Abstract
Aims
Spinal muscular atrophy (SMA) is a progressive neuromuscular disease associated with the degeneration of motor neurons in the brainstem and spinal cord. Studies examining the epidemiology and economic impact of SMA are limited in Canada. This study aimed to estimate the epidemiology as well as healthcare resource utilization (HRU) and healthcare costs for children with SMA in Alberta, Canada.
Materials and methods
We conducted a retrospective study using anonymized data from administrative healthcare databases provided by Alberta Health. Data from 1 April 2010 to 31 March 2018, were extracted for patients <18 years of age identified with SMA. Five-year incidence and prevalence were calculated for cases identified between 1 April 2012 and 31 March 2017. HRU and healthcare costs were assessed one year after SMA diagnosis, including hospitalizations, physician visits, ambulatory care visits and long-term care admissions.
Results
The five-year incidence and prevalence of pediatric onset SMA were 1.03 per 100,000 person-years and 9.97 per 100,000 persons, respectively. General practitioner, specialist, and ambulatory care visits were common among children with SMA in the first-year post-diagnosis. The mean (SD) total annual direct cost per patient in the first-year post-diagnosis was $29,774 ($38,407); hospitalizations accounted for 41.7% of these costs ($12,412 [$21,170]), followed by practitioner visits at 32.3% ($9,615 [$13,054]), and ambulatory care visits at 26.0% ($7,746 [$9,988]).
Conclusions
Children with SMA experience substantial HRU, particularly for hospitalizations and practitioner visits, following diagnosis. Given the high costs of SMA, timely access to effective treatment strategies, such as the novel survival motor neuron (SMN)-restoring treatments recently approved for use, are needed to improve health outcomes and HRU.
Background
Spinal muscular atrophy (SMA) is a progressive neuromuscular disease associated with degeneration of the motor neurons in the spinal cord and brainstem, leading to muscular weakness and paralysisCitation1,Citation2. Other organs and tissues, such as muscle and the neuromuscular junction, are also affectedCitation1,Citation2. It is caused by mutations or deletions of the survival motor neuron 1 (SMN1) gene on 5q13Citation3. Internationally, the estimated incidence of SMA is 1 per 10,000 newborns, with a prevalence of 1–2 per 100,000 persons, and a carrier frequency of 1 in 40–60 individualsCitation4; though the number of cases in Canada remains largely unknown.
SMA has a broad range of severity and is classified into four main types based on the age of onset of symptoms and the highest level of attained gross motor function; the majority (>95%) of cases present during childhood (<18 years of age)Citation5–7. Type 1 SMA, often referred to as infantile SMA, generally presents before six months of age with severe muscle weakness, hypotonia, and progressive bulbar and respiratory insufficiencyCitation8,Citation9. These infants are unable to sit and often die before their second birthday if untreatedCitation10. Children with type II SMA are symptomatic before 18 months of age; they can sit but are unable to stand or walk unassisted. Orthopedic complications, such as joint contractures or scoliosis, and respiratory complications are common in patients with type II SMA and can lead to reduced life expectancyCitation11. Children with type III SMA present after 18 months of age with the ability to walk unassisted. However, progressive muscle weakness may result in loss of independent ambulationCitation11. Adult (≥18 years) onset type IV SMA accounts for less than 5% of overall SMA cases; it is associated with slowly progressive muscle weakness but generally a normal life expectancyCitation12.
Due to the impact of SMA on respiratory, feeding, and motor functions, the management of SMA is resource-intensive, with substantial impact of care and healthcare resource utilization (HRU) among affected individuals. For example, in the United States (US), the annual median medical expenditures were estimated to be $83,652 US dollars (USD) across all study years under investigation (2003–2012), with an interquartile range of $29,620 to $228,754 USDCitation13. While studies from other countries and two recently published systematic reviews have indicated high costs for SMACitation13–17, little is known about healthcare expenditures for this disorder in Canada.
Previously, therapeutic options for SMA were limited to symptom managementCitation9,Citation18. However, novel therapies have become available, including nusinersen, the first treatment approved by Health Canada and recommended for public coverage by the Canadian Agency for Drugs and Technologies in Health (CADTH) in December 2017Citation19. More recently, onasemnogene abeparvovec and risdiplam received approval from Health CanadaCitation20,Citation21. As the therapeutic landscape continues to evolve, an understanding of the epidemiology of SMA in Canada is vital for strategic planning to provide more effective management strategies for individuals living with SMA.
Studies characterizing the impact of SMA are limited in CanadaCitation22,Citation23, and cost of illness studies conducted internationally may not be generalizable to the Canadian healthcare settingCitation6,Citation13,Citation24–29. Canada has a publicly funded healthcare system that is decentralized, whereby, individual provinces govern their own healthcare systems, leading to variability in the capture of patient and health administrative data. Compared to other provinces, Alberta has a single province-wide health authority, which provides an opportunity to use population-based administrative datasets to understand the impact of SMA in Canada. Therefore, the objective of this study was to determine the incidence, prevalence, HRU and direct healthcare costs for children with SMA in the province of Alberta, Canada.
Methods
We conducted a retrospective observational cohort study using administrative health databases from Alberta, with a population of approximately 4.3 million in 2018Citation30. Alberta Health, the provincial health authority, provided anonymized data from 1 April 2010 to 31 March 2018, from province-wide administrative datasets including Alberta Blue Cross claims (public drug plan), Alberta Continuing Care Information System (long-term care [LTC]), Discharge Abstract Database (DAD), National Ambulatory Care Reporting System (NACRS), Pharmaceutical Information Network (PIN) prescription dispenses, Population Registry, Practitioner Claims, and Vital Statistics (births and deaths). Based on a published algorithm for identifying patients with SMA from administrative data in the USCitation13, patients with SMA were identified in this study if they had at least one inpatient (DAD) or three ambulatory care or outpatient (from NACRS and Practitioner Claims datasets) visits attributed to a diagnostic code for SMA (first code within the study period was classified as their SMA index date). Inpatient (DAD) admissions included hospital visits, and hospital-based rehabilitation. Ambulatory care and outpatient visits included emergency department (ED) visits, outpatient visits, or practitioner claims. Codes from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) were used in the Practitioner Claims dataset. Codes from the International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Canada (ICD-10-CA), were used for the DAD and NACRS datasets. The distribution of these ICD-9-CM/ICD-10-CA codes within our study cohort is shown in Supplementary Table 1.
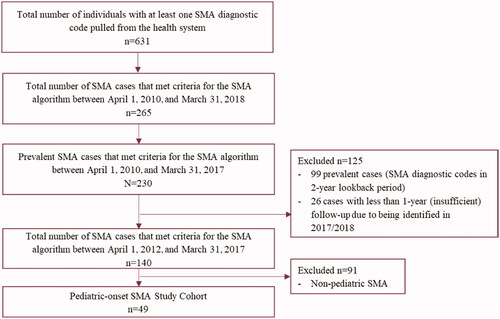
To estimate the epidemiology of SMA, a case ascertainment period was applied, between 1 April 2012 and 31 March 2017, allowing for a two-year lookback period (before SMA index) and a minimum of one year of follow-up data post-SMA identification. Prevalent SMA cases were defined as those with an SMA diagnostic code within the two-year lookback period, while incident cases were defined as those without an SMA diagnostic code within the lookback period. Only patients with pediatric-onset SMA (<18 years of age at index date) were included in this study. To examine the demographic and clinical characteristics, HRU and associated costs for incident patients with SMA, prevalent cases were excluded from the study cohort. The pediatric SMA sample was also stratified by age categories (<6 months, 6 to <18 months, and 18 months to <18 years) according to their age at the first SMA diagnostic code date within the case ascertainment period.
Study variables
Five-year and annual incidence and prevalence estimates were calculated for cases identified between 1 April 2012 and 31 March 2017. Demographic and clinical characteristics, including age and sex, were captured at the patient’s SMA index date. Comorbidities of interest, including respiratory failure, pulmonary infections, pneumonia, cardiomyopathy, congenital heart disease, dysphagia, scoliosis, spinal fusion, joint contractures, and failure to thrive were extracted from inpatient hospitalization data (DAD) one-year before and after the patient’s index date (see Supplementary Table 2 for the corresponding ICD-10-CA codes).
All-cause and SMA-specific (where any one of the SMA diagnostic codes were present as the most responsible diagnosis) HRU in the first year after a patient’s index date were extracted for hospitalizations, hospital length of stay (LoS), practitioner visits (general and specialist), ambulatory care visits (emergency department visits and day procedures), and LTC admissions.
Statistical analyses
For five-year prevalence, the numerator was the number of prevalent SMA cases as of 1 April 2012, plus incident cases between 1 April 2012 and 31 March 2017. The denominator was the average population within the five years, defined as the total mid-year Alberta population <18 years of age on 1 July 2014, based on Statistics Canada estimatesCitation30. To calculate the five-year incidence rate, the numerator was the number of new SMA cases between 1 April 2012 and 31 March 2017. The denominator was the sum of the Alberta mid-year population at risk on July 1 of each year based on Statistics Canada estimates between 2012 and 2016Citation30, representing the number of person-years of follow-up over the period. The assumption was made that each person based on the mid-year population contributed one year of follow-upCitation31. The denominator for the incidence rate calculation by age was based on the age-specific population estimates at risk. Annual incidence rates were calculated using a similar method based on annual population estimates. Corresponding confidence intervals (CI) were calculated based on a Poisson distribution, due to the small cohort sizes.
Demographic and clinical characteristics were reported using frequencies, means and standard deviations (SD). HRU was assessed in the first year post-SMA index. HRU was described by all-cause and SMA-specific utilization. The mean total cost per patient was estimated for each resource and overall, where the denominator was all patients even those with zero resource use. Additionally, event-based costs were calculated for hospitalizations and LTC, which included only patients that had at least one hospitalization or LTC admission, respectively. Practitioner visits cost data was extracted directly from the Alberta Health data, while hospitalization costs were calculated from the Canadian Institute for Health Information (CIHI) methodologyCitation32, ambulatory care costs were obtained from the Alberta Health Interactive Health Data ApplicationCitation33, and LTC costs were obtained from the Alberta Health Continuing Care Accommodation chargesCitation34. All costs were inflated to 2020 Canadian dollars (CAD) using component-specific price indices from Alberta HealthCitation35. All analyses were stratified by age at index (<6 months, 6 to <18 months, and 18 months to <18 years).
All statistical analyses were performed in SAS version 9.4Footnotei. This study was approved by the Health Research Ethics Board of Alberta – Community Health Committee.
Results
Epidemiological estimates
The five-year incidence and five-year prevalence of pediatric-onset SMA in Alberta were 1.03 (95% CI: 0.77–1.36) per 100,000 person-years and 9.97 (95% CI: 9.34–10.62) per 100,000 persons in Alberta, respectively. Across the results stratified by age, those <6 months of age had the highest five-year incidence at 7.44 (95% CI: 3.57–13.68) per 100,000 person-years ( and Supplementary Table 3).
Table 1. Five-year incidence of pediatric-onset SMA in Alberta between 2012 and 2016, stratified by age.
Patient demographic and clinical characteristics
For the HRU and cost analyses, the incident pediatric SMA cohort was used. A total of 49 children were identified with SMA between 1 April 2012 and 31 March 2017, in Alberta (). Among all patients with pediatric-onset SMA, the mean (SD) age at index was 5.5 (5.2) years and 57.1% were male. The most common comorbidities were failure to thrive (20.4%), pneumonia (14.3%), and respiratory failure (12.2%). The demographic and clinical characteristics, overall and by age categories, are reported in .
Table 2. Demographic and clinical characteristics for children with incident SMA, stratified by age.
Healthcare resource utilization
During the first year following SMA-diagnosis, 25 (51.0%) children were hospitalized, with a mean (SD) of 2.12 (1.72) hospitalizations and a mean LoS of 29.84 (41.59) days. In the first year after diagnosis, 48 (98.0%) children had specialist(s) visits (mean of 48.39 visits [70.10]), 30 (61.2%) saw a general practitioner (mean of 3.78 visits [5.93]), and 46 (93.9%) had other ambulatory care visits (mean of 25.59 visits [35.57]). No patients with pediatric SMA had a LTC admission in the first year following diagnosis (). The proportion of children requiring hospitalization was highest among those <6 months of age (100%), with a mean of 2.30 (1.95) hospitalizations and LoS of 44.30 (44.82) days during the first year following diagnosis among those with ≥1 visit (see ).
Table 3. HRU in the first year post-incident SMA diagnosis.
Table 4. All-cause HRU and costs in the first year post-incident SMA diagnosis, stratified by age.
Healthcare costs
During the first year of follow-up post-SMA diagnosis, the mean (SD) total all-cause costs per pediatric patient was $29,774 ($38,407). As shown in and , hospitalizations accounted for 41.7% of the mean total costs per patient ($12,412 [$21,170]), followed by practitioner visits at 32.3% ($9,615 [$13,054]) and ambulatory care visits at 26.0% ($7,746 [$9,988]). The mean total SMA-specific costs per pediatric patient in the first year of follow-up was $7,132 ($12,930). Relative to all-cause HRU costs, SMA-specific costs accounted for 36.2% of hospitalization costs, 25.2% of practitioner visit costs, and 2.8% of ambulatory care visit costs.
Figure 2. Contribution of types of healthcare resource use to total costs for the incident pediatric-onset SMA cohort. Abbreviation. SMA, spinal muscular atrophy.
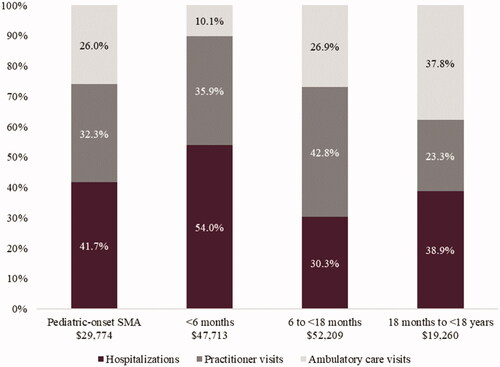
The highest mean (SD) total costs per patient were observed in those <6 months and 6 months to <18 months of age at $47,713 ($32,495) and $52,209 ($34,440), respectively (). Hospitalization costs accounted for the highest proportion of the mean total costs per patient among those <6 months of age (54.0% of total costs) and those 18 months to <18 years (38.9% of total costs) (). For patients 6 to <18 months of age, practitioner visits accounted for the highest proportion (42.8%) of mean total costs per patient.
Discussion
Our study found that the five-year incidence of pediatric-onset SMA in Alberta was 1.03 per 100,000 person-years and the five-year prevalence was 9.97 per 100,000 persons. The incidence rate was the highest for those <6 months of age relative to the other age categories. The first year after SMA diagnosis was associated with substantial HRU for both all-cause and SMA-specific resources in Alberta. The high costs reflect in part the broad range of symptoms and support needs of children with SMA. According to the updated International Standard of Care for SMA consensus statements, a multidisciplinary approach including neurologists, pediatricians, other specialists, and clinicians are needed to work along with the families of children with SMA to provide various aspects of careCitation9,Citation18.
When comparing our incidence and prevalence results to previous studies, there are some notable differences. An earlier study from Ontario during 2003 to 2014 reported a prevalence estimate of 0.7 per 100,000 persons for children <18 yearsCitation23. The Ontario study prevalence estimates were likely lower than our study because only hospitalizations and ED visits were included in the Ontario algorithm, whereas our study also included practitioner visits to identify patients. Several other studies have reported on the incidence and prevalence of pediatric SMA. One study in Ireland reported a point prevalence among SMA cases aged ≥ five years of 1.10 per 100,000 in December 2013Citation24. The Global Burden of Disease Study (2016) reported an incidence of 1.9 per 100,000 person-years in persons 28 days to 1 year of age, which varied by location (e.g. 0.9 per 100,000 person-years in sub-Saharan Africa to 13.1 per 100,000 person-years in Australia)Citation22. Similarly, a study using the TREAT-NMD Global SMA Patient Registry and the Care and Trial Sites Registry (CTSR) reported considerable variability between countries with prevalence estimates ranging from 0.01 to 2.43 per 100,000 in the Global Patient Registry to 0.00 to 4.11 per 100,000 in the CTSR for SMA types I-IIICitation6. The variation in incidence and prevalence estimates across studies may be due to differences in data sources, time periods, geography, ethnicity, and estimation methods and age range included in the estimateCitation36,Citation37. Newborn screening is being implemented in many countries, which will help provide clarity on the real-world incidence rates of SMA in particular regionsCitation38.
Outside of Canada, several studies have highlighted the high healthcare costs associated with SMA. A recently published systematic review, including nine studies, reported annual costs of SMA ranging from $27,157 to $196,429, with the highest costs reported among SMA type ICitation16. Another systematic review, including 14 studies, reported estimates for annual per-patient direct medical costs of $3,320 to $324,410, similarly with the highest cost estimates also associated with type I SMACitation17. In the US, one study reported the average annualized total direct healthcare expenditure for SMA to be $47,862 USD between 2013 and 2017Citation13, while another study reported mean direct healthcare costs per person per month of $25,517 for infantile-, $6,357 for childhood-, and $2,499 for late-onset SMA patients between 2006 and 2016Citation15. Two more recently published US studies found mean annual inpatient costs of children with SMA to be $104,197 per childCitation39, and mean net payments for inpatient and outpatient admissions for SMA of $39,398.91 and $49,067.83, respectivelyCitation40. A recent Australian study reported an average total annual cost of SMA at $143,705 (2017 USD) between 2016 and 2017, with direct costs accounting for 56% of total costsCitation41. In Spain, total average annual direct healthcare costs of €10,882 annually in 2015 have been reportedCitation42, while the average annual cost per SMA-related admission was €6,274 between 1997 and 2015Citation43. A cross-sectional study from Germany reported an overall mean direct cost of €54,721 annually per patientCitation14. Another study of 86 children and adolescents with types I to III SMA across Europe in 2015 found that the annual average cost associated with SMA (including both direct and non-direct costs) ranged from €32,042 in France to €54,295 in the UKCitation44. Though there is considerable variation in healthcare costs across these studies, the economic impact of SMA remains notably significant, aligning with the substantial direct costs we identified among patients with pediatric SMA in Alberta.
In the current study, mean annual costs in the year post diagnosis were over two times higher among those aged <6 months ($47,713) and 6 to < 18 months ($52,209) relative to those 18 months to < 18 years ($19,260). Higher expenditures among patients with infantile (i.e. type I) SMA have also been noted internationally. One US study reported a mean total cost of $50,190 USD per hospital admission among patients with type I SMACitation45, while another US study reported total mean health care costs at $137,627 per patient per year in patients with type 1 SMA between 2016 and 2018Citation46. Differences in costs in our study compared to those from the US likely reflect differences in the healthcare funding models between countries (public versus private healthcare models). The higher total costs and hospitalization rates may be attributed to patients with type I SMA being younger and having more severe disease, which may require more care.
By way of comparison to the general population, the Canadian Institute for Health Information estimated a total health expenditure of $6,604 per Canadian in 2017; driven largely by hospital, drug, and physician servicesCitation47. In addition, the average expenditure per patient for the general Alberta pediatric population was only $1,640 in 2017 according to the National Health Expenditures data from the Canadian Institute for Health InformationCitation47. Other studies confirmed that the average costs per patient associated with SMA were much higher relative to the average medical expenditure for general patient populationsCitation13,Citation45.
This study provides real-world, population-based insights into the impact of disease of pediatric-onset SMA in Alberta. By linking several province-wide administrative datasets, we were able to provide a comprehensive evaluation of direct HRU and associated costs, as well as insights into the demographic and clinical characteristics of those identified with SMA in Alberta. The first-year post-diagnosis HRU and costs were considered due to the shortened lifespan of children with severe SMA, particularly those <6 months of age. This approach enabled a clear understanding of resource use and costs following diagnosis. Although our study provides insights to the impact of SMA in Alberta, future studies conducted in other provinces or across Canada would provide evidence to understand any regional variation in SMA care. Finally, future investigations examining SMA HRU by different types of interventions (e.g. tracheostomy, gastrostomy, etc.) and how SMA HRU and associated costs compare to other chronic pediatric onset conditions would highlight the impact of SMA on the health system.
While administrative health data provides a unique opportunity for the real-world evaluation of patient populations, some limitations should be noted when interpreting the results of this study. These data are not collected for research purposes, but for healthcare administration, which may impact the type of health information collected. Diagnostic codes are not a confirmed presence of disease, and it was not possible to confirm whether the included patients had genetically confirmed 5q-linked SMACitation3. As a result, the identified cases may include other types of motor neuron disease. Other data sources that collect genetic test diagnostic information would be beneficial to confirm SMA diagnosis. Comorbidities of interest were extracted from inpatient hospitalization data within one year prior to the index date and may be underestimated if comorbidities developed after SMA diagnosis. Further, comorbidities could also be underestimated if they were captured in other sources, such as primary care. Additional investigations to examine subgroups of patients with SMA and notable comorbidities (e.g. cardiomyopathy or congenital heart disease) would be valuable to understand any differences in HRU and healthcare costs. Furthermore, this study only captured direct costs pertaining to specific sectors of the health system. Other costs such as compassionate release of novel therapies (e.g. nusinersen starting in 2016), allied healthcare providers, out-of-pocket expenses and caregiver support were not includedCitation48. Specifically, a societal perspective cannot be assessed using administrative data. According to a recently published Canadian patient and caregiver survey studyCitation49, the estimated median expenditure for assistive devices, health professional services and SMA-related travel and accommodations in the past 12 months were $4,500, $6,800, and $1,200 CAD, respectively. In addition, 45–63% of caregivers also reported needing respite care, physiotherapy from an injury due to a lift/transfer, or other health impacts. Furthermore, this current study also did not include the cost of SMA treatments, as the first approved SMA treatment was not added to the Alberta Health formulary for pediatric patients with type I-III SMA until December 2018Citation33,Citation50. Future studies using Alberta administrative data when the uptake of these newly-approved novel therapies for SMA have been established would be of great interest, especially considering that there have been investigations in other countries looking at the cost effectiveness of nusinersen in patients with SMACitation51. The algorithm used to identify patients with SMA was broad, which may have overestimated the incidence and prevalence estimates reported here. Although a two-year lookback period was used to exclude prevalent cases, two years may not be sufficient to truly identify incident SMA, particularly those with a later onset of disease. This limitation may especially affect the identification of patients older than two years of age, as there may be a longer time between symptom onset and a formal diagnosisCitation52. Lastly, we used age at the first SMA diagnostic code date to stratify the results by pediatric age categories of interest, which may not be representative of the true onset of disease. Further stratification of SMA by type was not feasible to derive from the administrative data. In addition, the implementation of newborn screening, survival motor neuron 2 (SMN2) gene copy number determination, and changing disease phenotypes with treatment create additional challenges for historical SMA typing when examining the outcomes of patients with SMA and should be accounted for in future studies.
Conclusion
Overall, this study provides novel insights into the HRU and associated healthcare costs among children with SMA in Alberta. The findings indicate that pediatric patients with SMA have many interactions with healthcare providers in the first year following diagnosis, particularly for hospitalizations and specialist practitioner visits. With recent advancements in treatment for SMA, including nusinersenCitation19, onasemnogene abeparvovecCitation20, and risdiplamCitation21, the impact of disease should improve. Future investigations are warranted to better understand the impact of these novel medications on health outcomes, HRU and associated costs.
Transparency
Declaration of funding
Funding for this project was provided by Hoffmann-La Roche Limited, Mississauga, Ontario, Canada.
Declaration of financial/other interests
GC is a consultant for Medlior Health Outcomes Research Ltd. He has also received research funding from the Canadian Institutes for Health Research. BS, BG, MSF, and TC are employed by Medlior Health Outcomes Research Ltd. CC, JWW, BM, and KKP are employed by Hoffmann-La Roche Limited who funded this study and hold Hoffmann-La Roche Limited stock. JKM received a consultant fee and research grant from Hoffmann-La Roche Limited.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
GC provided support with statistical analysis. BS provided support with economic analysis. BG and MSF provided project management support and medical writing support. TC provided project management support. CC, JWW, BM and KKP provided project management and editing support. JKM provided clinical expertise and editing support.
Supplemental Material
Download MS Word (65.7 KB)Acknowledgements
This study is based on data provided by Alberta Health and Alberta Precision Laboratories. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of the Government of Alberta. Neither the Government of Alberta nor Alberta Health expresses any opinion in relation to this study. We would also like to thank Wayne Khuu for his support with medical writing and analysis.
Notes
i SAS version 9.4 is a registered trademark of SAS Institute, Cary, NC, USA.
References
- Chaytow H, Faller KME, Huang YT, et al. Spinal muscular atrophy: from approved therapies to future therapeutic targets for personalized medicine. Cell Rep Med. 2021;2(7):100346.
- Wirth B. Spinal muscular atrophy: in the challenge lies a solution. Trends Neurosci. 2021;44(4):306–322.
- Lefebvre S, Bürglen L, Reboullet S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80(1):155–165.
- Verhaart IEC, Robertson A, Wilson IJ, et al. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy - a literature review. Orphanet J Rare Dis. 2017;12(1):124.
- Prior TW, Snyder PJ, Rink BD, et al. Newborn and carrier screening for spinal muscular atrophy. Am J Med Genet A. 2010;152A(7):1608–1616.
- Verhaart IEC, Robertson A, Leary R, et al. A multi-source approach to determine SMA incidence and research ready population. J Neurol. 2017;264(7):1465–1473.
- Wang CH, Finkel RS, Bertini ES, et al. Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol. 2007;22(8):1027–1049.
- Palomino MA, Castiglioni C. Respiratory care in spinal muscular atrophy in the new therapeutic era. Rev Chil Pediatr. 2018;89(6):685–693.
- Mercuri E, Finkel RS, Muntoni F, et al. Diagnosis and management of spinal muscular atrophy: Part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018;28(2):103–115.
- Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377(18):1723–1732.
- Zerres K, Rudnik-Schöneborn S, Forrest E, et al. A collaborative study on the natural history of childhood and juvenile onset proximal spinal muscular atrophy (type II and III SMA): 569 patients. J Neurol Sci. 1997;146(1):67–72.
- MedlinePLUS genetics. Spinal Muscular Atrophy. 2020; [cited 2020 Sep 25]. Available from: https://ghr.nlm.nih.gov/condition/spinal-muscular-atrophy.
- Armstrong EP, Malone DC, Yeh WS, et al. The economic burden of spinal muscular atrophy. J Med Econ. 2016;19(8):822–826.
- Klug C, Schreiber-Katz O, Thiele S, et al. Disease burden of spinal muscular atrophy in Germany. Orphanet J Rare Dis. 2016;11(1):58.
- Tan H, Gu T, Chen E, et al. Healthcare utilization, costs of care, and mortality among Patients with spinal muscular atrophy. J Health Econ Outcomes Res. 2019;6(3):185–195.
- Dangouloff T, Botty C, Beaudart C, et al. Systematic literature review of the economic burden of spinal muscular atrophy and economic evaluations of treatments. Orphanet J Rare Dis. 2021;16(1):47.
- Landfeldt E, Pechmann A, McMillan HJ, et al. Costs of illness of spinal muscular atrophy: a systematic review. Appl Health Econ Health Policy. 2021;19(4):501–520.
- Finkel RS, Mercuri E, Meyer OH, et al. Diagnosis and management of spinal muscular atrophy: Part 2: pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul Disord. 2018;28(3):197–207.
- Canadian agency for drugs and technologies in health. Nusinersen pharmacoeconomic review report. Ottawa, Ontario, Canada: Canadian Agency for Drugs and Technologies in Health; 2018.
- Health Canada. Regulatory Decision Summary – Zolgensma. 2020.
- Hoffman-La Roche. Roche presents new 2-year data for Evrysdi (risdiplam) in infants with Type 1 spinal muscular atrophy (SMA). 2020; [cited 2021 Jan 22]. Available from: https://www.roche.com/media/releases/med-cor-2020-09-28.htm.
- GBD 2016 Motor Neuron Disease Collaborators. Global, regional, and national burden of motor neuron diseases 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2018;17(12):1083–1097.
- Rose L, McKim D, Leasa D, et al. Trends in incidence, prevalence, and mortality of neuromuscular disease in Ontario, Canada: a population-based retrospective cohort study (2003–2014). PLoS One. 2019;14(3):e0210574.
- Lefter S, Hardiman O, Ryan AM. A population-based epidemiologic study of adult neuromuscular disease in the Republic of Ireland. Neurology. 2017;88(3):304–313.
- König K, Pechmann A, Thiele S, et al. De-duplicating patient records from three independent data sources reveals the incidence of rare neuromuscular disorders in Germany. Orphanet J Rare Dis. 2019;14(1):152.
- Husebye SA, Rebne CB, Stokland AE, et al. A hospital based epidemiological study of genetically determined muscle disease in south western Norway. Neuromuscul Disord. 2020;30(3):181–185.
- Okamoto K, Fukuda M, Saito I, et al. Incidence of infantile spinal muscular atrophy on Shikoku Island of Japan. Brain Dev. 2019;41(1):36–42.
- Vill K, Kölbel H, Schwartz O, et al. One year of newborn screening for SMA – results of a German pilot project. J Neuromuscul Dis. 2019;6(4):503–515.
- Draušnik Ž, Cerovečki I, Štefančić V, et al. The prevalence of muscular dystrophy and spinal muscular atrophy in Croatia: data from national and non-governmental organization registries. Croat Med J. 2019;60(6):488–493.
- Statistics Canada. Table 17-10-0005-01. Population estimates on July 1st, by age and sex. Ottawa (Ontario): Statistics Canada.
- Gerstman BB. Epidemiology kept simple: an introduction to traditional and modern epidemiology. Oxford: John Wiley & Sons; 2013.
- Canadian Institute for Health Information. Health care cost drivers: the facts. Ottawa (Ontario): CIHI; 2011.
- Strategic Services Division Alberta Health. Health care cost drivers. Edmonton (Alberta): Alberta Health, 2013.
- Alberta Health. Continuing care accomodation charges. Alberta, Canada: Alberta Health, 2018; [cited 2021 Dec 04]. Available from: http://www.health.alberta.ca/services/continuing-care-accommodation-charges.html.
- Statistics Canada. Table 18-10-0005-01 Consumer Price Index, annual average, not seasonally adjusted. Ottawa (Canada): Statistics Canada. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1810000501.
- Spronk I, Korevaar JC, Poos R, et al. Calculating incidence rates and prevalence proportions: not as simple as it seems. BMC Public Health. 2019;19(1):512.
- Zaldívar T, Montejo Y, Acevedo AM, et al. Evidence of reduced frequency of spinal muscular atrophy type I in the Cuban population. Neurology. 2005;65(4):636–638.
- Glascock J, Sampson J, Haidet-Phillips A, et al. Treatment algorithm for infants diagnosed with spinal muscular atrophy through newborn screening. J Neuromuscul Dis. 2018;5(2):145–158.
- Lee M, Jr., França UL, Graham RJ, et al. Pre-Nusinersen hospitalization costs of children with spinal muscular atrophy. Pediatr Neurol. 2019;92:3–5.
- Belter L, Cruz R, Kulas S, et al. Economic burden of spinal muscular atrophy: an analysis of claims data. J Mark Access Health Policy. 2020;8(1):1843277.
- Chambers GM, Settumba SN, Carey KA, et al. Prenusinersen economic and health-related quality of life burden of spinal muscular atrophy. Neurology. 2020;95(1):e1–e10.
- Lopez-Bastida J, Pena-Longobardo LM, Aranda-Reneo I, et al. Social/economic costs and health-related quality of life in patients with spinal muscular atrophy (SMA) in Spain. Orphanet J Rare Dis. 2017;12(1):141.
- Darbà J, Marsà A. Patient characteristics and hospitalisation costs of spinal muscular atrophy in Spain: a retrospective multicentre database analysis. BMJ Open. 2019;9(11):e031271-e.
- Peña-Longobardo LM, Aranda-Reneo I, Oliva-Moreno J, et al. The economic impact and Health-Related quality of life of spinal muscular atrophy: an analysis across Europe. Int J Enrivon Res Public Health. 2020;17(16):5640.
- Cardenas J, Menier M, Heitzer MD, et al. High healthcare resource use in hospitalized patients with a diagnosis of spinal muscular atrophy type 1 (SMA1): retrospective analysis of the Kids' Inpatient Database (KID). Pharmacoecon Open. 2019;3(2):205–213.
- Droege M, Sproule D, Arjunji R, et al. Economic burden of spinal muscular atrophy in the United States: a contemporary assessment. J Med Econ. 2020;23(1):70–79.
- Canadian Institute for Health Information. National Health Expenditure Trends, 1975 to 2019. Canadian Agency for Drugs and Technologies in Health: CIHI, 2019.
- Farrar MA, Carey KA, Paguinto SG, et al. Financial, opportunity and psychosocial costs of spinal muscular atrophy: an exploratory qualitative analysis of Australian carer perspectives. BMJ Open. 2018;8(5):e020907.
- McMillan HJ, Gerber B, Cowling T, et al. Burden of spinal muscular atrophy (SMA) on patients and caregivers in Canada. J Neuromuscul Dis. 2021;8(4):553–568.
- Alberta Health. Interactive drug benefit list: SPINRAZA 2.4 MG / ML Injection (Nusinersen Sodium). Edmonton (AB): Alberta Health; 2020.
- Zuluaga-Sanchez S, Teynor M, Knight C, et al. Cost effectiveness of nusinersen in the treatment of patients with Infantile-Onset and Later-Onset spinal muscular atrophy in Sweden. Pharmacoeconomics. 2019;37(6):845–865.
- Lin CW, Kalb SJ, Yeh WS. Delay in diagnosis of spinal muscular atrophy: a systematic literature review. Pediatr Neurol. 2015;53(4):293–300.