Abstract
Aims
To assess the effects of mavacamten on health-related quality-of-life (HRQoL) in symptomatic obstructive hypertrophic cardiomyopathy (HCM) and estimate health utilities by New York Heart Association (NYHA) functional class.
Materials and methods
Patients with symptomatic obstructive HCM were randomized to 30 weeks of mavacamten or to placebo treatment, with or without beta-blocker or calcium channel blocker monotherapy, in EXPLORER-HCM (ClinicalTrials.gov identifier: NCT03470545). Health utility was measured using the EuroQoL 5-dimension 5-level (EQ-5D-5L) index score with the US value set. The 30-week changes in EQ-5D-5L index score and EuroQoL visual analog scale (EQ-VAS) score were compared between the two arms using linear regression, and the proportions of patients with a meaningful improvement were compared using logistic regression. The meaningful change thresholds were estimated using both distribution- and anchor-based approaches. Mean utilities by NYHA class were estimated for each arm using a generalized estimating equation.
Results
Compared with placebo (N = 89), patients receiving mavacamten (N = 96) had significantly greater 30-week improvement in EQ-5D-5L index score (mavacamten = 0.084; placebo = 0.009; adjusted difference = 0.073 [95% confidence interval = 0.027–0.118]) and EQ-VAS score (mavacamten = 8.5; placebo = 0.7; adjusted difference = 7.5 [95% confidence interval = 1.8–13.2]), and a significantly higher proportion of these patients showed meaningful improvement in EQ-5D-5L index score and EQ-VAS score. Both outcomes were correlated with the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ CSS) and HCM Symptom Questionnaire Shortness-of-Breath (HCMSQ SoB) subscore, two patient-reported anchor variables. Additionally, mean utilities significantly decreased with higher NYHA functional class (values for NYHA class I, II, and III/IV – mavacamten = 0.950, 0.866, and 0.708; placebo = 0.952, 0.850, and 0.704).
Conclusions
Compared with placebo, mavacamten significantly improved EQ-5D-5L index score and EQ-VAS score – and thus HRQoL – among patients with symptomatic obstructive HCM. Patients with a higher NYHA functional class had a lower health utility value.
Introduction
Hypertrophic cardiomyopathy (HCM) is a primary myocardial disorder with complex pathophysiologyCitation1. It may be associated with a disease-causing sarcomere-related variant or unresolved genetic etiology and is predominantly characterized by left ventricular hypertrophy that cannot be explained by other cardiac or systemic diseasesCitation1–3. Approximately two-thirds of patients with HCM have obstructive disease in which outflow of blood from the left ventricle is blocked or reducedCitation4. Patients with HCM often experience a range of symptoms that can severely impair health-related quality-of-life (HRQoL), such as chest pain, difficulty breathing, heart palpitations, loss of consciousness, and fatigueCitation2,Citation5–7. Using the 36-item Short-Form Survey and the Hospital Anxiety and Depression Scale, a few studies found that patients with HCM had severe physical and mental impairments and poor HRQoLCitation6–8. Huff et al. also showed that patients with HCM had moderate reductions in score in all domains of the Kansas City Cardiomyopathy Questionnaire (KCCQ, a commonly used instrument for patient health status), with the largest reduction in quality-of-life correlating with New York Heart Association (NYHA) functional class (a widely used measure to assess patient symptoms and limitations in physical activity) and with peak oxygen consumptionCitation9.
Current pharmacologic therapies, including beta-blockers (BBs) and calcium channel blockers (CCBs), focus on symptom relief rather than treating the underlying cause of the diseaseCitation1. Many patients respond well to BB and CCB therapy, but there are limited treatment options for patients with refractory symptoms; such patients may receive disopyramide, an anti-arrhythmic with known pro-arrhythmic and anticholinergic properties, or invasive septal reduction therapyCitation1. Mavacamten is a selective allosteric inhibitor of cardiac myosin that may serve as an innovative treatment for patients with symptomatic obstructive HCMCitation10,Citation11. In the phase 3 EXPLORER-HCM trial, patients with symptomatic obstructive HCM were randomized to mavacamten with or without (±) BB or CCB monotherapy or to placebo ± BB or CCB monotherapy. Patients in the mavacamten arm showed significant improvements in hemodynamics, exercise capacity, biomarkers, health status, and shortness of breath compared with those in the placebo armCitation10. For example, compared with patients in the placebo arm at week 30, a significantly higher proportion of those in the mavacamten arm showed improvement in NYHA functional class of at least one classCitation10, a significantly larger improvement in the Overall Summary Score (OSS) and the Clinical Summary Score (CSS) of the KCCQ-23Citation10,Citation12, and a significantly larger reduction in the Hypertrophic Cardiomyopathy Symptom Questionnaire (HCMSQ) Shortness-of-Breath (SoB) subscoreCitation10.
Further investigations are required to fully understand the effects of mavacamten treatment on HRQoL in patients with obstructive HCM. While past studies have assessed the impact of mavacamten on the KCCQ and HCMSQ SoB, the effect of mavacamten on the EuroQoL 5-dimension 5-level (EQ-5D-5L) index score and EuroQoL visual analog scale (EQ-VAS) score has not been evaluated. In fact, the EQ-5D-5L index score and EQ-VAS are commonly used, generic metrics to quantify patient HRQoL and have been widely studied among patients with cardiovascular diseasesCitation13,Citation14. Using EXPLORER-HCM trial data, the present study compared 30-week changes in EQ-5D-5L index score and EQ-VAS in patients with obstructive HCM. In addition, the proportions of patients with improvements in EQ-5D-5L index score and EQ-VAS score experiencing at least the meaningful change threshold (MCT), in those who received mavacamten ± BB or CCB monotherapy versus placebo ± BB or CCB monotherapy were also compared. MCTs were estimated by distribution-based and anchor-based approaches using the KCCQ-23 CSS and HCMSQ SoB subscores as the candidate anchor variablesCitation15,Citation16. While addressing this objective, the analysis also evaluated the associations between EQ-5D-5L and EQ-VAS scores and the two anchor-based patient-reported outcomes (PROs). Furthermore, NYHA functional class is commonly used to evaluate symptoms and limitations of physical activity among patients with obstructive HCM; however, the association between NYHA functional class and health utilities among patients with symptomatic obstructive HCM has not been reported. To this end, health utility values by NYHA functional class were also estimated for each treatment arm in this study.
Methods
Study population
The EXPLORER-HCM trial was a phase 3, double-blind, randomized, placebo-controlled, multicenter, international, and parallel-group study that evaluated the safety, tolerability, and efficacy of mavacamten versus placebo in adult patients with symptomatic obstructive HCM (ClinicalTrials.gov identifier: NCT03470545). Background BB or CCB monotherapy was allowed in both treatment arms. Specifically, a total of 429 patients were assessed for eligibility. Among those assessed, a total of 251 patients with NYHA functional class II or III at baseline were randomized 1:1 by an interactive response system to receive mavacamten ± BB or CCB monotherapy (N = 123) or placebo ± BB or CCB monotherapy (N = 128) for 30 weeks. Among those randomized, 119 patients in the mavacamten arm and 125 patients in the placebo arm completed treatment. The details of the trial design have been described elsewhereCitation10,Citation17. Individual patient data from the EXPLORER-HCM trial were used in the present post hoc analysis. The EXPLORER-HCM trial fully adhered to the ethical principles of the Declaration of Helsinki and the specifications of Good Clinical Practice. Written informed consent was obtained from each fully anonymized participant in the study prior to treatment.
Assessments
HRQoL was measured using the EQ-5D-5L questionnaireCitation18, which was completed by patients on day 1 and weeks 6, 12, 18, 30 (or end of treatment), and 38 (or end of study). The present study considered both the EQ-5D-5L index score (health utility), calculated based on the value set for the US populationCitation19, and the EQ-VAS score, administered as part of the EQ-5D-5L questionnaire. EQ-5D-5L index scores are typically interpreted using a range from 1 (full health) to 0 (a health state as bad as being dead). The EQ-VAS score provides an assessment of overall health status ranging from 100 (best imaginable health state) to 0 (worst imaginable health state).
Participants also completed the KCCQ (23-item version)Citation20, which measures the impact of cardiovascular disease or its treatment on symptoms/signs, physical limitations, quality-of-life, social limitations, self-efficacy, and symptom stability, on day 1 and weeks 6, 12, 18, 30 (or end of treatment), and 38 (or end of study); and the HCMSQ, a novel questionnaire that assesses HCM core symptoms (tiredness/fatigue, heart palpitations, chest pain, dizziness, and shortness of breath), for a minimum of 7 consecutive days during the screening period, daily from day 1 through week 6, then for 7 consecutive days every 4 weeks until week 30 (or end of treatment), and then for 7 consecutive days at week 38 (or end of study).
NYHA functional class, a clinical assessment of the impact of cardiac symptoms on patients’ daily activities, was assessed by the physician at screening, on day 1, and at weeks 4, 6, 8, 12, 14, 18, 22, 26, 30 (or end of treatment or at early termination), and 38 (or end of study).
Statistical analyses
Thirty-week change in HRQoL: EQ-5D-5L index score and EQ-VAS score
For the comparison of changes in EQ-5D-5L index score and EQ-VAS score from baseline to week 30, a post-hoc analysis was conducted to include patients with EQ-5D assessments at both baseline (i.e. assessed on day 1) and week 30. This criterion was applied to (a) ensure consistency with the following analysis that examined the HRQoL improvements greater than or equal to MCT estimates at 30 weeks and (b) focus on analyzing the changes by the end of the 30-week dosing period. The mean changes from baseline to week 30 in EQ-5D-5L index score and EQ-VAS score were then compared between the two arms using a linear regression model with robust standard error estimation. Both unadjusted and adjusted models were estimated. The adjusted analyses included three variables used in stratifying treatment allocation: NYHA functional class (II or III), current treatment with BBs (yes or no), and planned type of ergometer used during the study (treadmill or exercise bicycle).
HRQoL improvements greater than or equal to MCT estimates at 30 weeks
Patients with EQ-5D assessments at both baseline (i.e. assessed on day 1) and week 30 were included in the post-hoc analyses. For the distribution-based approach, MCTs were calculated using half of the standard deviation of the baseline valueCitation21. For the anchor-based approach, Spearman correlation coefficients (R2) were first calculated between a candidate anchor variable (KCCQ-23 CSS or HCMSQ SoB subscore) and an HRQoL variable (EQ-5D-5L index score or EQ-VAS score). To qualify as an anchor variable, the R2 was required to be at least 0.25Citation22,Citation23. If the qualification criteria for an anchor variable were met, a random-effects linear model was fitted to predict the change in an HRQoL variable from baseline to a follow-up visit based on the corresponding change in the qualified anchor variable. Specifically, the MCTs for the KCCQ-23 CSS were 5, 10, and 20-point increases, representing the thresholds for small-to-moderate, moderate-to-large, and very large treatment effects, respectivelyCitation12. Similarly, the MCTs for the HCMSQ SoB subscore were 2, 2.5, and 3-point reductions (data on file; Bristol Myers Squibb). The anchor-based MCTs for EQ-5D-5L index score and EQ-VAS score were then estimated based on the coefficients from the regression models and the MCTs for the anchor variable. The models accounted for the repeated measurements from each patient.
For comparison of the proportions of patients with an improvement reaching the MCT in EQ-5D-5L index score or EQ-VAS score from baseline (i.e. assessed on day 1) to week 30, patients with EQ-5D assessments at both baseline and week 30 whose baseline score allowed them to potentially achieve an MCT improvement were included in the analysis. The proportions of patients with an improvement reaching the MCT at week 30 in EQ-5D-5L index score or EQ-VAS score were compared between the two arms using logistic regression. As with the analysis of mean change from baseline, both unadjusted and adjusted models were fitted. Odds ratios with 95% confidence intervals comparing mavacamten with placebo are presented.
Estimation of health utilities by NYHA functional class
In this analysis, health utilities were based on the EQ-5D-5L index score. All patients with at least one post-baseline assessment of EQ-5D-5L index score (non-missing) and NYHA functional class (non-missing) at weeks 6, 12, 18, and/or 30 were included in the analysis. Post-baseline EQ-5D-5L index score and NYHA functional class assessed at weeks 6, 12, 18, and 30 were all included. Based on the NYHA functional class at each visit, patients were classified into three groups – NYHA functional classes I, II, and III/IV. NYHA functional classes III and IV were combined into a single category because of the small number of patients who experienced NYHA functional class IV in the EXPLORER-HCM trial. Of note, while the EXPLORER-HCM trial included patients with NYHA functional classes II and III at baseline, their post-baseline NYHA functional classes may be NYHA functional class I, II, or III/IV, which made it possible to estimate the health utilities by NYHA functional class I, II, and III/IV.
Mean health utilities were estimated for each NYHA functional class in each arm. Specifically, a generalized estimating equation model was fitted including interaction terms between the three NYHA functional classes and the treatment variable. A robust standard error estimation was used to account for repeated measurements of the EQ-5D-5L index score within each patient. Mean health utilities were then compared across the NYHA functional classes within each arm and compared between the two treatment arms within each NYHA functional class. By estimating treatment-specific health utilities for each NYHA functional class, the model did not need to account separately for the impact of adverse events on utility.
Results
Thirty-week change in HRQoL: EQ-5D-5L index score and EQ-VAS score
Among the 123 patients who received mavacamten ± BB or CCB monotherapy, 101 had an EQ-5D assessment at baseline and 111 had an EQ-5D assessment at week 30. Among the 128 patients who received placebo ± BB or CCB monotherapy, 98 had an EQ-5D assessment at baseline and 113 had an EQ-5D assessment at week 30. A total of 96 patients in the mavacamten arm and 89 patients in the placebo arm had EQ-5D assessments at both baseline and week 30 and were included in subsequent analyses. The baseline characteristics of these patients were similar to those of the overall population enrolled in the trialCitation10 ().
Table 1. Baseline patient characteristics by treatment group.
The baseline mean EQ-5D-5L index scores were similar between the two arms (mavacamten = 0.814; placebo = 0.823). At week 30, patients in the mavacamten arm had a statistically significant improvement from the baseline EQ-5D-5L index score compared with patients in the placebo arm (mavacamten = 0.084; placebo = 0.009; unadjusted difference = 0.075 [95% confidence interval = 0.028–0.122], p-value < 0.05; adjusted difference = 0.073 [95% confidence interval = 0.027–0.118], p-value < 0.05) ().
Table 2. 30-week change in EQ-5D-5L index score and EQ-VAS score.
Similarly, the baseline mean EQ-VAS scores were similar between the two arms (mavacamten = 70.0; placebo = 68.6). A significantly greater improvement was also observed for the change in EQ-VAS score from baseline to week 30 among patients in the mavacamten versus the placebo arm (mavacamten = 8.5; placebo = 0.7; unadjusted difference = 7.8 [95% confidence interval = 2.0–13.6], p-value < 0.05; adjusted difference = 7.5 [95% confidence interval = 1.8–13.2], p-value < 0.05) ().
HRQoL improvements greater than or equal to MCT estimates at 30 weeks
At baseline, the standard deviation was 0.176 for the EQ-5D-5L index score and 19.7 for the EQ-VAS score. Thus, the distribution-based approach suggested an MCT of 0.088 for the EQ-5D-5L index score and of 9.9 for the EQ-VAS score ().
Table 3. Patients achieving an improvement of at least the MCT in EQ-5D-5L index score and EQ-VAS score at 30 weeks.
Both the KCCQ-23 CSS and HCMSQ SoB subscore had a sufficiently high correlation with EQ-5D-5L index score and EQ-VAS score and were therefore qualified as anchor variables for both HRQoL outcomes. Specifically, the R2 between the KCCQ-23 CSS and EQ-5D-5L index score was 0.492; it was 0.397 between the KCCQ-23 CSS and EQ-VAS score; 0.342 between the HCMSQ SoB subscore and EQ-5D-5L index score; and 0.291 between the HCMSQ SoB subscore and EQ-VAS score. With the anchor-based approach, the MCT for EQ-5D-5L index score ranged from 0.022 to 0.105, and the MCT for EQ-VAS score ranged from 1.34 to 9.89 ().
The proportion of patients achieving an improvement in EQ-5D-5L index score meeting the MCT was significantly higher in the mavacamten arm compared with the placebo arm, regardless of the MCT value used (adjusted odds ratios ranged from 2.48 to 4.05) (). Specifically, using the distribution-based MCT, 69.0% of patients in the mavacamten arm versus 39.3% in the placebo arm had an improvement of at least the MCT in EQ-5D-5L index score at week 30. Using KCCQ-23 CSS 20-point, 10-point, and 5-point increases as the MCTs for the anchor variable, the proportion of patients with an improvement meeting the MCT in the mavacamten arm was 66.0%, 74.6%, and 78.9%, respectively, compared with 32.1%, 53.7%, and 59.7% in the placebo arm. Analyses using anchor-based MCT values estimated with the HCMSQ SoB subscore generated similar results ().
The proportion of patients with a meaningful improvement in EQ-VAS score was significantly higher in the mavacamten arm compared with the placebo arm (adjusted odds ratios ranged from 1.82 to 2.31) at all MCTs evaluated, except for those estimated using a 10-point increase in the KCCQ-23 CSS and a 2.5-point reduction in HCMSQ SoB subscore (). Specifically, using the distribution-based MCT, 44.2% of patients in the mavacamten arm versus 29.3% of those in the placebo arm had an improvement of at least the MCT in EQ-VAS score at week 30. Using the KCCQ-23 CSS 20-point, 10-point, and 5-point increases as the MCTs for the anchor variable, the proportion of patients with an improvement of at least the MCT in the mavacamten arm was 44.2%, 57.1%, and 64.2%, respectively, compared with 29.3%, 42.4%, and 44.3% in the placebo arm. Analyses using the anchor-based MCT values estimated using the HCMSQ SoB subscore generated similar results ().
Estimation of health utilities by NYHA functional class
A total of 119 of the 123 patients who received mavacamten and 125 of the 128 patients who received placebo had at least one post-baseline assessment of EQ-5D-5L index score and NYHA functional class and were included in the analysis. Only seven patients (3%) were excluded; owing to the small proportion of patients excluded, this is not anticipated to substantially impact the results. The baseline characteristics of these patients were similar to those for the overall population enrolled in the trialCitation10 ().
For patients in the mavacamten arm, the mean utilities were 0.950, 0.866, and 0.708 for NYHA functional classes I, II, and III/IV, respectively. The corresponding mean utilities for patients in the placebo arm were 0.952, 0.850, and 0.704, respectively (). Within each treatment arm, the health utility decreased significantly with a higher NYHA functional class (p-values < 0.05) (). However, utility values were similar within the same NYHA functional class between treatments arms, suggesting that adverse events in the mavacamten treatment arm had very little impact on the utility level beyond what the NYHA class measures ().
Figure 1. Mean health utility (EQ-5D-5L index score) by NYHA functional class and treatment. Error bars represent the 95% CI of the estimates. Abbreviations. BB, beta-blocker; CCB, calcium channel blocker; CI, confidence interval; EQ-5D-5L, EuroQoL 5-dimension 5-level; NYHA, New York Heart Association.
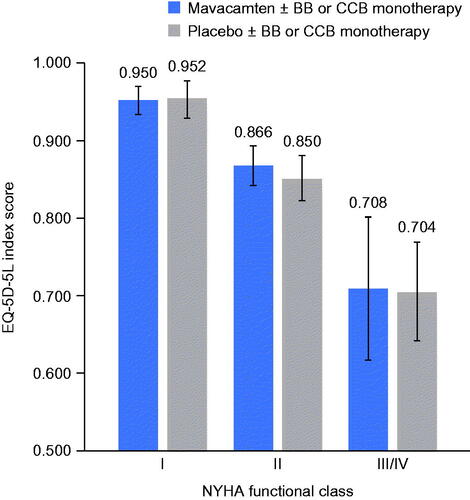
Table 4. Health utility differences (EQ-5D-5L index score) between NYHA functional classes.
Table 5. Health utility difference (EQ-5D-5L index score) between treatments within each NYHA functional class.
Discussion
Symptoms associated with obstructive HCM can lead to marked impairment of patient-reported HRQoL. The results of this study demonstrate the substantial HRQoL benefits associated with treatment with mavacamten in symptomatic obstructive HCM. Patients in the mavacamten arm had substantially greater improvements in EQ-5D-5L index score (health utilities) and EQ-VAS score (general well-being) after 30 weeks of treatment compared with those in the placebo arm. To date, mavacamten is the only pharmacologic treatment with a demonstrated beneficial impact on patient-reported HRQoL in patients with symptomatic obstructive HCM. By using a general (non-disease-specific) HRQoL measure, this study provides information that enables decision-makers to assess and compare the impact of novel treatments in different disease areas, which is important in health technology assessment for market access.
From baseline to week 30, patients receiving placebo ± BB or CCB monotherapy experienced a limited improvement in EQ-5D-5L index score and EQ-VAS score (30-week mean changes = 0.009 in EQ-5D-5L index score and 0.7 in EQ-VAS score). In contrast, the addition of the 30-week course of mavacamten was associated with greater changes in EQ-5D-5L index score and EQ-VAS score (30-week mean changes = 0.084 in EQ-5D-5L index score and 8.5 in EQ-VAS score). These results are consistent with previous findings on the impact of mavacamten on other PROs, including the KCCQ-23 and the HCMSQCitation10,Citation12. The magnitudes of between-arm differences in the 30-week changes are also substantial. By way of comparison, the difference in improvement between the mavacamten arm and the placebo arm was comparable to the difference seen between patients treated with tafamidis, a recently approved treatment for transthyretin amyloid cardiomyopathy, and patients treated with placebo. The ATTR-CM study showed that tafamidis reduced the 30-month decline in EQ-5D index score by 0.09 and in EQ-VAS score by 9.11 compared with placeboCitation24.
Additionally, the odds ratios comparing the proportions of patients experiencing a meaningful improvement between the two arms in this study were also large in magnitude – ranging from 2.48 to 4.05 for EQ-5D-5L index score and from 1.82 to 2.31 for EQ-VAS score. These data suggest that substantially more patients in the mavacamten treatment arm had clinically important improvements in health utilities and general health well-being than patients in the placebo treatment arm, irrespective of the methods used to define the MCT.
Furthermore, the present analysis identified strong correlations between the two HRQoL outcomes with the KCCQ-23 CSS and HCMSQ SoB subscore. Specifically, the correlations with the KCCQ-23 CSS suggest a link between HRQoL and health status in patients with symptomatic obstructive HCM. Notably, the KCCQ OSS has previously been found to strongly correlate with EQ-5D-3L index score and EQ-VAS score in a population with heart failureCitation25. A high validation R2 has also been found in the mapping of the KCCQ to EQ-5D-5L index score for patients with heart failureCitation26. Additionally, the strong correlations between the two HRQoL outcomes and the HCMSQ SoB subscore further suggest a link between patient HRQoL and one of the HCM-related symptoms – shortness of breath. Such evidence supports using the EQ-5D index score to measure health utilities in obstructive HCM.
This study also estimated heath utilities by NYHA functional class, a commonly used clinical measure to evaluate the symptoms and limitations in physical activity in symptomatic obstructive HCM. To our knowledge, this is the first study to evaluate the health utility of the NYHA functional class in this disease. The EQ-5D index score is the preferred utility measure in the appraisals by the National Institute for Health and Care Excellence in the UKCitation27 and the Institute for Clinical and Economic Review in the USCitation28. The results provide important information to support the economic evaluation of novel treatments in obstructive HCM. In addition, the analysis showed strong associations between NYHA functional class and HRQoL across treatment arms, an observation that has similarly been reported in previous HRQoL studies in heart-related disordersCitation29–31. The results are also consistent with those reported by Huff et al., which showed that another PRO measure, the KCCQ, was correlated with NYHA functional class among patients with HCMCitation9. Additionally, the limited residual between-arm differences in health utility values suggest that the marked differences in EQ-5D-5L index score between the two arms could be predominantly explained by NYHA functional classes, suggesting that mavacamten was not associated with a substantial impact on the safety profile when compared with placebo.
The present study is subject to several limitations. First, patients with symptomatic obstructive HCM were treated for only 30 weeks in the EXPLORER-HCM trial. Therefore, the current data cannot be used to evaluate the long-term effects of mavacamten on HRQoL beyond 30 weeks. Future research with longer follow-up may shed light on the long-term HRQoL benefit of mavacamten. Second, the study combined NYHA functional classes III and IV in the analysis because of the small number of patients with NYHA functional class IV in the EXPLORER-HCM trial. Studies have shown that HRQoL may differ greatly between NYHA classes III and IVCitation30,Citation31. Future studies may consider including more patients with severe symptoms and limitations of physical activity to distinguish the health utilities between NYHA functional classes III and IV. Third, this study utilized data from the EXPLORER-HCM clinical trial, and so additional studies may be needed to validate the generalizability of these results to the real-world patient population with symptomatic obstructive HCM. Fourth, due to missing EQ-5D assessments, the sample of the present study was a smaller subset of the intention-to-treat population.
Conclusions
Treatment with mavacamten ± BB or CCB monotherapy significantly improved EQ-5D-5L index score and EQ-VAS score – and thus HRQoL – compared with placebo ± BB or CCB monotherapy in patients with symptomatic obstructive HCM. Patients with more severe functional limitations, as assessed by NYHA functional class, showed poorer HRQoL compared with patients assessed to be in a lower NYHA functional class. Additionally, both EQ-5D-5L index score and EQ-VAS score were positively correlated with other PROs, such as the KCCQ-23 CSS and HCMSQ SoB subscore.
Transparency
Declaration of funding
Funding for this study was provided by MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb.
Declaration of financial/other interests
JX and YW are employees of Analysis Group, Inc., which has received consulting fees from MyoKardia, Inc. a wholly owned subsidiary of Bristol Myers Squibb. YX and JTF are employees of MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb, and hold stock/options. JL is an employee of Bristol Myers Squibb and holds stock/options. LPG is an independent consultant and partner in VeriTech Corporation which has received consulting fees from MyoKardia Inc., a wholly owned subsidiary of Bristol Myers Squibb. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
Editorial assistance was provided by Oxford PharmaGenesis and Shelley Batts, Ph.D., an employee of Analysis Group, Inc. Support for this assistance was funded by MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb.
Data availability statement
Data not available due to ethical, legal, and commercial restrictions. Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
References
- Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2020;142(25):e558–e631.
- Marian AJ, Braunwald E. Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res. 2017;121(7):749–770.
- Cirino AL, Ho C. Hypertrophic cardiomyopathy overview. GeneReviews®. [Internet]: Seattle (WA): University of Washington; 2021.
- Maron MS, Olivotto I, Zenovich AG, et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114(21):2232–2239.
- Maron BJ. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med. 2018;379(7):655–668.
- Cox S, O'Donoghue AC, McKenna WJ, et al. Health related quality of life and psychological wellbeing in patients with hypertrophic cardiomyopathy. Heart. 1997;78(2):182–187.
- Magnusson P, Mörner S, Gadler F, et al. Health-related quality of life in hypertrophic cardiomyopathy patients with implantable defibrillators. Health Qual Life Outcomes. 2016;14(1):1–9.
- Jacquelinet M, Pentiah AD, Coisne A, et al. Quality of life in outpatients with hypertrophic cardiomyopathy. Arch Cardiovasc Dis Suppl. 2017;9(1):34.
- Huff CM, Turer AT, Wang A. Correlations between physician-perceived functional status, patient-perceived health status, and cardiopulmonary exercise results in hypertrophic cardiomyopathy. Qual Life Res. 2013;22(3):647–652.
- Olivotto I, Oreziak A, Barriales-Villa R, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;396(10253):759–769.
- Maron MS, Ommen SR. Exploring new and old therapies for obstructive hypertrophic cardiomyopathy: mavacamten in perspective. Circulation. 2021;143(12):1181–1183.
- Spertus JA, Fine JT, Elliott P, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): health status analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397(10293):2467–2475.
- Dyer MT, Goldsmith KA, Sharples LS, et al. A review of health utilities using the EQ-5D in studies of cardiovascular disease. Health Qual Life Outcomes. 2010;8:13.
- Batóg P, Rencz F, Péntek M, et al. EQ-5D studies in cardiovascular diseases in eight Central and Eastern European countries: a systematic review of the literature. Kardiol Pol. 2018;76(5):860–870.
- Food and Drug Administration. Patient-reported outcome measures: use in medical product development to support labeling claims [Internet] 2009. [cited August 06 2021]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims
- Revicki D, Hays RD, Cella D, et al. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61(2):102–109.
- Ho CY, Olivotto I, Jacoby D, et al. Study design and rationale of EXPLORER-HCM: evaluation of mavacamten in adults with symptomatic obstructive hypertrophic cardiomyopathy. Circ Heart Fail. 2020;13(6):e006853.
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–1736.
- Pickard AS, Law EH, Jiang R, et al. United States valuation of EQ-5D-5L health states using an international protocol. Value Health. 2019;22(8):931–941.
- Green CP, Porter CB, Bresnahan DR, et al. Development and evaluation of the Kansas City cardiomyopathy questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245–1255.
- Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592.
- Streiner D, Norman GR, Cairney J. Health measurement scales: a practical guide to their development and use. Vol. 5th ed. New York: Oxford University Press; 2015.
- Simpson E, Eckert L, Gadkari A, et al. Validation of the atopic dermatitis control tool (ADCT©) using a longitudinal survey of biologic-treated patients with atopic dermatitis. BMC Dermatol. 2019;19(1):1–10.
- Hanna M, Damy T, Grogan M, et al. Impact of tafamidis on health-related quality of life in patients with transthyretin amyloid cardiomyopathy (from the tafamidis in transthyretin cardiomyopathy clinical trial). Am J Cardiol. 2021;141:98–105.
- Gallagher AM, Lucas R, Cowie MR. Assessing health-related quality of life in heart failure patients attending an outpatient clinic: a pragmatic approach. ESC Heart Fail. 2019;6(1):3–9.
- Thomas M, Jones PG, Cohen DJ, et al. Predicting the EQ-5D utilities from the Kansas City Cardiomyopathy Questionnaire in patients with heart failure. Eur Heart J Qual Care Clin Outcomes. 2021;7(4):388–396.
- Brazier J, Longworth L. NICE DSU technical support document 8: an introduction to the measurement and valuation of health for NICE submissions. Report by the decision support unit. London: National Institute for Health and Care Excellence (NICE); 2011.
- Institute for Clinical and Economic Review (ICER). 2020–2023. Value Assessment Framework [Internet]. 2020 Jan 21 [updated 2021 Oct 25; cited 2021 Nov 11]. Available from: https://icer.org/wp-content/uploads/2021/10/ICER_2020_2023_VAF_102521.pdf
- Sandtröm A, Sandberg C, Rinnström D, et al. Factors associated with health-related quality of life among adults with tetralogy of fallot. Open Heart. 2019;6(1):e000932.
- Griffiths A, Paracha N, Davies A, et al. Analyzing health-related quality of life data to estimate parameters for cost-effectiveness models: an example using longitudinal EQ-5D data from the SHIFT randomized controlled trial. Adv Ther. 2017;34(3):753–764.
- Göhler A, Geisler BP, Manne JM, et al. Utility estimates for decision–analytic modeling in chronic heart failure—health states based on New York heart association classes and number of rehospitalizations. Value Health. 2009;12(1):185–187.
