Abstract
Aims
To examine healthcare utilization and costs in a US Medicare population diagnosed with Huntington’s disease (HD).
Methods
This was a retrospective matched cohort study using Medicare fee-for-service (FFS) claims data using 2013–2017 Research Identifiable Files. Medicare beneficiaries diagnosed with HD based on the presence of at least one medical claim with an International Classification of Diseases, Ninth or Tenth Revision, Clinical Modification (ICD-9/10-CM) diagnosis code for HD (ICD-9-CM: 333.4; ICD-10-CM: G10) during the identification period (2014–2016). Beneficiaries without HD were drawn from a 5% random sample of Medicare beneficiaries and 1:1 matched to those with HD for comparison. All-cause and HD-related (any utilization related to HD diagnosis or symptoms associated with HD) healthcare utilization and costs were reported.
Results
We identified 3,688 matched pairs of beneficiaries with and without HD. Of those with HD, 1,922 (52.1%) were late-stage, 916 (24.8%) were middle-stage, and 850 (23.1%) were early-stage. Mean [SD] annual total healthcare costs were higher for HD beneficiaries than beneficiaries without HD ($41,631 [57,393] vs. $17,222 [31,218], p < .001) and were primarily driven by outpatient pharmacy costs ($19,182 [45,469] vs. $4,318 [11,553], p < .001). In the stratified analysis, total healthcare costs were highest among beneficiaries with late-stage HD (mean [SD] cost: $20,475 [$41,122] for early-stage vs. $29,733 [$44,977] for middle-stage vs. $56,657 [$64,185] for late-stage; p < .001).
Limitations
Results are not generalizable to beneficiaries enrolled in other non-FFS Medicare plans. Administrative claims are intended for billing purposes, not research, and may not capture all symptoms, comorbidities, and other adverse events.
Conclusions
This original, comprehensive analysis of healthcare utilization and economic burden among Medicare beneficiaries with HD found that healthcare needs and associated costs are substantially higher among Medicare beneficiaries who are diagnosed with HD compared to beneficiaries without HD.
Subject classification codes:
Introduction
Huntington’s disease (HD) is a genetic, progressive, neurodegenerative disease characterized by cognitive and motor decline, and behavioral symptoms that lead to loss of independence and severe disabilityCitation1. While the onset of this eventually terminal disease usually occurs between 30 and 50 years of ageCitation2, the onset of HD can occur earlier or later in life. The median and mean survival time after a diagnosis of HD is 15 yearsCitation3. Treatment for HD is limited to symptomatic treatment as there are no currently available disease-modifying therapies. Due to this, the expected and, potentially, prolonged disability associated with HD has generated concern about the associated economic burden.
Currently, few studies have investigated the healthcare utilization and cost burden among HD populations in the United States (US). Prior studies have investigated cost burden by disease stage in HD populations comprised of individuals with commercial or Medicaid insurance coverageCitation4,Citation5, or before and after a diagnosis of HD in patients with commercial or supplemental Medicare insurance coverage who initiated tetrabenazineCitation6. Another study investigated hospitalizations in a small population with juvenile HD, primarily covered by private and Medicaid insuranceCitation7. While these studies shed light on various aspects of the burden of HD, none have comprehensively examined healthcare utilization and costs among all fee-for-service (FFS) Medicare beneficiaries diagnosed with HD.
Given the dearth of evidence on the economic burden associated with HD in other US populations, the objective of this study was to examine healthcare utilization and costs in a US Medicare population diagnosed with HD. Medicare beneficiaries who are diagnosed with HD may experience pronounced burden resulting from the manifestation of symptoms or conditions associated with both HD and increasing age, making them an important population for study.
Methods
Study design and data source
This was a retrospective cohort study using the five most recent years (2013–2017) of Medicare FFS claims from the 100% Research Identifiable Files (RIFs) to examine healthcare use and cost burden associated with HD. Medicare RIFs contain beneficiary-level information on demographics, enrollment, and administrative claims for healthcare received by Medicare beneficiaries, including Part A (e.g. institutional claims), Part B (non-institutional claims), and Part D (drug claims). Medicare provides coverage to all US citizens who are at least 65 years old, are less than 65 years old who have received Social Security Disability Insurance (SSDI) for 24 months, or have other qualifying circumstances. For conditions such as HD, patients younger than 65 years of age must wait at least five months to qualify for SSDI before later becoming eligible for Medicare as described aboveCitation8. This study received approval for a full waiver of Health Insurance Portability and Accountability Act (HIPAA) authorization from the Western Institutional Review Board.
Patient population
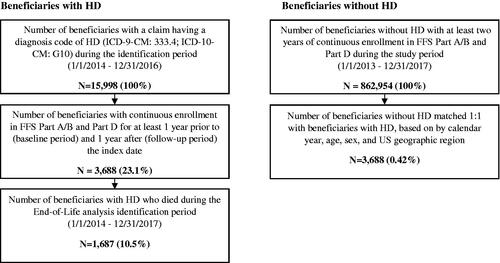
We identified Medicare beneficiaries who were diagnosed with HD based on the presence of at least one medical claim with an International Classification of Diseases, Ninth or Tenth Revision, Clinical Modification (ICD-9/10-CM) diagnosis code for HD (ICD-9-CM: 333.4; ICD-10-CM: G10) during the identification (ID) period of January 1, 2014 to December 31, 2016. The date of the first HD claim was assigned as the index date. If a patient had multiple claims with an HD diagnosis code during the ID period which met the enrollment criteria, one was randomly chosen as the index date to capture all disease stages. Patients were required to have continuous enrollment in FFS Parts A/B and Part D plans for at least one year prior to (baseline period) and one year after (follow-up period) the index date (); beneficiaries with Medicare Managed Care (Part C) were not included in this analysis because healthcare utilization and cost information for this population may be incomplete in RIFs. Beneficiaries with HD were compared to beneficiaries without HD (i.e. beneficiaries without a diagnosis of HD during the entire study period) who were drawn from a separate, 5% random sample of Medicare beneficiaries. The beneficiaries without HD were exactly matched to beneficiaries diagnosed with HD on a 1:1 ratio by age, sex, and US geographic region; beneficiaries without HD were then assigned the same index date as their matched case and had to meet the same enrollment requirement.
Figure 1. Study time frame. Abbreviations. EOL, end-of-life; ID, identification; Pre Q1 and Pre Q2, the 3-month periods immediately preceding death and Pre Q1, respectively.
To investigate outcomes along a continuum of HD progression, we stratified beneficiaries with HD by disease stage (early, middle, late) and examined those with HD at the end of life. The disease stage was measured in the 1-year post-index period based on a published hierarchical algorithm that assigns stage based on the presence of disease markers (i.e. diagnoses or services) received in claims during the index yearCitation4. Beneficiaries with the late-stage disease were identified first based on the presence of any of the following markers: nursing home, feeding tube, incontinence, bedsore, hospice care, at least two falls within a one-month period, and dysphagia. Among the remaining beneficiaries, those with the following markers were classified as having middle-stage disease: home assistance, physical therapy, dementia, gait disorder, dysarthria, speech therapy, and having two falls in a one-month period. Finally, beneficiaries without late- or middle-stage disease markers were defined as having early-stage diseaseCitation4.
For the end-of-life analysis, HD beneficiaries who died between January 1, 2014 and December 31, 2017 were observed in the last two quarters prior to their death (end-of-life period); such patients were required to have continuous enrollment in Medicare FFS Parts A/B and Part D in the one year preceding the date of death.
Study measures and analysis
Demographic characteristics, HD symptoms, and comorbidities, including the Charlson comorbidity index (CCI), a proxy measure for underlying health status and risk of mortality, and the number of chronic conditionsCitation9–11, were measured during the baseline period. Annual healthcare utilization was measured during the follow-up period and was reported by setting or type of care, which included emergency department (ED) visits, inpatient hospitalizations, physician office visits, skilled nursing facility (SNF) care, hospice care, and use of durable medical equipment (DME). Medication use was reported for HD treatment (i.e. tetrabenazine, deutetrabenazine, neuroleptics, amantadine, riluzole, donepezil, minocycline, nabilone, coenzyme Q10, and energy metabolites such as creatine and omega-3 fatty acids), antidepressants, anxiolytics, antiepileptic agents, and antispasticity agents (). Costs were calculated for inpatient (e.g. acute hospitalization, SNF, hospice, or other inpatients), outpatient (e.g. ED, physician office, lab, or other outpatient facilities), and outpatient pharmacy services (all-cause and HD pharmacy). Costs were adjusted to 2017 US dollars using the medical component of the Consumer Price IndexCitation12. Outcomes were reported as all-cause and HD-related; HD-related claims were defined as claims that contained a diagnosis code for HD or for an HD symptom (i.e. chorea, dementia, depression, anxiety, cachexia, dysphagia) in any position.
Among HD beneficiaries who died between 2014 and 2017, outcomes were examined at the end of life in the last two quarters before death: The first quarter (Pre Q1) was defined as the 3 months immediately preceding death, while Pre Q2 was defined as the 3 months preceding Pre Q1. In addition, the comorbidity and disease stage variables were measured in the year preceding death.
Descriptive statistics were reported for all outcome measures (means and standard deviations for continuous variables; frequencies and proportions for categorical variables) for the main comparison groups, by disease stage, and at the end of life. Chi-square tests were used for comparison of categorical outcomes and t-tests were used for continuous outcomes at a significance level of 0.05. All statistical analyses were conducted using SAS version 9.4.
Results
Healthcare utilization and costs in beneficiaries with vs. without Huntington’s disease
We identified a total of 3,688 Medicare beneficiaries with HD and 3,688 matched beneficiaries without HD who met all eligibility criteria and were included in the analysis (). As shown in , the matched cohorts had a mean (standard deviation, SD) age of 67.8 (12.4) years, the majority were female (53.6%), and represented all geographic regions in the US: Midwest (33.2%), South (30.4%), Northeast (23.1%), and West (13.3%). Both cohorts were predominantly white, although more so among the beneficiaries diagnosed with HD compared to beneficiaries without HD (90.6% vs. 86.9%, p < .001). Beneficiaries with HD compared to those without had similar mean (SD) CCI (2.0 [2.5] vs. 2.0 [2.4], p = .366), but slightly more chronic conditions (5.4 [2.6] vs. 5.0 [2.5], p < .001) at baseline. The occurrence of symptoms usually associated with HD was more frequent among beneficiaries with HD versus those without (81.3% vs. 28.1%, p < .001), with the most common diagnoses being chorea (58.5%), anxiety (33.6%), and dysphagia (29.4%).
Figure 3. Annual healthcare costs among Medicare beneficiaries, with vs. without Huntington's diseasea.
aBeneficiaries without HD were exactly matched to beneficiaries diagnosed with HD on a 1:1 ratio by age, sex, and US geographic region.
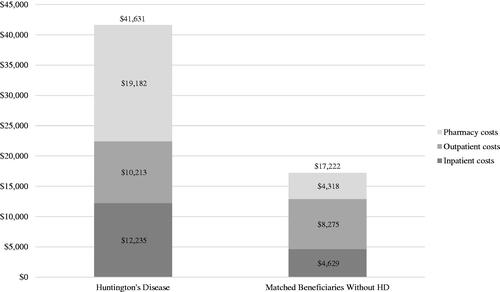
Table 1. Medication categories (GPI 10).
Compared to beneficiaries without HD, HD beneficiaries had a higher proportion of annual all-cause inpatient hospitalization (26.8% vs. 16.8%) and emergency department (ED) visits (36.1% vs. 26.2%), but fewer mean office visits (10.9 [13.6] vs. 12.8 [13.0]) (all p < .001; ). HD beneficiaries had significantly higher prescription drug use for antidepressants (60.0% vs. 32.8%), anxiolytics (28.1% vs. 18.6%), and antiepileptics (40.0% vs. 23.0%) (all p < .001), but not for antispasticity agents (10.8% vs. 10.6%; p = .792). Mean annual all-cause total costs were higher in beneficiaries with HD ($41,631 [57,393] vs. $17,222 [31,218]), which were driven by pharmacy costs ($19,182 [45,469] vs. $4,318 [11,553]), followed by inpatient costs ($12,235 [26,796] vs. $4,629 [15,467]) and outpatient costs ($10,213 [15,521] vs. $8,275 [17,535]) (all p < .001) ().
Table 2. Baseline characteristics among medicare beneficiaries, with vs. without Huntington's disease.
Healthcare utilization and costs by stage of Huntington’s disease
Of the 3,688 beneficiaries with HD included in the main analysis, 1,922 (52.1%) qualified as late-stage, 916 (24.8%) qualified as middle-stage, and the remaining 850 (23.1%) qualified as an early-stage disease (). The mean (SD) age was significantly lower for early-stage, while beneficiaries with the middle and late-stage disease had similar ages (64.6 [12.2], 69.3 [11.5], 68.5 [12.7]; p < .001). Beneficiaries with late and middle-stage HD had a higher proportion of female beneficiaries compared to those with early-stage disease (48.8%, 57.0%, 54.1%; p < .002). The mean (SD) CCI increased by disease stage (1.3 [2.1], 2.1 [2.4], 2.2 [2.6]; p < .001), as did the mean (SD) number of chronic conditions (4.3 [2.4], 5.5 [2.4], 6.0 [2.6]; p < .001). Finally, the proportion of beneficiaries with symptoms of HD increased by disease stage (67.9%, 76.4%, 89.6%; p < .001).
Table 3. Annual healthcare utilization and costs among Medicare beneficiaries, with vs. without Huntington's disease.
Annual all-cause healthcare utilization increased with disease stage (early, middle, late) (). Late-stage beneficiaries had the highest proportion of hospitalization (8.4%, 20.3%, 38.1%) and ED visits (19.8%, 36.7%, 43.1%). Late-stage beneficiaries had higher use of HD treatment (42.0%, 51.4%, 64.5%), including tetrabenazine (13.4%, 13.5%, 18.6%) and neuroleptics (30.1%, 35.8%, 49.7%), and higher use of supportive medications such as antidepressants (49.5%, 58.4%, 65.3%), anxiolytics (22.2%, 27.1%, 31.2%), antiepileptics (30.1%, 38.4%, 45.1%), and, to a lesser extent, antispasticity agents (9.3%, 10.6%, 11.6%). Mean (SD) annual all-cause total costs were higher for late-stage beneficiaries ($20,475 [$41,122], $29,733 [$44,977], $56,657 [$64,185]), driven by pharmacy costs ($14,831 [38,774], $15,898 [40,503], $22,672 [49,964]), followed by outpatient costs ($4,484 [10,185], $9,811 [13,478], $12,939 [17,549]) and inpatient costs ($1,160 [6,149], $4,024 [11,510], $21,047 [33,678]) (, ).
Figure 4. Annual and quarterly healthcare costs by disease stagea and at end of lifeb (all-cause)c.
aLate-stage disease markers identified first: nursing home, feeding tube, incontinence, bedsore, hospice care, at least two falls within one month, and dysphagia. Middle-stage disease markers identified second: home assistance, physical therapy, dementia, gait disorder, dysarthria, speech therapy, and having two falls in one month. Beneficiaries without late or middle-stage disease markers were defined as an early-stage diseases.
bAt the end of life, Pre Q1 was defined as the 3 months immediately preceding death and Pre Q2 was defined as the 3 months preceding Pre Q1.
cCosts reported by disease stage reflect annual costs; end-of-life costs reported in Q1 and Q2 reflect quarterly costs.
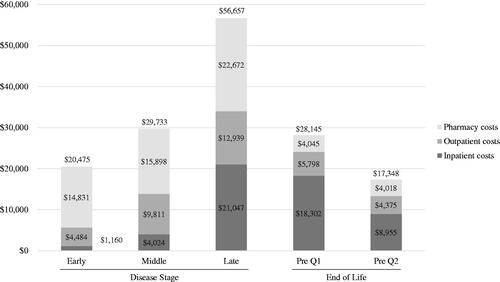
Table 4. Baseline characteristics among Medicare beneficiaries with Huntington’s disease, by disease stage and at end of life.
Table 5. Annual and quarterly healthcare utilization and costs among Medicare beneficiaries with Huntington’s disease, by disease stage and at end of lifea.
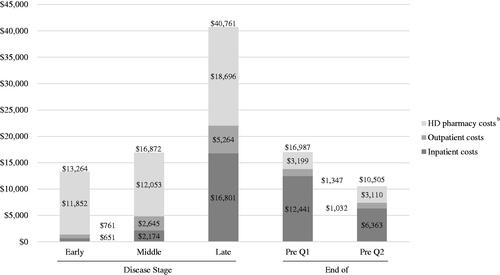
Annual HD-related utilization also increased with disease stage (early, middle, late) (). Late-stage beneficiaries had the highest proportion of hospitalization (5.6%, 14.4%, 33.5%) and ED visits (7.8%, 17.6%, 25.8%). As shown in (), mean annual HD-related total costs were higher for late-stage beneficiaries ($13,264 [$37,254], $16,872 [37,154], $40,761 [56,990]), driven by HD pharmacy costs ($11,852 [36,748], $12,053 [36,203], $18,696 [49,006]), followed by outpatient costs ($761 [1,296], $2,645 [3,910], $5,264 [7,132]) and inpatient costs ($651 [3,316], $2,174 [7,574], $16,801 [28,137]).
Figure 5. Annual and Quarterly Healthcare Costs by Disease Stage and at End of Life (Huntington's Disease-Related)a.
aCosts reported by disease stage reflect annual costs; end-of-life costs reported in Q1 and Q2 reflect quarterly costs.
bCosts reported for full HD pharmacy category including tetrabenazine, deutetrabenazine, neuroleptics, amantadine, riluzole, donepezil, minocycline, nabilone, coenzyme Q10, and energy metabolites.
Healthcare utilization and costs in beneficiaries with Huntington’s disease at the end-of-Life
A total of 1,687 Medicare beneficiaries diagnosed with HD died between 2014 and 2017 and were included in the end-of-life analysis. Of these beneficiaries, 1,513 (89.7%) had late-stage disease prior to death (results not shown) and 1,605 (95.1%) had symptoms of HD (). Mean (SD) age at death was 73.2 (12.9) years, the majority were female (50.3%) and white (91.6% white, 6.2% black, 2.2% unknown), with all regions represented (): Midwest (33.6%), South (31.2%), Northeast (24.0%), West (11.2%). Beneficiaries in the end-of-life cohort had a mean CCI of 4.4 (4.0) and a mean number of chronic conditions of 7.5 (3.0).
Healthcare utilization and costs were elevated in the quarter immediately prior to death (Q1), while less so in the quarter prior (Q2). Beneficiaries in Q1 and Q2 had a high proportion of hospitalization (48.7% and 20.0%) and ED visits (30.8% and 20.3%) (). Similarly, the proportion of HD-related hospitalization was greater in Q1 (40.7% and 15.5%) as was the proportion of HD-related ED visits (15.6% and 9.1%).
Mean (SD) all-cause total costs in Q1 and Q2 were $28,145 (31,932) and $17,348 (24,759), respectively, both composed mainly of inpatient costs ($18,302 [25,696] and $8,955 [19,631]) (, ). For HD-related costs, mean (SD) total costs in Q1 and Q2 were $16,987 (21,710) and $10,505 (18,812), respectively, again driven by inpatient costs ($12,441 [18,531] and ($6,363 [15,951]) (, ).
Discussion
This comprehensive analysis of healthcare utilization and economic burden among Medicare beneficiaries with HD is the first of its kind. We analyzed administrative claims databases from Medicare RIFs, the largest and most comprehensive data source for Medicare beneficiaries, which is ideal for capturing patients with rare diseases such as HD, and found that healthcare needs and associated costs are substantially higher among Medicare beneficiaries who are diagnosed with HD compared to their matched beneficiaries without HD. Broadly, our findings in the Medicare FFS population underscore the substantial economic burden facing those diagnosed with HD and are consistent with previously published literature. Among Medicare beneficiaries with HD, nearly two-thirds of whom were in the late stage of disease, more than one-quarter were hospitalized during the year, which was 1.5 times that of matched beneficiaries without HD. In addition, the use of prescription medications, including antidepressants and anxiolytics, was higher in HD beneficiaries compared to beneficiaries without HD, corresponding to prevailing comorbid depression and anxiety that has been reported in individuals with HDCitation13–16. Similarly, annual healthcare costs were also substantial for beneficiaries with HD, with mean annual total costs of about $41,000, which is more than twice that of costs for beneficiaries without HD and ostensibly driven by inpatient costs and pharmacy costs. In particular, the mean annual HD pharmacy cost was over $15,000 and 37% of the total all-cause cost burden.
We also observed progressive burden among beneficiaries with HD throughout each stage of disease, including at the end of life; this is consistent with previous literatureCitation4. A 2013 study using 2002–2009 commercial and Medicaid claims found that direct medical costs increased with disease progression in beneficiaries with HD and were driven by outpatient servicesCitation4; this study employed the same hierarchical algorithm for measuring HD disease stage in claims as our study. In our study, beneficiaries in the late stage of HD had the highest level of utilization and costs relative to earlier stages, with one-third experiencing an HD-related hospitalization in one year compared to 6% and 14% of beneficiaries with early and middle-stage disease, respectively. Medication use was elevated across all stages, but even more so among beneficiaries in the late stage of HD. While we found that total costs increased by stage, in contrast with the 2013 study, we found this cost pattern to be driven by inpatient services, reflecting the hospitalization patterns described above. In the end-of-life analysis, we found that acute hospitalization and hospice care were very common (49% and 57%) in the last few months before death, corresponding to high inpatient costs during the same period. A 2005 study using 1996–2002 inpatient hospital data reported that 53.5% of HD patients with inpatient hospitalizations were transferred to a long-term care facilityCitation17. Although our study did not examine long-term nursing care, use of skilled nursing facilities and hospice care was observed in over 40% of beneficiaries with late-stage HD. Additionally, a 2019 literature review estimated that costs at the end of life account for 13–25% of the general Medicare population’s expendituresCitation18, which is consistent with our findings of elevated costs in the final months of life among beneficiaries with HD who died.
In this study, we found all-cause costs were higher than those previously reported for other neurodegenerative diseases. A 2017 investigation of direct annual medical expenditures associated with Alzheimer's disease and related dementias estimated annual total costs of $14,508 in this populationCitation19. In a 2010 study using commercial insurance claims from 2004 to 2006, patients with multiple sclerosis were estimated to have an annual all-cause cost burden of $18,829 (adjusted to 2010 US dollars); this would be equivalent to $21,166 in 2017 US dollarsCitation20. In a 2005 study using commercial claims from 1999 to 2002, patients with Parkinson’s disease were estimated to have annual direct medical costs of $23,101 (adjusted to 2002 US dollars) Citation21; this would be equivalent to $30,775 in 2017 US dollars, although changes in the treatment of Parkinson’s disease since this period would likely contribute to further shifting of costs. In a more recent 2020 study, the annual direct medical costs in beneficiaries with Parkinson’s disease were estimated to be $24,439 (2017 US dollars)Citation22.
We believe our findings have importance for various reasons. Comparatively, our cost estimates associated with HD are higher than previously published estimates in other HD populationsCitation4, which may reflect the unique needs and disease burden in the Medicare HD population due to disability or age. Medicare beneficiaries who are diagnosed with HD have an additional clinical burden and intensity in healthcare utilization compared to the general Medicare population, pointing to an increased vulnerability among the small, but the older population of adults with HD. Furthermore, we observed that most (66.6% of) beneficiaries identified as having HD in our sample were not newly diagnosed, based on a prior diagnosis code for HD during the baseline period (results not shown); as the onset of HD typically occurs earlier in life, this finding may indicate prolonged burden in the HD Medicare population. In addition, this burden increases substantially by disease stage; our findings reflect the need for disease-modifying therapy to delay disease progression and early diagnosis of HD to reduce healthcare burden.
This study has several limitations. First, our measure of disease stage was constructed, in part, using variables for healthcare utilization. As a result, the observed increase in costs by stage is, to some degree, an artifact of the construction of the disease stage measure. However, in the absence of clinical measures in claims data, healthcare utilization is a useful proxy for the disease stage. Second, we did not examine the full Medicare population, as only beneficiaries in FFS plans (∼65% of the Medicare plans) were included in our sampleCitation23; therefore, our findings may not be representative of patients with HD in non-FFS Medicare plans (i.e. Medicare managed care) or those with other types of insurance (e.g. commercial, Medicaid) who may be younger and with different clinical characteristics. Third, our analysis of Medicare claims data may not have uncovered the entire burden of HD among beneficiaries; for example, resource burden among beneficiaries who receive long-term nursing care, which beyond 100 days of SNF care is not covered by Medicare, would not be captured in these claims. Additionally, our analysis did not assess indirect costs, such as informal nursing or personal care, which has been found to be a major driver of costs in HD outside of the USCitation24, or functional status, which might further elucidate the burden of HD in the late-stage of disease or end of life. Fourth, we matched the study cohorts based on demographic, but not clinical characteristics, which may have led to confounding in our results. Fifth, similar to prior studies, our eligibility criteria required only one diagnosis of HD without a second confirmatory diagnosisCitation4–6. While the final cohort may contain beneficiaries with a misdiagnosis of HD, the rate of misdiagnosis is expected to be low due to the availability of genetic testing that can confirm the diagnosis. Finally, administrative claims are intended for billing purposes, not research. Claims data capture events relevant to medical billing and pertinent diagnoses, and may not capture all underlying symptoms, comorbidities, and other adverse events.
Conclusions
The results of this study demonstrate the substantial economic burden associated with HD. Medicare beneficiaries with HD have significantly higher healthcare utilization and costs compared with beneficiaries without HD, which were primarily driven by higher inpatient hospitalization and pharmacy utilization. As expected, such clinical and economic burden increases with the progression of HD among Medicare beneficiaries and is most intensive at the end of life. Our findings reflect the need for effective treatments that delay or prevent disease progression, which may reduce the burden associated with HD.
Transparency
Declaration of funding
This research was sponsored by Genentech, Inc. and funded by F. Hoffmann-La Roche Ltd.
Declaration of financial/other relationships
AE is an employee of Genentech, Inc., and shareholder of F. Hoffmann-La Roche Ltd. SRR is an employee of PHAR, LLC, which was paid by Genentech, Inc. to conduct this research. EC is an employee of PHAR, LLC, which was paid by Genentech, Inc. to conduct this research. JTT is an employee of Genentech, Inc. AMP is an employee and shareholder of Genentech, Inc. CP is an employee of PHAR, LLC, which was paid by Genentech, Inc. to conduct this research. GJY has no conflicts to disclose. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors fulfilled the ICMJE authorship requirements.
Previous presentations
Figures and tables in this manuscript have been included in posters presented at the 2020 Academy of Managed Care Pharmacy (AMCP) conference.
Acknowledgements
None stated.
Data availability statement
Data is available from the Centers for Medicare and Medicaid Services (CMS).
References
- Peavy GM, Jacobson MW, Goldstein JL, et al. Cognitive and functional decline in Huntington's disease: dementia criteria revisited. Mov Disord. 2010;25(9):1163–1169.
- Myers RH. Huntington's disease genetics. NeuroRx. 2004;1(2):255–262.
- Keum JW, Shin A, Gillis T, et al. The HTT CAG-Expansion mutation determines age at death but not disease duration in Huntington disease. Am J Hum Genet. 2016;98(2):287–298.
- Divino V, DeKoven M, Warner JH, et al. The direct medical costs of Huntington's disease by stage. A retrospective commercial and Medicaid claims data analysis. J Med Econ. 2013;16(8):1043–1050.
- Anderson KE, Divino V, DeKoven M, et al. Interventional differences among Huntington’s disease patients by disease progression in commercial and Medicaid populations. J Huntingtons Dis. 2014;3:355–363.
- Sung VW, Iyer RG, Gandhi SK, et al. Retrospective analysis of healthcare resource use, treatment patterns, and treatment-related events in patients with Huntington's disease-associated chorea initiated on Tetrabenazine. J Health Econ Outcomes Res. 2018;6(1):15–24.
- Mendizabal A, Ngo Vu A-T, Thibault D, et al. Hospitalizations of children with Huntington’s disease in the United States. Mov Disord Clin Pract. 2017;4(5):682–688.
- Kaiser Family Foundation. Medicare’s Role for People under Age 65 with Disabilities. Kaiser Family Foundation – Medicare. 2016. [cited 2021 Oct 7]. Available from: https://www.kff.org/medicare/issue-brief/medicares-role-for-people-under-age-65-with-disabilities/
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- Agency for Healthcare Research and Quality. HCUP Chronic Condition Indicator. Healthcare Cost and Utilization Project (HCUP). 2015. Available from: www.hcup-us.ahrq.gov/toolssoftware/chronic/chronic.jsp
- United States Department of Labor, Bureau of Labor Statistics. Consumer Price Index – December 2017. United States Department of Labor, Bureau of Labor Statistics; 2018. [cited 2021 Jan 7]. Available from: https://www.bls.gov/news.release/archives/cpi_01122018.pdf
- Anderson KE, van Duijn E, Craufurd D, et al. Clinical management of neuropsychiatric symptoms of Huntington disease: expert-based consensus guidelines on agitation, anxiety, apathy, psychosis and sleep disorders. J Huntingtons Dis. 2018;7(3):355–366.
- Epping EA, Paulsen JS. Depression in the early stages of Huntington disease. Neurodegener Dis Manag. 2011;1(5):407–414.
- Paulsen JS, Nehl C, Hoth KF, et al. Depression and stages of Huntington's disease. J Neuropsychiatry Clin Neurosci. 2005;17(4):496–502.
- Ishihara L, Oliveri D, Wild EJ. Neuropsychiatric comorbidities in Huntington's and Parkinson's disease: a United States claims database analysis. Ann Clin Transl Neurol. 2021;8(1):126–137.
- Dubinsky RM. No going home for hospitalized Huntington's disease patients. Mov Disord. 2005;20(10):1316–1322.
- Duncan I, Ahmed T, Dove H, et al. Medicare cost at end of life. Am J Hosp Palliat Care. 2019;36(8):705–710.
- Deb A, Sambamoorthi U, Thornton JD, et al. Direct medical expenditures associated with Alzheimer's and related dementias (ADRD) in a nationally representative sample of older adults – an excess cost approach. Aging Ment Health. 2018;22(5):619–624.
- Asche CV, Singer ME, Jhaveri M, et al. All-cause health care utilization and costs associated with newly diagnosed multiple sclerosis in the United States. JMCP. 2010;16(9):703–712.
- Huse DM, Schulman K, Orsini L, et al. Burden of illness in Parkinson's disease. Mov Disord. 2005;20(11):1449–1454.
- Yang W, Hamilton JL, Kopil C, et al. Current and projected future economic burden of Parkinson's disease in the U.S. NPJ Parkinsons Dis. 2020;6:15.
- Centers for Medicare & Medicaid Services. CMS Fast Facts. 2020. [cited 2020 Nov 30]. Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/CMS-Fast-Facts
- Jones C, Busse M, Quinn L, et al. The societal cost of Huntington's disease: are we underestimating the burden? Eur J Neurol. 2016;23(10):1588–1590.