Abstract
Background
Multiple interventions may be used to treat symptomatic knee osteoarthritis (OA), but concerns have been raised about the safety and efficacy of some therapies. Clinical trials have shown that hyaluronic acid (HA) can provide pain relief up to 6 months and possibly to 12 months, while real-world data has shown that pain medication and intra-articular corticosteroid (CS) injection utilization are reduced within 6 months after HA.
Objective
To examine changes in prescription pain medication and CS utilization during 1 year after multimodal therapy that included high molecular weight, bio-fermentation derived HA (Bio-HA) use for knee OA.
Methods
Commercial and Medicare Supplemental claims data (IBM MarketScan Research Databases) (1 January 2012, through 31 December 2018) was used to identify unilateral Bio-HA patients using multimodal therapy (any combination of CS injection, opioids, and non-opioid pain medication). Monthly therapy utilization was compared in the 12 months after Bio-HA therapy initiation to the 4-month intra-multimodal period.
Results
A total of 13,999 patients underwent Bio-HA therapy with concurrent multimodal therapy. The number of filled opioid prescriptions decreased from 2,913.0/month to 2,861.5/month after Bio-HA, with a reduction in mean monthly prescriptions from 0.60 to 0.43 per user (p < 0.001). A number of opioid days supplied also decreased from 48,914/month to 39,730/month, with a decrease from 10.1/month to 6.0/month per user (p < 0.001). Bio-HA patients had prescription pain medication-free days for 71% of the time post-multimodal period compared to 53% during the intra-multimodal period (p < 0.001). The proportion of patients with CS injections after Bio-HA decreased from 53.8% to 29.6% (p < 0.001). Total monthly CS injections decreased from 2,292 to 663.
Conclusions
Our data suggest that high molecular weight Bio-HA, as part of multimodal therapy, may be effective in providing longer-term pain relief with the reduction in pain therapy (CS injections and opioids) and increase in prescription pain medication-free days.
© 2022 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group
Introduction
A number of non-surgical treatments are available for relieving symptoms among knee osteoarthritis (OA) patients, including opioids, non-steroidal anti-inflammatory drugs (NSAIDs), intra-articular corticosteroids (CS), and intra-articular hyaluronic acid (HA)Citation1–4. The choice of a single or combined intervention may vary depending on the course of the disease or symptoms, and through shared decision-making between the patient and clinicianCitation4,Citation5. However, the selection of therapies is complicated by inconsistent recommendations or guidelines due to questions regarding safety (for NSAIDs, opioids, and CS)Citation2,Citation3,Citation6,Citation7 and efficacy (for CS and HA)Citation1,Citation4.
Regarding HA, the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) task force has recommended the use of HA for knee OA patients with mild-to-moderate disease and for more severe patients who are either contraindicated to knee arthroplasty or wish to delay surgeryCitation3. HA was recommended alongside intra-articular CS if patients were still symptomatic after topical and oral NSAIDs. As a last pharmacological option before surgery, short-term weak opioids were recommended. On the other hand, the American College of Rheumatology (ACR) conditionally recommended against the use of HA that was motivated by the demonstration of clinical benefits in studies with a higher risk of bias, though they strongly recommended knee brace, intra-articular CS, topical NSAIDs, and oral NSAIDs among other approaches to manage knee OACitation4. In their latest 2021 clinical practice guidelines, the American Academy of Orthopaedic Surgeons (AAOS) indicates that HA is not recommended for routine use, with a moderate strength of recommendation, but states that a specific subset of patients might benefit from its useCitation8. This contrasts from the previous 2013 guidelines that strongly recommended against the use of HACitation1. Their latest guidelines also strongly recommend oral or topical NSAIDs, while the guidelines state that intra-articular CS could provide short-term reliefCitation8. They further state that opioids result in a significant increase in adverse events and are not effective at improving pain or function.
A multimodal approach that includes HA as part of the armamentarium may provide therapeutic value to managing knee OA pain. There are several mechanisms in which HA injections may provide clinical benefit in knee OA, with chondroprotection being the most frequently reported mechanismCitation9. HA therapy is also reported to stimulate proteoglycan and glycosaminoglycan synthesis and provide anti-inflammatory, mechanical, subchondral, and analgesic effectsCitation9. Individual clinical trials have shown that intra-articular HA therapy can provide significant knee OA pain relief at up to 6 monthsCitation10,Citation11, and may even extend out to 12 monthsCitation12. Real-world data has also provided evidence that pain medication and CS injection utilization are reduced during the 6-month period after HA, although these were mainly limited to the entire product classCitation13–15. Furthermore, the outcomes following the use of HA may be dependent on molecular weight, source, and method of synthesisCitation9. The present study sought to examine changes in prescription pain medication and intra-articular CS utilization after a period of multimodal therapy including Bio-HA use for knee OA.
Methods
An administrative claims dataset (Commercial and Medicare Supplemental claims data; IBM MarketScan Research Databases, IBM Corporation, Somers, NY), from 1 January 2012 to 31 December 2018, was used to compile data for knee OA patients, including those who either underwent HA injections or KA. The dataset is constructed by collecting commercial claims data for over 273 million covered lives from over 120 employers and 40 health plans in routine clinical practice and encompasses employees, spouses, and dependents covered by employer-sponsored private health insurance. Medicare Supplemental data was also compiled from retirees with Medicare supplemental insurance paid by employers, including Medicare-covered and employer-paid portions, as well as out-of-pocket expenses. Claims for inpatient and outpatient services, and outpatient prescription drugs, including claims for mail-order prescriptions and specialty pharmacies, are included in the dataset. The data is fully de-identified and adheres to the requirements of the Health Insurance Portability and Accountability Act of 1996 (HIPAA) with additional verification by a third party to meet the requirements for a fully de-identified data set. Each individual has a unique de-identified enrollee identifier to allow for tracking of beneficiary-level claims for the same individual over time. This study was based on a data set that was available for licensed use. This database has been used by others for real-world evidence research, including for prescription pain medication and HA studiesCitation13,Citation14,Citation16. The reliability of the database is enhanced by the inclusion of only claims that have been fully paid and adjudicated. The data set was de-identified, did not involve human subject research, and therefore did not require oversight by an institutional review board.
Knee OA patients were identified for the dataset using International Classification of Diseases (ICD) diagnosis codes (Supplementary Table S1), from which those who underwent unilateral Bio-HA (Euflexxa; Ferring Pharmaceuticals Inc., Parsippany, NJ) as a first-line HA therapy with no other HA between enrollment or start of the data period and the first Bio-HA injection were included in the study. The instructions for use for Bio-HA specify that it is to be administered once a week, for a total of three injectionsCitation17.
The study inclusion criteria were: (1) at least 18 years old; (2) at least 6 months claim history prior to first Bio-HA injection and following the last of the index Bio-HA injections; (3) only three Bio-HA injections in the same knee during the index Bio-HA treatment within a 6-week period; (4) with 12 months follow-up after initiation of the index Bio-HA treatment; and (5) with multimodal therapy during a 4-month period centered around the first of the index Bio-HA injections (i.e. 2 months before through 2 months after the first injection). Multimodal therapy was defined as any combination of intra-articular CS injection, opioids, and non-opioid pain medication (Supplementary Table S1). The exclusion criteria were: (1) unknown laterality to any of the Bio-HA injections during the index treatment; (2) without pharmacy benefits in the 6 months prior to the last of the index Bio-HA injections and during the follow-up period; (3) with HA treatment prior to the index Bio-HA treatment; (4) with primary or revision KA prior to the index Bio-HA treatment; (5) without a completed course of Bio-HA treatment during the index Bio-HA treatment (less than three per knee within a 6-week period); and (6) who exceeded the course of Bio-HA treatment during the index Bio-HA treatment (more than three per knee within a 6-week period). The latter two criteria were intended to improve the robustness of the study to focus on patients who completed a full course, rather than those who did not complete their treatment or underwent multiple treatments, to reduce any potential confounding effects.
Opioid prescription fills, prescription pain medication pain-free days, and CS injections were assessed during the 4-month intra-multimodal period and compared to the utilization in the 12 months after initiation of Bio-HA therapy excluding the first 2 months of the multimodal period. The 12-month period after initiation of Bio-HA therapy was based on clinical trial findings of significant knee OA pain relief up to 12 months after intra-articular HA therapyCitation10–12. The number of filled opioid prescriptions, number of opioid days supplied, prescription pain medication-free days, the proportion of patients who filled opioid prescriptions, and number of CS injections were normalized by calculating an average monthly utilization. The changes in the use of these therapies were used as surrogate pain relief markers. Univariate statistics (t-test) was used to compare the mean number of filled opioid prescriptions per user per month and the mean number of opioid days supplied per user per month between the intra-multimodal period and after Bio-HA use. The proportion of patients who filled opioid prescriptions and had CS injections during the intra-multimodal period were compared to the corresponding proportions after Bio-HA therapy using the Chi-square test. The proportion of the time period that the Bio-HA patients were prescription pain medication-free between the intra-multimodal period and post-Bio-HA therapy period was recorded.
Results
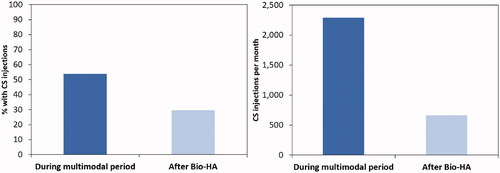
During the study period, a total of 13,999 patients received Bio-HA therapy with concurrent multimodal therapy of intra-articular CS injection, opioids, and/or non-opioid pain medication (). Non-opioid pain medication prescriptions were filled by 62.7% (n = 8,773) of the cohort during the 4-month intra-multimodal period, while 53.8% (n = 7,527) had CS injections and 34.7% (n = 4,857) filled opioid prescriptions. In addition to the Bio-HA, 42.3% (n = 5,915) of the cohort used a combination of the selected multimodal therapies, while 26.0% (n = 3,636) only filled non-opioid pain medication prescriptions, 22.7% (n = 3,174) only had CS injections, and 9.1% (n = 1,274) only filled opioid prescriptions. Approximately two-thirds of the cohort were women, while the most common age groups were 55–59 (20.7%) and 60–64 (20.7%) years, followed by 50–54 years (15.8%) (). Physical therapy, knee arthroscopy, and knee brace were utilized by 9.5%, 11.6%, and 9.6% of the cohort in the 6 months prior to initiation of Bio-HA treatment, respectively.
Table 1. Study cohort patient characteristics.
Compared to the time period during the multimodal therapy with Bio-HA, patients were less reliant on pain medications after treatment. The monthly number of filled opioid prescriptions decreased from 2,913.0 to 2,861.5 after Bio-HA (). Of those who filled opioid prescriptions, the average prescriptions per month decreased from 0.60 ± 1.05 prescriptions to 0.43 ± 1.55 prescriptions (t-test, p < 0.001; ). The monthly number of opioid days supplied also decreased from 48,914 to 39,730 after Bio-HA. Of those who filled prescriptions, the average opioid days supplied per month decreased from 10.1 ± 28.3 to 6.0 ± 27.2 (t-test, p < 0.001). Bio-HA patients also had a mean of 21.7 prescription pain medication-free days per month (or 71% of the time period) following Bio-HA therapy compared to 16.0 days (or 53% of a time period) during the intra-multimodal period (chi-square, p < 0.001). This corresponded to a total of 3,037,617 days of not using any prescription pain medication over 10 months compared to 897,460 days over 4 months for the 13,999 patients. The proportion of patients who filled opioid prescriptions following Bio-HA increased from 34.7% over the 4-month intra-multimodal period to 47.5% over the 10-month post-multimodal period (chi-square, p < 0.001), but normalized to a monthly rate of 8.7% and 4.8%, respectively. Correspondingly, 14.2% of the patients in the Bio-HA cohort were found to have undergone subsequent knee arthroplasty in the 12 months after initiating Bio-HA therapy.
Figure 1. Opioid utilization during the multimodal period and after Bio-HA use (top left: mean opioid use per month; top right: opioid days supplied per month; bottom: percent with opioid use).
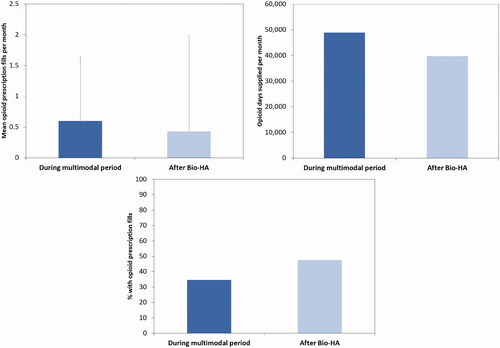
Table 2. Changes in utilization during the multimodal period and after Bio-HA use.
Patients who received Bio-HA were less reliant on CS injections after their index treatment regimen. The proportion of Bio-HA patients who had CS injections after Bio-HA decreased from 53.8% to 29.6% (Chi-square, p < 0.001; ). The total number of CS injections decreased from 9,167 to 6,628 following Bio-HA therapy, which corresponded to a decrease from 2,292 per month to 663 per month.
Discussion
Our analysis of patients who received multimodal therapy with high molecular weight Bio-HA patients indicated they were subsequently less reliant on pain medication and CS injections. Specifically, Bio-HA patients who had concurrent use of prescription pain medication and/or intra-articular CS demonstrated decreases in the monthly number of opioid prescriptions and opioid days supplied, the proportion of CS injection use, and a monthly number of CS injections, as well as an increase in prescription pain medication-free days, after completion of their Bio-HA course.
The reduction in pain therapy at 12 months following initiation of the Bio-HA therapy in routine clinical practice, including having prescription pain medication-free days for about three-quarters of the period after Bio-HA use, was also reported in the clinical trial results by Altman et al.Citation12 The authors reported a 34.9% reduction in acetaminophen use at 52 weeks for patients who underwent repeated series of Bio-HA. Others have also observed a reduction in the need for analgesic or rescue medication, albeit up to 6 months after HA therapy, but longer follow-up was not examined. Chitnis et al.Citation13 used real-world data to examine the utilization of NSAIDs, CS injections, and opioids in the 6 months before and after HA treatment period for patients who received non-avian high molecular weight HA. In their cohort of 29,076 patients, the proportion of patients filling NSAIDs, CS injection, and opioid prescriptions decreased by 6%, 28%, and 4%, respectively. Another analysis using the same commercial claims database as the present study found a 15%, 57%, and 6% reduction in the number of patients filling NSAIDs, CS injection, and opioid prescriptions, respectively, in the 6 months after 152,953 patients received any HA injections compared to the 6-month pretreatment periodCitation14. Niazi et al.Citation15 evaluated claims data for 128,658 HA users and identified a 54% reduction in the number of opioid users 6 months after receiving a HA injection, with 82% of the HA patients not using opioids during that period. Three-quarters of the HA patients were also found to not receive additional CS injections in the 6 months after HA. A retrospective review of prospectively collected data for patients who initiated high molecular weight avian-based HA treatment demonstrated reductions of 68%, 68%, and 57% in oral medication taken for knee OA pain at weeks 4, 12, and 26, respectivelyCitation11. The reduction in opioid use following Bio-HA therapy can have a significant impact on the patient population as prolonged opioid use can contribute to increased misuseCitation18 and loss of analgesic efficacyCitation19. Furthermore, opioid use has been linked to adverse eventsCitation20–22, which can lead to longer hospital stays, higher costs, readmissions, and mortalityCitation21,Citation22.
Although the present study demonstrated decreases in many of the surrogate pain relief markers, the proportion of patients who filled opioid prescriptions increased following Bio-HA, which could be due to the 2.5-times longer duration of assessment during the post-multimodal period compared to the intra-multimodal period. The increase in the proportion of patients could also be related to the patients who underwent knee arthroplasty in the follow-up period. Researchers have found an 85% increase in the proportion of patients filling opioid prescriptions after total knee arthroplastyCitation14. Previous researchers also observed that two-thirds of knee arthroplasty patients filled opioid prescriptions within 6 months post-surgery, with 78% of opioid users continuing fills and 62% of nonusers initiating useCitation15. The percent of patients who underwent knee arthroplasty in the 12 months after initiating Bio-HA was also consistent with other studies. McIntyre et al.Citation14 observed that 7.5% of HA patients had total knee arthroplasty within 6 months of the first HA injection, which is 6 months shorter than our study, but consistent when extrapolated out to 12 months. Moreover, HA has been shown to be associated with a delay to knee arthroplastyCitation23–28.
Various national and international healthcare groups have developed clinical practice guidelines regarding treatment options for managing knee OACitation2–4,Citation8, but have conflicting recommendations, making it difficult for clinicians to fully evaluate available options. In particular, a systematic review of clinical practice guidelines identified 27 guidelines that provided a statement on the use of HA for treating knee OACitation29. HA was recommended in most guidelines, with the review reporting that 37% of the guidelines provided strong recommendations and 37% provided conditional recommendations for HA use, while 7.4% had uncertain recommendations, 7.4% had weak recommendations against, and 11.1% had strong recommendations against HA use. The typical reason for an unfavorable recommendation against HA use was for the lack of certainty and risk of bias within the literature, despite the relatively large number of trials that have evaluated this intervention. The inconsistent results following the use of HA may be attributed to the role of different intrinsic properties of specific HA products, such as their molecular weightCitation9,Citation30,Citation31. HA products with an average molecular weight of at least 3,000 kDa have been found to provide more favorable efficacy results than products with lower average molecular weightCitation30. Unlike low molecular weight HA, high molecular weight HA also exceeded the minimal clinically important improvement threshold or minimally important difference for pain reliefCitation31,Citation32. However, the different characteristics of specific HA products are not rigorously addressed in a number of the recommendations.
Limitations
Knee OA severity could not be directly evaluated in the administrative claims data, but all patients in the study used one or more CS, opioids, and non-opioid prescription pain medication, which may be representative of patients with moderate-to-severe knee OACitation33. Although different durations were compared in the period following multimodal therapy (10 months) and during the intra-multimodal therapy period (4 months), the findings were normalized to a monthly basis to account for this difference. This study focused on Bio-HA patients, but it is unclear if these results are generalizable to patients using alternative HA therapies. The present study focused on Bio-HA as the varying and inconsistent results following the use of HA may be related to differences in the intrinsic properties between specific HA productsCitation9,Citation30,Citation31. Differences in product characteristics can include rheological properties (e.g. molecular weight), source (e.g. avian origin, bacterial cells, etc.), and method of synthesis (e.g. cross-linked)Citation30,Citation31,Citation34. Specifically, Bio-HA is a high molecular weight, linear chain, and bio-fermented HA sourced from bacterial cells. Furthermore, focusing on Bio-HA allowed for a real-world comparison against clinical trial results, which reported a reduction in acetaminophen use at 12 monthsCitation12. The dataset also does not account for over-the-counter pain medications, such as NSAIDs and topical analgesics, thus we were unable to evaluate overall (over-the-counter and prescription) pain medication-free days. In addition, the prescription pain medication-free days observed in the present study may overestimate the number of overall pain medication-free days. Filled prescriptions do not equate to actual consumption. However, our combined assessment of a number of days supplied based on filled prescriptions still provides an assessment of the accessibility to prescription pain medications. The prescription fill date was used to identify whether a claim was filled during the intra-multimodal period or following that period. As a result of this criteria, there may be potential misclassification if patients had remaining supplies from a prior prescription before the observational window for the multimodal period definitions or fill the prescription in one period but consume the medication in the next period. However, this would apply to both periods. Due to the observational nature of the study, one must exercise caution to assert causal inferences.
Conclusions
Using surrogate markers, our data suggest that high molecular weight Bio-HA, as part of multimodal therapy, may be effective in providing longer-term pain relief for patients in routine clinical practice and may be associated with subsequent reduction in pain therapy (CS injections and opioids) and increase in prescription pain medication-free days.
Transparency
Declaration of funding
Exponent, Inc. received funding from Ferring Pharmaceuticals Inc. for this study. Role of sponsor: Two of the authors (FN, WNN) are employees of the study sponsor and were involved in the design of the study, interpretation of data, critical revision of the manuscript, and the decision to submit the report for publication. The study sponsor was not involved in the collection and analysis of the data or initial drafting of the paper.
Declaration of financial/other interests
KO reports other from Ferring Pharmaceuticals, during the conduct of the study; other from Bioventus, Medtronic, Stryker Orthopaedics, Sanofi, Pacira Pharmaceuticals, St. Jude Medical, Relievant Medsystems, the International Society for the Advancement of Spine Surgery, SI-Technology, LLC, Zimmer Biomet, Joerns Healthcare, SpineFrontier, ot Ethicon, DJO, Ossur, Karl Storz Endoscopy-America, Rex Medical, Smith & Nephew, and Covidien, outside the submitted work. SK reports grants from Ferring Pharmaceuticals, during the conduct of the study; grants from Stryker, Zimmer Biomet, Invibio, Wright Medical Technology, Ceramtec, Celanese, Ferring Pharmaceuticals, Formae, Lima Corporate, SINTX Technologies, Orthoplastics, Osteal Therapeutics, 3Spine, DJO Global, Carbofix, DePuy Synthes, other from Exponent, Inc., outside the submitted work. MN has nothing to disclose. FN reports being employed by Ferring Pharmaceuticals Inc. WN was an employee of Ferring Pharmaceuticals. EL reports other from Ferring Pharmaceutical, during the conduct of the study; other from Osteal Therapeutic, Ceramtec Inc., and Relievant Medsystem Inc., outside the submitted work. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (15.3 KB)References
- American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee: evidence-based guideline. 2nd edition. [Published 2013; cited 2018 March 15]. Available from: https://www.aaos.org/research/guidelines/TreatmentofOsteoarthritisoftheKneeGuideline.pdf
- Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578–1589.
- Bruyere O, Cooper C, Pelletier JP, et al. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis-From evidence-based medicine to the real-life setting. Semin Arthritis Rheum. 2016;45(4 Suppl):S3–S11.
- Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020;72(2):220–233.
- Bruyere O, Cooper C, Pelletier JP, et al. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum. 2014;44(3):253–263.
- Kijowski R. Risks and benefits of intra-articular corticosteroid injection for treatment of osteoarthritis: what radiologists and patients need to know. Radiology. 2019;293(3):664–665.
- Wernecke C, Braun HJ, Dragoo JL. The effect of intra-articular corticosteroids on articular cartilage: a systematic review. Orthop J Sports Med. 2015;3(5):232596711558116.
- American Academy of Orthopaedic Surgeons. Management of osteoarthritis of the knee (non-arthroplasty). Evidence-based clinical practice guideline. 3rd Edition. [Published 2021; cited 2021 Oct 8]. Available from: https://www.aaos.org/globalassets/quality-and-practice-resources/osteoarthritis-of-the-knee/oak3cpg.pdf
- Altman RD, Manjoo A, Fierlinger A, et al. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015;16:321.
- Altman RD, Rosen JE, Bloch DA, et al. A double-blind, randomized, saline-controlled study of the efficacy and safety of EUFLEXXA for treatment of painful osteoarthritis of the knee, with an open-label safety extension (the FLEXX trial). Semin Arthritis Rheum. 2009;39(1):1–9.
- Waddell DD, Bricker DC. Clinical experience with the effectiveness and tolerability of hylan G-F 20 in 1047 patients with osteoarthritis of the knee. J Knee Surg. 2010;19(01):19–27.
- Altman RD, Rosen JE, Bloch DA, et al. Safety and efficacy of retreatment with a bioengineered hyaluronate for painful osteoarthritis of the knee: results of the open-label extension study of the FLEXX trial. Osteoarthritis Cartilage. 2011;19(10):1169–1175.
- Chitnis AS, Etter K, Holy CE, et al. Real world impact of the high concentration non-avian high molecular weight hyaluronan on pain medication use among osteoarthritis patients. Curr Med Res Opin. 2019;35(9):1523–1527.
- McIntyre LF, Beach W, Bhattacharyya S, et al. Impact of hyaluronic acid injections on utilization of pain management medications. Am J Pharma Benefits. 2017;9(6):195–199.
- Niazi F, Ong KL, Kidd VD, et al. Decrease in opioid and intra-articular corticosteroid burden after intra-articular hyaluronic acid for knee osteoarthritis treatment. Pain Manag. 2020;10(6):387–397.
- Ong KL, Stoner KE, Yun BM, et al. Baseline and postfusion opioid burden for patients with low back pain. Am J Manag Care. 2018;24(8):e234–e240.
- Ferring Pharmaceuticals Inc. Product information. Euflexxa (1% sodium hyaluronate). [Published 2016; Cited 2020 Nov 3]. Available from: http://www.euflexxa.com/wp-content/uploads/2019/09/Patient-Information.pdf
- Atluri S, Sudarshan G, Manchikanti L. Assessment of the trends in medical use and misuse of opioid analgesics from 2004 to 2011. Pain Physician. 2014;17(2):E119–128.
- Ballantyne JC, Shin NS. Efficacy of opioids for chronic pain: a review of the evidence. Clin J Pain. 2008;24(6):469–478.
- Herzig SJ, Rothberg MB, Cheung M, et al. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73–81.
- Kessler ER, Shah M, Gruschkus SK, et al. Cost and quality implications of opioid-based postsurgical pain control using administrative claims data from a large health system: opioid-related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy. 2013;33(4):383–391.
- Manchikanti L, Abdi S, Atluri S, et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: part I–evidence assessment. Pain Physician. 2012;15(3 Suppl):S1–S65.
- Altman R, Fredericson M, Bhattacharyya SK, et al. Association between hyaluronic acid injections and time-to-Total knee replacement surgery. J Knee Surg. 2016;29(7):564–570.
- Altman R, Lim S, Steen RG, et al. Hyaluronic acid injections are associated with delay of total knee replacement surgery in patients with knee osteoarthritis: evidence from a large U.S. Health claims database. PLOS One. 2015;10(12):e0145776.
- Dasa V, Lim S, Heeckt P. Real-World evidence for safety and effectiveness of repeated courses of hyaluronic acid injections on the time to knee replacement surgery. Am J Orthop. 2018;47(7). DOI:10.12788/ajo.2018.0058
- Etter K, Chitnis AS, Holy CE, et al. High-concentration nonavian high-molecular weight hyaluronan injections and time-to-total knee replacement surgery. J Comp Eff Res. 2020;9(11):795–805.
- Ong KL, Anderson AF, Niazi F, et al. Hyaluronic acid injections in medicare knee osteoarthritis patients are associated with longer time to knee arthroplasty. J Arthroplasty. 2016;31(8):1667–1673.
- Ong KL, Runa M, Lau E, et al. Is Intra-Articular injection of synvisc associated with a delay to knee arthroplasty in patients with knee osteoarthritis? Cartilage. 2019;10(4):423–431.
- Phillips M, Bhandari M, Grant J, et al. A systematic review of current clinical practice guidelines on intra-articular hyaluronic acid, corticosteroid, and platelet-rich plasma injection for knee osteoarthritis: an international perspective. Orthop J Sports Med. 2021;9(8):23259671211030272.
- Altman RD, Bedi A, Karlsson J, et al. Product differences in intra-articular hyaluronic acids for osteoarthritis of the knee. Am J Sports Med. 2016;44(8):2158–2165.
- Phillips M, Vannabouathong C, Devji T, et al. Differentiating factors of intra-articular injectables have a meaningful impact on knee osteoarthritis outcomes: a network meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2020;28(9):3031–3039.
- Hummer CD, Angst F, Ngai W, et al. High molecular weight intraarticular hyaluronic acid for the treatment of knee osteoarthritis: a network meta-analysis. BMC Musculoskelet Disord. 2020;21(1):702.
- Shewale AR, Barnes CL, Fischbach LA, et al. Comparative effectiveness of intra-articular hyaluronic acid and corticosteroid injections on the time to surgical knee procedures. J Arthroplasty. 2017;32(12):3591–3597 e3524.
- Nicholls M, Manjoo A, Shaw P, et al. Rheological properties of commercially available hyaluronic acid products in the United States for the treatment of osteoarthritis knee pain. Clin Med Insights Arthritis Musculoskelet Disord. 2018;11:1179544117751622.