?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objectives
To perform a systematic review and meta-analysis to pool the incremental net benefit (INB) of each herpes zoster vaccine [i.e. Zoster Vaccine Live (ZVL) and Recombinant Zoster Vaccine (RZV)].
Methods
We initially identified individual studies by hand-searching reference lists of the relevant systematic review articles. An updated comprehensive search was performed in Medline, Scopus, and Embase until June 2020 for additional studies. Studies were eligible if they assessed the cost-effectiveness/utility of any pair among ZVL and RZV, and no vaccine and reported economic outcomes. Details of the study characteristics, economic model inputs, costs, and outcomes were extracted. INB was calculated with monetary units adjusting for purchasing power parity for 2019 US dollars and pooled by meta-analysis.
Results
A total of 37 studies were pooled for meta-analysis stratified by perspectives [i.e. societal (SP) and third-party payer (TPP)] and vaccine types. In SP, ZVL was cost-effective compared to no vaccine when vaccinated at ages of 50–59 and 70–79 years with INBs (95% CI) of $0.61 (0.37, 0.85) and $9.67 (5.20, 14.14), respectively. RZV was cost-effective for those aged 60–69 and 70–79 years with INBs of $75.61 (17.98, 133.23) and $85.01 (30.02, 140.01), respectively. In TPP, ZVL was cost-effective compared to no vaccine when vaccinated at age 70–79 years with INB of $7.57 (0.27, 14.86) and RZV was cost-effective at 60–69 years with INB $220.87 (47.80, 393.93). The cost-effectiveness of RZV was robust across a series of sensitivity analyses, but ZVL differs on different vaccination ages.
Conclusions
RZV may be cost-effective for vaccination in ages of 60–79 years for both SP and TPP perspectives, while ZVL might be cost-effective in some age groups, but results are not robust.
Introduction
Herpes zoster (HZ), also known as shingles, caused by reactivation of latent varicella-zoster virus (VZV) or chickenpox reservoir, occurs frequently in individuals aged 50 years or olderCitation1,Citation2. HZ and its complications, such as post-herpetic neuralgia (PHN), can have a substantial negative impact on quality of life. The incidence of herpes zoster has been increasing throughout the world, causing substantial morbidityCitation1. In 2013, there were more than 1 million cases of HZ in the United States (US) alone, with the direct and indirect medical costs of infection estimated at $1.3 billion and $1.7 billion, respectively, annuallyCitation3,Citation4. This burden is expected to rise significantly due to an aging population and an increasingly large pool of at-risk individuals worldwideCitation5,Citation6. Hence, the prevention of HZ is an important public health objective.
Currently, two HZ vaccines are available [i.e. the non-live subunit Recombinant Zoster Vaccine (RZV) and the attenuated Zoster Vaccine Live (ZVL)] which are approved by the Food and Drug Administration (FDA) for adults aged 50 years or older. ZVL is a single-dose regimen, whereas RZV is a two-dose regimen, in which a booster should be administered 2–6 months after the first dose. Current recommendations from the Advisory Committee on Immunization Practices (ACIP) prefer RZV over ZVL for most patients because it provides greater protection, especially among people in their seventh to the ninth decadeCitation7. Moreover, ZVL is gradually being replaced by RZV in many countriesCitation8. For example, as of November 2020, ZVL is no longer available in the USCitation9; however, ZVL continues to be used in many high-income countries since RZV is not yet widely available because of its limited supplyCitation8. Additionally, a few countries still incorporated ZVL in their immunization programs funded through National Health System (e.g. Australia, Italy, France, etc.)Citation8. Meanwhile, HZ vaccination has not become routine practice in many low- and middle-income countries (LMICs) because of the limited availability of both vaccines, and ZVL remains the first option in these countriesCitation10,Citation11.
Cost-effectiveness analyses (CEAs) provide information to decision-makers for efficient use of available resources for maximizing health benefits. A previous systematic review of CEAs of HZ vaccination summarized that most included studies found HZ vaccination cost-effectiveCitation12. However, the evidence was largely available for only ZVL because of the limited number of CEAs on RZV in these reviews. The cost-effectiveness of HZ vaccination seemed to be influenced by vaccine types and their price, age groups, perspectives of analyses, region or country-level income, and many other factorsCitation12. It is important for global decision-makers from both resource-rich and resource-poor countries to know how these factors influence the cost-effectiveness of HZ vaccination. However, previous reviews provided only an overall descriptive summary of evidence from individual cost-effectiveness studies. Recently, Crespo et al. suggested a meta-analysis method of CEAsCitation13. This approach is now getting recognition by the wider scientific communityCitation14–18. The evidence generated from a meta-analysis of CEAs was found to be useful to decision-makers in several aspects as it provides (1) an overall summary of all economic evidence quantitatively, and (2) a summary stratified by perspectives [e.g. societal (SP), third-party payer (TPP), etc.], vaccine types, age groups, and other factors as mentioned above, to provide specific evidence for each group with less heterogeneity. Immunization and Vaccine-related Implementation Research Advisory Committee (IVIR-AC)-WHO (March 2021) agrees that meta-analysis of CEAs is useful and could facilitate decision-making in countries where context-specific CEAs are not availableCitation19.
Therefore, we conducted a systematic review and meta-analysis of CEAs by pooling incremental net benefit (INB) to assess the cost-effectiveness of ZVL and RZV compared to no vaccine (NoV) and each other in patients aged 50 years and older. The purpose of this research is to emphasize the economic values of HZ vaccines and inform policymakers especially in those countries where economic evidence is limited for the selection of two choices of HZ vaccine.
Methods
This study was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (Supplementary Appendix I). The review protocol was registered with Open Science Framework (OSF) and can be found at osf.io/4f867.
Data source and searches
We performed an updated comprehensive search to identify additional individual studies using MEDLINE via PubMed, Scopus, and EMBASE from the date of the last search conducted by Chiyaka et al. in March 2018 to 9 June 2020Citation12. Search terms used were “herpes zoster,” “vaccination,” and “economic evaluation” (Supplementary Appendix II).
Study selection
Titles, abstracts, and full texts of the studies identified by the updated search were screened by two independent reviewers (SU and MR). Individual studies from the updated search and Chiyaka et al. were included if they met the following criteria: conducted CEA or cost-utility analysis (CUE) by comparing any pair among ZVL, RZV, and NoV and reported economic outcomes. Studies were excluded if they did not provide sufficient economic data. Any disagreement in study selection was discussed with a third reviewer (NC).
Data extraction and quality assessment
We extracted data including vaccine data (i.e. type, dose, and administration schedule), comparator, World Health Organization (WHO)-epidemiological regions, country income level or GDP (per the World Bank 2019 report), time horizon, study perspective, discount rate for costs and effects, model used, population of interest, data source, sensitivity analysis results, cost (C), incremental cost (ΔC), willingness-to-pay threshold (K), effectiveness/utility (E), incremental effectiveness/utility (ΔE), and the incremental cost-effectiveness ratio (ICER), along with their dispersions [standard deviation (SD), standard error (SE), or 95% confidence interval (CI)]. Effectiveness was measured as quality-adjusted life years (QALYs) or life-years (LYs) gained. The risk of bias was independently assessed at the study level by the same reviewers above using the modified Economic Evaluations Bias (ECOBIAS) checklist, which consists of 22 itemsCitation20. Each item was graded as yes, partly, unclear, no, or not applicable.
Data analysis and synthesis
Interventions
There were three comparisons of interest: ZVL vs. NoV, RZV vs. NoV, and RZV vs. ZVL. ZVL was a single-dose regimen, while RZV was a two-dose regimen. Other regimens of ZVL and RZV were also included, such as single-dose ZVL vs. ZVL with booster and RZV in the previous ZVL vs. ZVL.
Outcome of Interest
An incremental net benefit (INB) (also recognized as an incremental net monetary benefit) was the primary outcome of interest which was calculated using EquationEquation (1) or (2) depending on the parameters reported in the individual studyCitation13,Citation21,Citation22. The variances of INB were calculated using EquationEquations (3) or (4).
(1)
(1)
(2)
(2)
(3)
(3)
(4)
(4)
K is the willingness-to-pay threshold, ΔE and ΔC are the incremental effectiveness and cost, and ΔCΔE is the covariance between ΔC and ΔE. A positive INB indicates the intervention is cost-effective, whereas a negative INB indicates the intervention is not cost-effective and the comparator is preferred.
Data preparation
Data were prepared according to five scenarios described elsewhere and in Supplementary Appendix IIIaCitation14–18,Citation22. INB and its variance were then calculated accordingly. All cost data including K, ΔC, ICER, and their variances in each currency were standardized by converting to 2019 United States Dollar (USD) using consumer price index (CPI)Citation23 and purchasing power parity (PPP)Citation24 (Supplementary Appendix IIIb).
Statistical analysis
A meta-analysis was applied to pool INBs across studies using the Dersimonian-Laid random-effects model if heterogeneity was present (I2 > 25% or the Q test p < .1)Citation25; otherwise, a fixed-effect inverse-variance model was applied (Supplementary Appendix IIIc). Heterogeneity was assessed by Cochrane’s Q test and I2 statisticCitation26. Sources of heterogeneity were explored by considering the cost of vaccine and comparator covariables in a meta-regression model one by one. If the I2 decreased by 50% or more, such a covariable was considered a source of heterogeneity. Publication bias was assessed using a funnel plot and Egger’s test. The source of asymmetry was further explored using a contour-enhanced funnel plot by contouring the funnel as significant (i.e. <0.01, <0.05) and non-significant areas (i.e. >0.05–0.1)Citation27. If missing studies were in a non-significant area, publication bias might be a source of heterogeneity; if studies were mostly in both significant and non-significant areas, heterogeneity might play a role.
If studies reported the cost-effectiveness of vaccines for different perspectives, vaccine types, and age groups, the studies were considered and accounted for more than once according to the number of comparisons reported. All analyses were stratified by country-level income, perspectives (SP and TPP), and vaccine types (ZVL and RZ). Vaccination age was used in subgroup analyses. A series of prespecified sensitivity analyses were performed as follows: (1) imputation of variance values by borrowing values from other studies with similar characteristics, (2) analysis using the average values of vaccination age and INB across different vaccination age groups reported within-study, (3) excluding studies with missing variances from the analyses, and (4) excluding studies with a high risk of bias. Analyses were performed using Microsoft Excel and STATA version 16.0 and results were considered statistically significant for all analyses at p < .05 (two-sided).
Results
Study selection
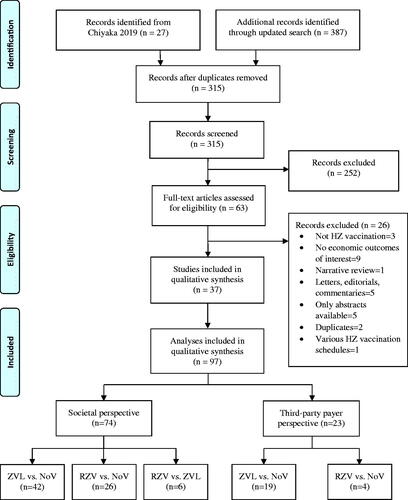
Twenty-seven individual studies were identified from a previous systematic reviewCitation12. We then performed an updated search and identified additional 387 studies. After screening 387 studies, 10 met the inclusion criteria and were added to the 27 previously identified studies. Therefore, a total of 37 studies consisting of 110 comparisons were included in the meta-analysis (). However, 13 comparisons were not included in the quantitative analysis because 12 were comparisons of various schedules of ZVL and RZV, and only one study compared RZV vs. ZVL in TPP, therefore there was not an adequate number of included studies for pooling. Finally, 97 comparisons from 37 studies were included in the synthesis ().
Study characteristics
According to WHO-epidemiological regionsCitation28, 17 studies were from European Region (EUR), 14 from the Americas Region (AMR), and 6 from Western Pacific Region (WPR) (). All studies were conducted in high-income countries. Most studies used decision-analytic or Markov model (35, 94.6%) and lifetime time horizon (33, 89.2%). Non-lifetime time horizons of 7.5, 10, and 15 years were used in 10.8% of the studiesCitation31,Citation37,Citation56.
Table 1. Characteristics of included studies (n = 37).
Of the 37 studies, 97 comparisons of HZ vaccines were identified according to different vaccines, perspectives, and age groups reported within the same study. Of the 97 comparisons, 74 (76.3%) used SP and 23 (23.7%) used TPP (Supplementary Appendix IV). Comparison pairs were single-dose ZVL with NoV (n = 61), 2-dose RZV with NoV (n = 30), and RZV with ZVL (n = 6). Vaccination ages were diversely reported as distinct ages, i.e. 50, 60, and up to 99 years, in ranges of 50–59, 60–69, and 70–79, and in open ranges of 50+, 60+, and 70+. Therefore, vaccination ages from all studies were combined and grouped into five subgroups including 50+ to 70+, 50–59, 60–69, 70–70, and 80–99 years. Data pooling was finally performed to compare INBs of different perspectives and vaccines; these included SP and TPP of ZVL vs. NoV, RZV vs. NoV, and RZV vs. ZVL, resulting in a total of 97 comparisons from 37 studies.
Risk of bias assessment
Risk of bias assessment was performed using the ECOBIAS checklist (Supplementary Appendix V). Overall, most studies had a similar bias profile. Bias related to reporting and dissemination were unclear for most studies. Studies that did not demonstrate bias related to cost measurement, structural assumptions, treatment comparator, model type, time horizon, data identification, baseline data, treatment effects, quality-of-life weights (utilities), data incorporation, and limited scope were determined to have a low risk of bias [15 studies (39.5%)].
Pooled INBs based on societal perspective
ZVL vs. NoV
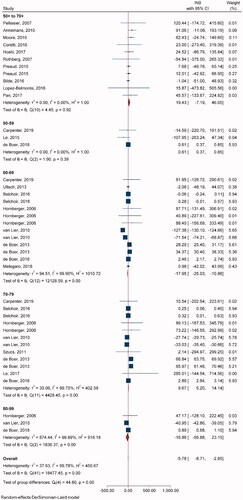
Twenty-one studies with 42 comparisons of single-dose ZVL vs. NoV were included in pooling INBs (). All studies used the decision-analytic or Markov model and lifetime time horizon. One study used a stochastic individual-based modelCitation60 and three studiesCitation31,Citation37,Citation56 used non-lifetime time horizon. Overall INB indicated that ZVL was not cost-effective relative to NoV [pooled INB (95% CI) −$5.78 (−8.71, −2.85)] (). For age subgroups, ZVL was cost-effective compared to NoV in ages 50–59 and 70–79 years with INBs of $0.61 (0.37, 0.85) and $9.67 (5.20, 14.14), respectively. Egger’s test showed asymmetry of the funnel in only 70–79 years (coefficient = 1.08, SE = 0.446, p = .0158), and a contour-enhanced funnel plot showed that most fell in the non-significant area, which indicated that asymmetry was likely due to heterogeneity rather than publication bias (Supplementary Appendix VIIa).
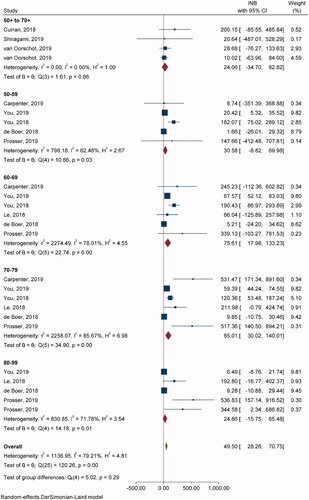
Table 2. Meta-analysis results for main groups.
RZV vs. NoV
Nine studies with 26 comparisons were included in pooling INBs of RZV vs. NoV (). Most of the studies used the Markov model and lifetime time horizon, except one study used simulation (state-transition model)Citation61 and another studyCitation56 used non-lifetime time horizon. Overall INB indicated that RZV was cost-effective compared to NoV with the INB (95% CI) of $49.5 (28.2, 70.7) (). In addition, RZV was more cost-effective in age groups 60–69 and 70–79 years with INBs of $75.61 (17.98, 133.23) and $85.01 (30.02, 140.01), respectively. Egger’s test showed significant publication bias for 70–79 (coefficient = 2.48, SE = 0.645, p = .001), and 80–89 years (coefficient = 2.25, SE = 0.610, p = .0002). The contour-enhanced funnel plot showed studies in both significant and non-significant areas which suggested that asymmetry was likely due to heterogeneity rather than publication bias (Supplementary Appendix VIIb).
RZV vs. ZVL
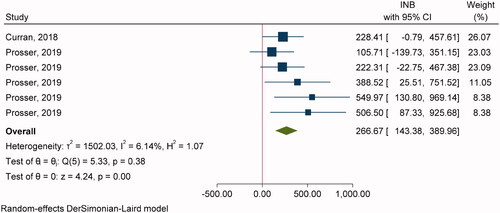
Two studiesCitation54,Citation61 with six comparisons were included in pooling INBs of RZV vs. ZVL with SP (). The overall pooling indicated that RZV was cost-effective compared to ZVL with the pooled INB (95% CI) of $266.67 (143.38, 389.97) (). Egger’s test showed significant publication bias (coefficient = 3.50, SE = 1.662, p = .0352) but contour-enhanced funnel plot showed studies in both significant and non-significant areas which suggested that asymmetry was likely due to heterogeneity rather than publication bias (Supplementary Appendix VIIc).
Pooled INBs based on third-party perspective (TPP)
ZVL vs. NoV
Fourteen studies with 19 comparisons of ZVL vs. NoV were included with TPP (). Overall pooling indicated that ZVL was cost-effective relative to NoV with the pooled INB (95% CI) of $8.21 (4.10, 12.32) (Supplementary Appendix VIa). Subgroup analysis by age groups indicated that ZVL was cost-effective in 50+ to 70+ and 70–79 years with INBs of $8.63 (3.62, 13.63) and $7.57 (0.27, 14.86), respectively. Egger’s test resulted in non-significant publication bias (coefficient = 0.19, SE = 0.264, p = .4754) (Supplementary Appendix VIId).
RZV vs. NoV
Four comparisons from four studies were included in pooling overall INBs showing RZV as cost-effective with the pooled INB (95% CI) of $133.25 (14.26, 252.25) (Supplementary Appendix VIc). Egger’s test resulted in non-significant publication bias (coefficient = −1.50, SE = 1.359, p = .2708) (Supplementary Appendix VIIe).
Sources of heterogeneity
Sources of heterogeneity were explored considering the cost of vaccines and comparators according to the comparisons ZVL vs. NoV, RZV vs. NoV, and RZV vs. ZVL (Supplementary Appendix VIII). None of them were identified as a source of heterogeneity from meta-regression results.
Sensitivity analyses
Sensitivity analysis showed that cost-effective results of RZV compared to NoV were robust in all sensitivity analyses, especially in the 70–79 age group (Supplementary Appendix IX). Cost-effectiveness results of ZVL differ across sensitivity analyses, especially when restricted to studies with non-missing variances and those with a low risk of bias. In addition, vaccination in 50–59 and 70–79 age groups was no longer cost-effective.
Discussion
In this study, we reviewed 37 published cost-effectiveness studies of HZ vaccine and performed meta-analyses by pooling INB to assess the cost-effectiveness of ZVL and RZV in patients aged 50 years and older. All included studies were conducted in high-income countries. Our findings demonstrated that RZV was found to be cost-effective as seen from positive INBs compared with NoV in both SP and TPP, especially for age 60 years and above. Comparing the two vaccines, RZV was dominant over ZVL in SP. However, the number of individual studies that directly compared two vaccines was limited, and even lacking in TPP. Meanwhile, ZVL was also cost-effective compared with NoV when vaccinated at ages 70–79 years from SP and TPP. ZVL from SP was also cost-effective in 50–59 years.
In our main analysis, we stratified the studies by different perspectives and vaccine types with subgroups of age resulting in more than one comparison and weights from one study. One of the sensitivity analyses performed to assess the robustness of our findings was utilizing the average values of age and INB to weight one study once and to avoid violation of the independence assumption. The results from sensitivity analyses were robust for RZV, but not for ZVL. In addition, we found RZV’s superiority over ZVL with positive INBs for all the age groups analyzed. Our findings for RZV are consistent with recommendations from the AICP as the preferred type of vaccineCitation7. However, It is important to note that the age for which we consistently saw greater INB benefit in RZV vaccination, 60 and above, is older than the current ACIP recommendation of 50 years and older. Additional research should be conducted to assess the impact of delaying vaccination, as aging is an important risk factor for HZ infection. As the world population continues to age and an increasing number of individuals are at risk for developing HZ, extending the vaccination age may be more cost-effective in those countries that authorized the use of RZV but confront limited supply.
With FDA approval and ACIP’s recommendation for the preference of RZV over ZVL, the market share of the RZV is predicted to change drastically and is expected to be more expensive. Moreover, RZV is now only available in a few countries including the United States, Canada, Belgium, Germany, Sweden, the Netherlands, etc. because of limited supplyCitation66. Although it is licensed in the European Union, Japan, New Zealand, Hong Kong, China, and Singapore, it is not yet available, and ZVL continues to be used in these countries and in LMICs. From ZVL comparisons, we have recognized that it is cost-effective compared to NoV when vaccinated at ages 70–79 years. Although we found a positive INB for ZVL for patients aged 50–59 years, the ACIP does not recommend ZVL in this age groupCitation67. This is partly due to the limited long-term protection provided by ZVL and because of the lower rate of herpes zoster in this age group compared with persons ≥60 years oldCitation68. Although high and rising healthcare costs of treating HZ documented in several LMICs suggest that it would probably be cost-effective to immunize elders from this region than othersCitation10. Due to the specific economic evaluation data are lacking in these countries; our findings on HZ vaccines at different age groups may provide information to decision-makers for efficient use of available resources for maximizing health benefits in the future and to resolve current uncertainties. In the context of unavailability and the expected high cost of RZV, our findings on ZVL at different age groups may support researchers from LMICs to conduct country-specific CEA to inform evidence-based decision-making.
Our study is the first to perform a synthesis on economic outcomes by quantifying INBs across studies for HZ vaccination. Although vaccine prices and costs differ between countries, we considered country-specific thresholds embedded into INB calculation. Additionally, meta-regression that incorporated both vaccine and comparator costs found neither of the variables to be a significant source of heterogeneity. Meta-analyses of economic evaluation studies do not aim to conclude the cost-effectiveness profiles for every country universally. Instead, we aim to broadly summarize how precise the cost-effectiveness values of HZ vaccines are based on all existing studies answering the same question across different settings. Our study is also strengthened by many included economic evaluation studies compared to previous reviews. Further, we were able to standardize monetary units to 2019 USD and used INB instead of ICER to indicate cost-effectiveness and non-cost-effectiveness for decision-making or policy analysisCitation69,Citation70.
Our study has some limitations. Our study used INB calculation to quantify the cost-effectiveness of the vaccines. It is assumed that the ICER is calculated from QALYs or life-years gained. Some of the cost-effectiveness studies may use different health outcome measures that do not fit the INB formula we used. However, these studies were not included in our pooled analyses and may not represent all existing evidence related to health economic evaluation studies investigating Herpes Zoster vaccination.
Some studies assessed various schedules of ZVL and RZV, including ZVL with boosters in varying time periods and additional RZV vaccination in those previously vaccinated with ZVLCitation51,Citation52,Citation54–56,Citation60,Citation61. However, the number of these studies was limited, thus prohibiting us from pooling them using meta-analysis. We could not validate the ACIP’s recommendation to vaccinate RZV in individuals who previously received ZVL. Based on the efficacy of clinical trials, the 2017 recommendations suggested that additional vaccination would be beneficial with a more pronounced benefit in those over the age of 70. One of the included CEAs evaluated this revaccination strategy with five years between administering ZVL and RZVCitation56. Vaccination of adults aged 60+ with RZV compared to no additional vaccination resulted in an ICER of $58,793 and was cost-saving compared to a ZVL booster. While these results originated from a single study, it added to the evidence behind the ACIP recommendation. More research on the direct comparison of RZV and ZVL and other vaccination schedules may be needed to determine the cost-effectiveness profiles for the elderly population.
Conclusions
Our study showed that RZV might be cost-effective compared to no vaccine as quantified by positive incremental net benefits across different age groups. Further, RZV was cost-effective for 60–79 years from the societal perspective, while ZVL compared to no vaccine may be cost-effective for 70–79 years, but the cost-effectiveness profile differs across sensitivity analyses. Future health care policies should be reviewed to determine whether vaccination at 60 may be more appropriate than the currently recommended 50 or older.
Transparency
Declaration of funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declaration of financial/other relationships
The authors declare that they have no known conflicts of interest that are directly relevant to the content of this article.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
S.U. and M.G.R. reviewed literatures, extracted data for the study, synthesis, and quality assessment with input from N.C., A.T., and T.A. S.U. did the meta-analysis. S.U., M.G.R., and S.V. wrote the first drafts of the manuscript and all authors made substantial contribution. All authors contributed to the study design, interpretation of findings, and critical revision of the manuscript.
Acknowledgements
None reported.
Consent for publication
All authors approved the final version of the manuscript for submission.
Supplemental Material
Download MS Word (279.8 KB)Data availability statement
All data are available from the corresponding author upon request.
Code availability
The data analysis file is available from the corresponding author upon request.
References
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6):e004833.
- Yawn BP, Gilden D. The global epidemiology of herpes zoster. Neurology. 2013;81(10):928–930.
- Cohen JI. Clinical practice: herpes zoster. N Engl J Med. 2013;369(3):255–263.
- McLaughlin JM, McGinnis JJ, Tan L, et al. Estimated human and economic burden of four major adult vaccine-preventable diseases in the United States, 2013. J Prim Prev. 2015;36(4):259–273.
- Centers for Disease Control and Prevention (CDC). Shingles (herpes zoster): overview; 2016 [cited 2020 Sep 23]. http://www.cdc.gov/shingles/about/overview.html
- Varghese L, Standaert B, Olivieri A, et al. The temporal impact of aging on the burden of herpes zoster. BMC Geriatr. 2017;17(1):30.
- Dooling KL, Guo A, Patel M, et al. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. Am J Transplant. 2018;18(3):756–762.
- Gabutti G, Bolognesi N, Sandri F, et al. Varicella zoster virus vaccines: an update. Immunotargets Ther. 2019;8:15–28.
- Centers for Disease Control and Prevention (CDC). Vaccines and preventable diseases: what everyone should know about zostavax; 2020 [cited 2021 Jul 15]. https://www.cdc.gov/vaccines/vpd/shingles/public/zostavax/index.html
- Chen LK, Arai H, Chen LY, et al. Looking back to move forward: a twenty-year audit of herpes zoster in Asia-Pacific. BMC Infect Dis. 2017;17(1):213.
- Piot P, Larson HJ, O'Brien KL, et al. Immunization: vital progress, unfinished agenda. Nature. 2019;575(7781):119–129.
- Chiyaka ET, Nghiem VT, Zhang L, et al. Cost-Effectiveness of herpes zoster vaccination: a systematic review. Pharmacoeconomics. 2019;37(2):169–200.
- Crespo C, Monleon A, Díaz W, et al. Comparative efficiency research (COMER): meta-analysis of cost-effectiveness studies. BMC Med Res Methodol. 2014;14(1):139.
- Bagepally BS, Chaikledkaew U, Gurav YK, et al. Glucagon-like peptide 1 agonists for treatment of patients with type 2 diabetes who fail metformin monotherapy: systematic review and meta-analysis of economic evaluation studies. BMJ Open Diabetes Res Care. 2020;8(1):e001020.
- Bagepally BS, Gurav YK, Anothaisintawee T, et al. Cost utility of sodium-glucose cotransporter 2 inhibitors in the treatment of metformin monotherapy failed type 2 diabetes patients: a systematic review and meta-analysis. Value Health. 2019;22(12):1458–1469.
- Haider S, Chaikledkaew U, Thavorncharoensap M, et al. Systematic review and meta-analysis of cost-effectiveness of rotavirus vaccine in low-income and lower-middle-income countries. Open Forum Infect Dis. 2019;6(4):ofz117.
- Noparatayaporn P, Thavorncharoensap M, Chaikledkaew U, et al. Incremental net monetary benefit of bariatric surgery: systematic review and meta-analysis of cost-effectiveness evidences. Obes Surg. 2021;31(7):3279–3290.
- Chaiyakittisopon K, Pattanaprateep O, Ruenroengbun N, et al. Evaluation of the cost-utility of phosphate binders as a treatment option for hyperphosphatemia in chronic kidney disease patients: a systematic review and meta-analysis of the economic evaluations. Eur J Health Econ. 2021;22(4):571–584.
- World Health Organization (WHO). Meeting of the immunization and vaccine related Implementation Research Advisory Committee (IVIR-AC). Weekly Epidemiological Record, No 17, 2021, 96, 133–144; 2021 [cited 2021 Jul 15]. https://www.who.int/publications/i/item/weekly-epidemiological-record-no-17-2021-96-133-144
- Adarkwah CC, van Gils PF, Hiligsmann M, et al. Risk of bias in model-based economic evaluations: the ECOBIAS checklist. Expert Rev Pharmacoecon Outcomes Res. 2016;16(4):513–523.
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making. 1998;18(2 Suppl):S68–S80.
- Bagepally BS, Chaikledkaew U, Chaiyakunapruk N, et al. Meta-analysis of economic evaluation studies: data harmonisation and methodological issues. Research Square Web site; 2020 [Updated 2021 Sep 8; cited 2021 Jul 15].
- The World Bank. Consumer price index (2010 = 100); 2019 [cited 2020 Oct 8]. https://data.worldbank.org/indicator/FP.CPI.TOTL?end=2014&start=2014&view=map&year=2019
- The World Bank. PPP conversion factor, private consumption (LCU per international $); 2019 [cited 2020 Oct 8]. https://data.worldbank.org/indicator/PA.NUS.PRVT.PP?end=2018&start=2018&view=map
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560.
- Peters JL, Sutton AJ, Jones DR, et al. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991–996.
- World Health Organization (WHO). Working with the regions; 2020 [cited 2021 Jan 14]. https://www.who.int/chp/about/regions/en/
- Hornberger J, Robertus K. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Ann Intern Med. 2006;145(5):317–325.
- Pellissier JM, Brisson M, Levin MJ. Evaluation of the cost-effectiveness in the United States of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Vaccine. 2007;25(49):8326–8337.
- Rothberg MB, Virapongse A, Smith KJ. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Clin Infect Dis. 2007;44(10):1280–1288.
- Brisson M, Pellissier JM, Camden S, et al. The potential cost-effectiveness of vaccination against herpes zoster and post-herpetic neuralgia. Hum Vaccin. 2008;4(3):238–245.
- Najafzadeh M, Marra CA, Galanis E, et al. Cost effectiveness of herpes zoster vaccine in Canada. Pharmacoeconomics. 2009;27(12):991–1004.
- van Hoek AJ, Gay N, Melegaro A, et al. Estimating the cost-effectiveness of vaccination against herpes zoster in England and Wales. Vaccine. 2009;27(9):1454–1467.
- Annemans L, Bresse X, Gobbo C, et al. Health economic evaluation of a vaccine for the prevention of herpes zoster (shingles) and post-herpetic neuralgia in adults in Belgium. J Med Econ. 2010;13(3):537–551.
- Moore L, Remy V, Martin M, et al. A health economic model for evaluating a vaccine for the prevention of herpes zoster and post-herpetic neuralgia in the UK. Cost Eff Resour Alloc. 2010;8:7.
- van Lier A, van Hoek AJ, Opstelten W, et al. Assessing the potential effects and cost-effectiveness of programmatic herpes zoster vaccination of elderly in The Netherlands. BMC Health Serv Res. 2010;10:237.
- Szucs TD, Kressig RW, Papageorgiou M, et al. Economic evaluation of a vaccine for the prevention of herpes zoster and post-herpetic neuralgia in older adults in Switzerland. Hum Vaccin. 2011;7(7):749–756.
- Bresse X, Annemans L, Préaud E, et al. Vaccination against herpes zoster and postherpetic neuralgia in France: a cost-effectiveness analysis. Expert Rev Pharmacoecon Outcomes Res. 2013;13(3):393–406.
- de Boer PT, Pouwels KB, Cox JM, et al. Cost-effectiveness of vaccination of the elderly against herpes zoster in The Netherlands. Vaccine. 2013;31(9):1276–1283.
- Ultsch B, Weidemann F, Reinhold T, et al. Health economic evaluation of vaccination strategies for the prevention of herpes zoster and postherpetic neuralgia in Germany. BMC Health Serv Res. 2013;13:359.
- Préaud E, Uhart M, Böhm K, et al. Cost-effectiveness analysis of a vaccination program for the prevention of herpes zoster and post-herpetic neuralgia in adults aged 50 and over in Germany. Hum Vaccin Immunother. 2015;11(4):884–896.
- Le P, Rothberg MB. Cost-Effectiveness of herpes zoster vaccine for persons aged 50 years. Ann Intern Med. 2015;163(7):489–497.
- Belchior E, Lévy-Bruhl D, Le Strat Y, et al. Cost-effectiveness of a herpes zoster vaccination program among the French elderly people. Hum Vaccin Immunother. 2016;12(9):2378–2382.
- Bilde L, Nielsen TT, Rønholt F, et al. The cost-effectiveness of introducing a vaccine for the prevention of herpes zoster and post-herpetic neuralgia in elderly Danes. Nordic J Health Eco. 2016;5(2):7–27.
- Coretti S, Codella P, Romano F, et al. Cost-effectiveness analysis of herpes zoster vaccination in Italian elderly persons. Int J Technol Assess Health Care. 2016;32(4):233–240.
- Lopez-Belmonte JL, Cisterna R, Gil de Miguel A, et al. The use of zostavax in Spain: the economic case for vaccination of individuals aged 50 years and older. J Med Econ. 2016;19(6):576–586.
- Blank PR, Ademi Z, Lu X, et al. Herpes zoster vaccine: a health economic evaluation for Switzerland. Hum Vaccin Immunother. 2017;13(7):1495–1504.
- Hoshi SL, Kondo M, Okubo I. Cost-effectiveness of varicella vaccine against herpes zoster and post-herpetic neuralgia for elderly in Japan. Vaccine. 2017;35(24):3264–3271.
- Pan J, Hsu T, Johnson KD, et al. Cost-effectiveness analysis of herpes zoster vaccine in adults above 50 in Singapore. Dermatol Sin. 2017;35(4):177–181.
- Le P, Rothberg MB. Determining the optimal vaccination schedule for herpes zoster: a cost-effectiveness analysis. J Gen Intern Med. 2017;32(2):159–167.
- Le P, Rothberg MB. Cost effectiveness of a shingles vaccine booster for currently vaccinated adults in the U.S. Am J Prev Med. 2017;53(6):829–836.
- Carpenter CF, Aljassem A, Stassinopoulos J, et al. A cost-effectiveness analysis of an adjuvanted subunit vaccine for the prevention of herpes zoster and post-herpetic neuralgia. Open Forum Infect Dis. 2019;6(7):ofz219.
- Curran D, Patterson B, Varghese L, et al. Cost-effectiveness of an adjuvanted recombinant zoster vaccine in older adults in the United States. Vaccine. 2018;36(33):5037–5045.
- Curran D, Patterson BJ, van Oorschot D, et al. Cost-effectiveness of an adjuvanted recombinant zoster vaccine in older adults in the United States who have been previously vaccinated with zoster vaccine live. Hum Vaccin Immunother. 2019;15(4):765–771.
- de Boer PT, van Lier A, de Melker H, et al. Cost-effectiveness of vaccination of immunocompetent older adults against herpes zoster in The Netherlands: a comparison between the adjuvanted subunit and live-attenuated vaccines. BMC Med. 2018;16(1):228.
- Drolet M, Zhou Z, Sauvageau C, et al. Effectiveness and cost-effectiveness of vaccination against herpes zoster in Canada: a modelling study. CMAJ. 2019;191(34):E932–E939.
- Hoshi SL, Seposo X, Shono A, et al. Cost-effectiveness of recombinant zoster vaccine (RZV) and varicella vaccine live (VVL) against herpes zoster and post-herpetic neuralgia among adults aged 65 and over in Japan. Vaccine. 2019;37(27):3588–3597.
- McGirr A, Van Oorschot D, Widenmaier R, et al. Public health impact and cost-effectiveness of non-live adjuvanted recombinant zoster vaccine in Canadian adults. Appl Health Econ Health Policy. 2019;17(5):723–732.
- Melegaro A, Marziano V, Del Fava E, et al. The impact of demographic changes, exogenous boosting and new vaccination policies on varicella and herpes zoster in Italy: a modelling and cost-effectiveness study. BMC Med. 2018;16(1):117.
- Prosser LA, Harpaz R, Rose AM, et al. A cost-effectiveness analysis of vaccination for prevention of herpes zoster and related complications: input for national recommendations. Ann Intern Med. 2019;170(6):380–388.
- Shiragami M, Mizukami A, Kaise T, et al. Cost-effectiveness of the adjuvant recombinant zoster vaccine in Japanese adults aged 65 years and older. Dermatol Ther. 2019;9(2):281–297.
- Van Oorschot D, Anastassopoulou A, Poulsen Nautrup B, et al. Cost-effectiveness of the recombinant zoster vaccine in the German population aged ≥60 years old. Hum Vaccin Immunother. 2019;15(1):34–44.
- You JHS, Ming WK, Lee CF, et al. Potential cost-effectiveness of adjuvanted herpes zoster subunit vaccine for older adults in Hong Kong. Vaccine. 2018;36(31):4610–4620.
- You JHS, Ming WK, Tsang OT, et al. Optimal gender-specific age for cost-effective vaccination with adjuvanted herpes zoster subunit vaccine in Chinese adults. PLOS One. 2019;14(1):e0210005.
- Lecrenier N, Beukelaers P, Colindres R, et al. Development of adjuvanted recombinant zoster vaccine and its implications for shingles prevention. Expert Rev Vaccines. 2018;17(7):619–634.
- Kim DK, Riley LE, Hunter P, et al. Recommended immunization schedule for adults aged 19 years or older, United States, 2018. Ann Intern Med. 2018;168(3):210–220.
- Marra F, Parhar K, Huang B, et al. Risk factors for herpes zoster infection: a meta-analysis. Open Forum Infect Dis. 2020;7(1):ofaa005.
- Willan AR. Incremental net benefit in the analysis of economic data from clinical trials, with application to the CADET-Hp trial. Eur J Gastroenterol Hepatol. 2004;16(6):543–549.
- Willan AR, Lin DY. Incremental net benefit in randomized clinical trials. Stat Med. 2001;20(11):1563–1574.