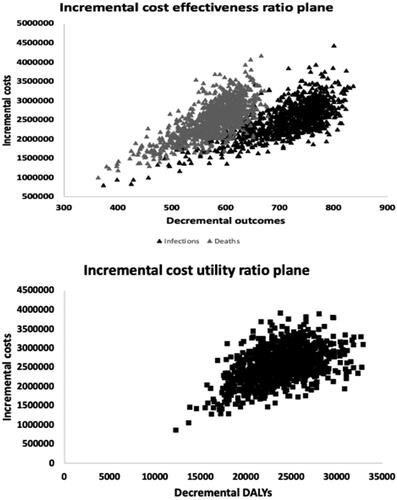
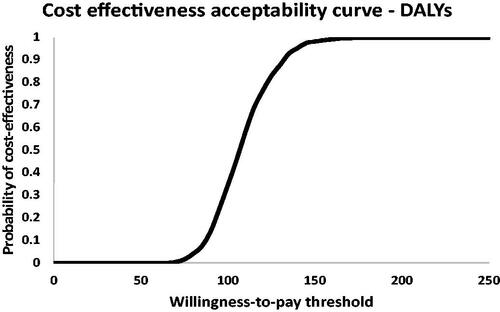
Probabilistic sensitivity analysis
The results of the PSA are shown in the ICER planes (; northeast quadrant of better outcomes at higher costs only) for the outcomes of infections and deaths (panel A) and DALYs everted (Panel B) and the CEAC (). reveals clustering of simulation results in the lower region of the northeast quadrants, an indication of the cost-effectiveness of vaccinating compared to not vaccinating in terms of infections, deaths, and DALYs averted. In terms of the latter, there was no probability of cost-effectiveness below a WTP threshold of $65, beyond which the probability of cost-effectiveness steadily increases. Vaccination demonstrated a 100% probability of cost-effectiveness at a WTP threshold of approximately $175 per DALY averted.
Discussion
While several studies have evaluated the impact of an EVD vaccine, ours assesses the cost-effectiveness of an EVD vaccine in terms of infections, deaths, and DALYs averted. Summarized, we evaluated the cost-effectiveness of an EVD vaccine in a hypothetical town with a population of 1,000 using epidemiological data from the DRC. Using a vaccine of 100% effectiveness and assuming coverage of 50%, a vaccination program would be cost-effective from a payer perspective in terms of infections, deaths, and DALYs prevented. In our model, EVD vaccination reduced infections, deaths as well as DALYs by about 99%. At a mere incremental cost of $94.51, EVD vaccination averts 1 DALY, proving to be highly cost-effective per the WHO recommendation. With about 22,000 DALYs averted in a population of 1,000, this means that, on average, vaccination grants each of the 1,000 persons an additional 22 life-years free of EVD-related disability – arguably, an unmeasurable human, economic, and societal benefit. Thus, although vaccinating a proportion of the population against EVD requires extra investments, these additional costs are largely offset by the benefits in terms of preventing infections, deaths, and DALYs. More generally, both our model and the results of our analyses provide stakeholders, policymakers and payers in particular, a readily available and easily interpretable approach to assessing the impact of a vaccine in anticipation of an outbreak and would be instrumental for rapid resource allocation decision-making in such an event.
Extensive sensitivity analyses were conducted given that there were several uncertainties. For example, vaccine coverage is considered an important determinant of cost-effectivenessCitation15,34–36. To assess its effect on our model, sensitivity analyses demonstrated that increasing coverage translates into greater cost-effectiveness critically, the highest cost-effectiveness was observed at a coverage equivalent to the herd immunity threshold of about 45%. This underscores economically that EVD vaccination is not only cost-effective but also the most cost-effective right at the herd immunity threshold – a double win. Further, this finding is in line with other studies in which the cost-effectiveness of vaccines have been seen to be optimized at the coverage level required to attain the herd immunity threshold; that is, the level of coverage at which vaccination confers indirect protection on the population and beyond which scarcely any incremental impacts on cases averted are achievedCitation34,Citation36. Conversely, as our findings also show, there is a diminishing return in terms of vaccination outcomes when vaccination is beyond the herd immunity threshold.
In the case of EVD, a highly fatal yet rare disease with an infinitesimally low risk of infection at the general population level if outbreaks are geographically contained, our findings provide compelling support for the assertion by Bausch that there is no evidence for including vaccination against EVD into routine vaccination of populations at large, even in endemic regionsCitation12. In addition to this, our findings reinforce Bausch’s recommendation of immediate population-based vaccination as soon as an outbreak occursCitation12. Further, our finding that the vaccine is not cost-effective at coverage levels below 40% is consistent with that of a study that analyzed the impact of vaccination against EVD in Sierra LeoneCitation37. In this study, Bodine and colleagues concluded that vaccination coverage of 40% is sufficient to prevent the catastrophic effects of an outbreakCitation37.
.Of note also, is the inelasticity of vaccination in terms of cost-effectiveness. As the sensitivity analysis showed, variations in the cost of vaccination have minimal effect on the ICER per DALY averted. Even at cost of vaccination as high as $250, the increase in the ICER to $99.40 to avert one DALY is low. Considering the economic impact of an outbreak of ebola, along with the inestimable burden of lives lost, influence on healthcare and the healthcare system, among others, there is a case to be made for vaccination even at high costs of vaccination. Huber et al. estimated the 2014 EVD outbreak to have caused the loss in GDP ranging from $2.8 billion to $32.6 billion with a more comprehensive estimate of the economic and social burden pegged at a total of $53.19 billionCitation38. In estimating the monetary value of human lives lost through EVD in DRC using the human capital approach, Kirigia and colleagues valued a life lost to EVD at Int$13,801 averagelyCitation39. The price of vaccination is minuscule compared to the burden in the absence of vaccination. In this light, our findings reinforce calls for timely vaccination in the event of an outbreak.
We see an increase in cost-effectiveness with increasing treatment costs for EVD. While this appears counterintuitive, it reflects the value of vaccination where the prevention of infections is concerned. In the absence of vaccination, more infections are projected to occur resulting in more costs for treating infected persons. With lower treatment costs, the value of the vaccine in terms of how much total costs it averts appears less substantial. As the cost of treatment increases, however, it becomes more apparent how infections averted translate into total costs saved by the vaccination program. This is particularly important for EVD where management also depends on the presenting symptomsCitation40 and thus varies across disease severity and setting.
Another parameter examined in our sensitivity analyses was the size of the population. Since we assumed a hypothetical population, we queried the outcome of our model over a range of population sizes and found that increasing the population size results in lower costs for averting infections, deaths, and DALYs. In part, this reflects the base costs for launching a vaccination program. More importantly, though, this demonstrates the role of economies of scale in achieving cost-effectiveness. Larger populations require more vaccine doses to attain the target vaccination coverage, and this provides payers bargaining leverage for securing favorable vaccine prices and value for money. Our results, thus, buttress the attribution of economies of scale to vaccination programs in developing countriesCitation41, including in EVD vaccination and similar vaccinations that do not require vaccination at the general population level.
The transmission rate is crucial to the cost-effectiveness of a vaccine, and this was evidenced also in the one-way sensitivity analyses in our model. The force of infection, a measure of the rate at which susceptible individuals in a population contract the disease and a parameter-dependent also on the transmission rate of the disease, drives infectious disease outbreaksCitation42. Another driver of cost-effectiveness revealed in our analyses is the cost of screening. With the highly fatal nature of EVD and the potential for spread within the population at risk, screening is critical for infection prevention and controlCitation43,Citation44. In the light of this, our model accounted for daily screening of all persons living in the population during the assessment duration. The impact in our model attributed to the cost of screening could be caused by the cumulative cost of this daily testing routine, which depending on the affected country’s protocol, may differ from one population to another.
In terms of effectiveness, our results align with those from previous studies. Vaccination provides a significant benefit in preventing infections and deaths, and ultimately preventing or controlling a potentially catastrophic outbreakCitation37,Citation45,Citation46. In their study modeling the daily risk of EVD in the presence and absence of a potential vaccine, Abo and Smith found that even at high transmission rates, a vaccine is effective at controlling an outbreakCitation41. The efficacy of a vaccine and the proportion of people in the population receiving the vaccine are critical to the vaccine’s effectiveness with higher proportions required to be vaccinated when less efficacious vaccines are usedCitation38,Citation45. Potluri and colleagues found that the benefits of EVD vaccination in terms of cases and deaths prevented increase with greater vaccine coverage, although this happens at a lower incremental rateCitation46. Generally, vaccines are cost-effective and help to ease the burden on the economies of affected communitiesCitation41 and our results lend further evidence in support. Note that vaccines against diseases such as influenzaCitation36, cervical cancerCitation35, herpes zosterCitation47, malariaCitation33, and the recent coronavirus (COVID-19)Citation34 have all been found to be cost-effective.
Our model is parsimonious but robust for conducting an economic evaluation of an infectious disease vaccine for inclusion in an emergency preparedness plan. The use of the DTM approach allowed us to account for the force of infection and the indirect effects of the vaccineCitation48. This ensures that decisions made based on this model consider the entire scope of the benefits, expressed in natural as well as generic units, obtained from a vaccination program. Our model assessed both natural outcomes (infections and deaths) and a generic utility-based outcome (DALYs). While natural outcomes are easier to understand and better appreciated, especially by decision-makers who are not economists, generic outcomes permit comparison of the cost-effectiveness of programs in other disease areas that may be endemic in the same populationCitation49. This is essential especially for LMICs where various outbreaks may be occurring simultaneously and where limited resources make such decision-making even more crucial.
Despite these strengths, our study has some limitations. Like most models, it assumes a rather homogenous target population in its composition and characteristics. We did not consider background births, deaths, immigration, and emigration; in good part, because we considered the zoonotic nature of EVD and outbreaks tending to be in often small isolated rural locations. We opted against highly granular models, as the gains in precision would have been offset by our aim to provide a parsimonious and workable model for integration into emergency preparedness Our study was from a payer as opposed to a societal perspective because we aimed to provide evidence mainly to donor organizations who typically pay for vaccines in LMICs. With no data on the waning immunity from EVD vaccination, we could not account for this factor. Using a time horizon of one year, we did not evaluate the benefits of the vaccine on the long-term (beyond one year) complications of EVD. The model did not account for other infection prevention methods that may reduce transmission and infection such as isolation and use of personal protective equipment. Future studies that account for these would be beneficial and help to better understand the effects of EVD vaccinations under various conditions.
Conclusion
In conclusion, EVD vaccination in our hypothetical population was found to be highly cost-effective from the payer perspective, with extensive sensitivity analyses underscoring our model’s robustness and elucidating key determinants of vaccination-related outcomes. For fast response and mitigation of a potentially infectious disease outbreak, the model presents an efficient approach for economic evaluations of vaccination programs for EVD and other infectious disease outbreaks as part of an emergency preparedness plan.
Transparency
Declaration of funding
This study received no funding.
Declaration of financial/other interests
IA holds equity in Matrix45, LLC, which provides research and consulting services to, among others, the pharmaceutical industry. Matrix45, LLC previously was contracted by Celgene for work unrelated to the subject of this Technical Note. By company policy, associates of Matrix45, LLC cannot provide services to nor receive compensation independently from sponsor organizations. IA has no other disclosures related to the work reported herein. All other authors have no disclosures related to the work reported herein. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Authors contributions
MOK and MH contributed to study design, collection, analyses and interpretation of data, revision, and final approval of the manuscript. DJR and BE contributed to revision and final approval of the manuscript. IA contributed to study design, revision, and final approval of the manuscript.
Previous presentations
This study was never presented previously.
Supplemental Material
Download MP4 Video (340.5 MB)Acknowledgements
No assistance in the preparation of this article is to be declared.
References
- World Health Organization (WHO). A strategic framework for emergency preparedness [Internet]. [cited 2021. Aug 10]. Available from: https://www.who.int/publications-detail-redirect/a-strategic-framework-for-emergency-preparedness
- World Health Organization (WHO). International health regulations: 2005. 2nd. ed. Geneva: WHO; 2008.
- Miglietta A, Waure C. d, Chronaki C, et al. Health technology assessment applied to emergency preparedness: a new perspective. International Journal of Technology Assessment in Health Care. 2021. [cited 2021 Aug 9]. p. 37. Available from: http://www.cambridge.org/core/journals/international-journal-of-technology-assessment-in-health-care/article/health-technology-assessment-applied-to-emergency-preparedness-a-new-perspective/7B483504919FEFAB582A3A7FB3FA2C4C.
- Boutayeb A. The burden of communicable and non-communicable diseases in developing countries. In: Preedy VR, Watson RR, editors. Handbook of disease burdens and quality of life measures. New York (NY): Springer; 2010. Available from: https://doi.org/https://doi.org/10.1007/978-0-387-78665-0_32
- Remais JV, Zeng G, Li G, et al. Convergence of non-communicable and infectious diseases in low- and middle-income countries. Int J Epidemiol. 2013;42(1):221–227.
- Lee ACK, Booth A, Challen K, et al. Disaster management in low- and middle-income countries: scoping review of the evidence base. Emerg Med J. 2014;31(e1):e78–e83.
- Tanveer F, Khalil AT, Ali M, et al. Ethics, pandemic and environment; looking at the future of low middle income countries. Int J Equity Health. 2020;19(1):182.
- Tansley G, Schuurman N, Amram O, et al. Spatial access to emergency services in low- and middle-income countries: a GIS-based analysis. PLOS One. 2015;10(11):e0141113.
- Zhang J, Lu X, Jin Y, et al. Hospitals' responsibility in response to the threat of infectious disease outbreak in the context of the coronavirus disease 2019 (COVID-19) pandemic: implications for low- and middle-income countries. Glob Health J. 2020;4(4):113–117.
- Mitiku AD, Argaw MD, Desta BF, et al. Pertussis outbreak in Southern Ethiopia: challenges of detection, management, and response. BMC Public Health. 2020;20(1):1223.
- Aruna A. Ebola virus disease outbreak — democratic republic of the Congo, August 2018–November 2019. MMWR Morb Mortal Wkly Rep [Internet]. 2019. [cited 2021 Aug 6];68. Available from: https://www.cdc.gov/mmwr/volumes/68/wr/mm6850a3.htm.
- Bausch DG. The need for a new strategy for ebola vaccination. Nat Med. 2021;27(4):580–581.
- 2014-2016 Ebola Outbreak in West Africa | History | Ebola (Ebola Virus Disease) | CDC [Internet]. 2020 [cited 2021 Sep 11]. Available from: https://www.cdc.gov/vhf/ebola/history/2014-2016-outbreak/index.html.
- Chowell G, Hengartner NW, Castillo-Chavez C, et al. The basic reproductive number of ebola and the effects of public health measures: the cases of Congo and Uganda. J Theor Biol. 2004;229(1):119–126.
- Lekone PE, Finkenstädt BF. Statistical inference in a stochastic epidemic SEIR model with control intervention: Ebola as a case study. Biometrics. 2006;62(4):1170–1177.
- Congo Population 2021. Worldometer [Internet]. [cited 2021 Aug 11]. Available from: https://www.worldometers.info/world-population/congo-population/.
- Democratic Republic of the Congo Life expectancy at birth, 1950-2020 - knoema.com [Internet]. Knoema. [cited 2021. Aug 11]. Available from: https://knoema.com//atlas/Democratic-Republic-of-the-Congo/topics/Demographics/Age/Life-expectancy-at-birth.
- 92 low- and middle-income economies eligible to get access to COVID-19 vaccines through Gavi COVAX AMC [Internet]. [cited 2021. Sep 12]. Available from: https://www.gavi.org/news/media-room/92-low-middle-income-economies-eligible-access-covid-19-vaccines-gavi-covax-amc.
- Haakenstad A, Birger M, Singh L, et al. Vaccine assistance to low- and middle-income countries increased to $3.6 billion in 2014. Health Aff. 2016;35(2):242–249.
- Tagoe ET, Sheikh N, Morton A, et al. COVID-19 vaccination in lower-middle income countries: national stakeholder views on challenges, barriers, and potential solutions. Front Public Health. 2021;9:709127.
- Wells CR, Pandey A, Parpia AS, et al. Ebola vaccination in the democratic republic of the Congo. Proc Natl Acad Sci USA. 2019;116(20):10178–10183.
- Fleurence RL, Hollenbeak CS. Rates and probabilities in economic modelling: transformation, translation and appropriate application. Pharmacoeconomics. 2007;25(1):3–6.
- Emerson J, Kim DD. Global Health CEA – DALY Calculator [Internet]. Center for the Evaluation of Value and Risk in Health, Tufts Medical Center, Boston, MA.; 2018. [cited 2021 Aug 9]. Available from: http://ghcearegistry.org/orchard/daly-calculator.
- Fox-Rushby JA, Hanson K. Calculating and presenting disability adjusted life years (DALYs) in cost-effectiveness analysis. Health Policy Plan. 2001;16(3):326–331.
- GEVIT_guidance_AppendixK.pdf [Internet]. [cited 2021. Aug 6]. Available from: https://www.who.int/csr/resources/publications/ebola/GEVIT_guidance_AppendixK.pdf?ua=1.
- Ebola-vaccines-prices-11012021.pdf [Internet]. [cited 2021. Aug 6]. Available from: https://www.unicef.org/supply/media/6956/file/Ebola-vaccines-prices-11012021.pdf.
- US Consumer Price Index: Medical Care [Internet]. [cited 2021. Sep 11]. Available from: https://ycharts.com/indicators/us_consumer_price_index_medical_care.
- Marseille E, Larson B, Kazi DS, et al. Thresholds for the cost–effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93(2):118–124.
- Vos T, Allen C, Arora M, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388(10053):1545–1602.
- Henao-Restrepo AM, Camacho A, Longini IM, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (ebola Ça suffit!). The Lancet. 2017;389(10068):505–518.
- Vogel G. Rapid test for Ebola now available [Internet]. Science AAAS. 2015 [cited 2021 Aug 11]. Available from: https://www.sciencemag.org/news/2015/02/rapid-test-ebola-now-available
- Bartsch SM, Gorham K, Lee BY. The cost of an ebola case. Pathog Glob Health. 2015;109(1):4–9.
- Seo MK, Baker P, Ngo KN-L. Cost-effectiveness analysis of vaccinating children in Malawi with RTS,S vaccines in comparison with long-lasting insecticide-treated nets. Malar J. 2014;13:66.
- Hagens A, İnkaya AÇ, Yildirak K, et al. COVID-19 vaccination scenarios: a cost-effectiveness analysis for Turkey. Vaccines. 2021;9(4):399.
- Suárez E, Smith JS, Bosch FX, et al. Cost-effectiveness of vaccination against cervical cancer: a multi-regional analysis assessing the impact of vaccine characteristics and alternative vaccination scenarios. Vaccine. 2008;26:F29–F45.
- Ibuka Y, Paltiel AD, Galvani AP. Impact of program scale and indirect effects on the cost-effectiveness of vaccination programs. Med Decis Making. 2012;32(3):442–446.
- Bodine EN, Cook C, Shorten M. The potential impact of a prophylactic vaccine for ebola in Sierra Leone. Math Biosci Eng. 2018;15(2):337–359.
- Huber C, Finelli L, Stevens W. The economic and social burden of the 2014 ebola outbreak in West Africa. J Infect Dis. 2018;218(suppl_5):S698–S704.
- Kirigia JM, Muthuri RNDK, Muthuri NG. The monetary value of human lives lost through ebola virus disease in the democratic republic of Congo in 2019. BMC Public Health. 2019;19(1):1218.
- Treatment | Ebola (Ebola Virus Disease) | CDC [Internet]. 2021. [cited 2021 Sep 17]. Available from: https://www.cdc.gov/vhf/ebola/treatment/index.html.
- Ehreth J. The global value of vaccination. Vaccine. 2003;21(7–8):596–600.
- Kaslow DC. Force of infection: a determinant of vaccine efficacy? Npj Vaccines. 2021;6(1):1–7.
- Screening Patients | For Clinicians | Ebola Virus Disease | CDC [Internet]. 2021. [cited 2021 Aug 22]. Available from: https://www.cdc.gov/vhf/ebola/clinicians/evaluating-patients/index.html.
- Faherty LJ, Doubeni CA. Unintended consequences of screening for ebola. Am J Public Health. 2015;105(9):1738–1739.
- Abo SMC, Smith? R. Modelling the daily risk of ebola in the presence and absence of a potential vaccine. Infect Dis Model. 2020;5:905–917.
- Potluri R, Kumar A, Maheshwari V, et al. Impact of prophylactic vaccination strategies on ebola virus transmission: a modeling analysis. PLOS One. 2020;15(4):e0230406.
- van Hoek AJ, Gay N, Melegaro A, et al. Estimating the cost-effectiveness of vaccination against herpes zoster in England and Wales. Vaccine. 2009;27(9):1454–1467.
- Pitman R, Fisman D, Zaric GS, et al. Dynamic transmission modeling: a report of the ISPOR-SMDM modeling good research practices task force-5. Value Health. 2012;15(6):828–834.
- Lorgelly PK, Lawson KD, Fenwick EAL, et al. Outcome measurement in economic evaluations of public health interventions: a role for the capability approach? Int J Environ Res Public Health. 2010;7(5):2274–2289.