Abstract
Aims
Proliferating hematopoietic stem and progenitor cells (HSPCs) are susceptible to chemotherapy-induced damage, resulting in myelosuppressive adverse events (AEs) such as neutropenia, anemia, and thrombocytopenia that are associated with high health care costs and decreased quality of life (QoL). In this study, a trial-based cost-effectiveness analysis was performed to help assess the economic impact of administering trilaciclib, a myeloprotective therapy that protects multilineage HSPCs from chemotherapy-induced damage, prior to standard first-line chemotherapy, using data from a pivotal Phase II study of trilaciclib in the setting of extensive-stage small cell lung cancer (ES-SCLC, NCT03041311).
Method
The aim of this study was to assess the cost-effectiveness of administering trilaciclib prior to chemotherapy versus chemotherapy alone among patients with ES-SCLC from a United States payer perspective. Data on the rate and frequency of myelosuppressive AEs and health utility were derived from the pivotal study of trilaciclib. Costs of managing myelosuppressive AEs and costs of chemotherapy treatment were sourced from published literature. Outcomes included the number of myelosuppressive AEs, costs (in 2021 US dollars), quality-adjusted life-years (QALYs), incremental cost, incremental QALY, and an incremental cost-effectiveness ratio.
Results
Administering trilaciclib prior to chemotherapy was associated with a reduction in neutropenia (82%), febrile neutropenia (75%), anemia (43%), and thrombocytopenia (96%) compared with chemotherapy alone. Additionally, trilaciclib prior to chemotherapy was cost-saving compared with chemotherapy alone ($99,919 vs $118,759, respectively) and associated with QALY improvement (0.150 vs 0.145, respectively). Probabilistic sensitivity analyses showed 58% of iterations projecting cost savings and QALY improvement with trilaciclib.
Conclusions
The findings suggest that the use of trilaciclib prior to first-line chemotherapy in patients with ES-SCLC can be cost-beneficial owing to fewer myelosuppressive AEs and lower costs, together with a favorable QoL profile.
Introduction
Chemotherapy-induced myelosuppression is a common toxicity that increases the risk of morbidity and mortality in patients with cancerCitation1. Although cancer cells are the intended targets of chemotherapy, these regimens are generally nonspecific and affect healthy tissue, resulting in unintended adverse effectsCitation2. Rapidly dividing healthy tissue types, such as hematopoietic stem and progenitor cells (HSPCs), are particularly susceptible to unintended sequelae from chemotherapyCitation3. This often results in multilineage myelosuppression that manifests as neutropenia, anemia, and/or thrombocytopeniaCitation3,Citation4.
A systematic review on the burden of myelosuppressive adverse events (AEs) reported that patients with cancer who received chemotherapy had 14.6-fold greater odds of developing neutropenia or thrombocytopenia, and 3.0-fold greater odds of developing anemia, compared with those who did not receive chemotherapyCitation5. The occurrence of myelosuppression during cancer therapy may also lead to dose delays, dose reductions, or discontinuation of chemotherapy, potentially jeopardizing tumor control and patient outcomesCitation3,Citation5,Citation6. These observations underscore the importance of multilineage myeloprotection among cancer patients receiving chemotherapy.
Data from the United States suggest that myelosuppressive AEs exert a substantial economic burdenCitation5. A retrospective analysis of the 2012 National Inpatient Database revealed that 5.2% of all cancer-related hospitalizations and 8.3% of all cancer-related hospitalization costs are attributable to cancer-related neutropeniaCitation7. Moreover, one study from the United States reported that 94% of emergency department visits for febrile neutropenia ended in hospitalizationCitation8. The direct cost of neutropenia ranges from $2,632 (2006 United States dollars (USD); $4,108 in 2021 USD)Citation9 per outpatient episode to $49,917 (2006 USD; $77,909 in 2021 USD) per febrile neutropenia hospitalization episode in the United StatesCitation5,Citation7. The total annualized cost of cancer-related neutropenia hospitalizations in the United States is estimated to be $2.3 billion (2012 USD; $3.0 billion in 2021 USD) for adultsCitation7. The cost of anemia management ranges from $22,775 to $93,454 (2006 USD; $35,547 to $145,861 in 2021 USD) per patient per yearCitation5. In addition, it is reported that approximately 15% of the available blood resources in the United States are allocated to patients with hematology and/or oncology indicationsCitation6. Cost estimates of thrombocytopenia management have extended from $1,395 (2006 USD; $2,177 in 2021 USD) per cycle to $22,698 (2015 USD; $26,660 in 2021 USD) per episodeCitation5,Citation10.
Currently, standard interventions for managing myelosuppressive AEs fall short of the ideal. Treatments are specific to a single hematopoietic lineage (e.g. utilization of granulocyte colony-stimulating factors [G-CSFs] for the management of neutropenia; red blood cell transfusions, administration of erythropoiesis-stimulating agents, and/or iron supplementation for the management of anemia; and platelet transfusion and antifibrinolytic agents for the management of thrombocytopenia)Citation3,Citation6,Citation11,Citation12. Of note, these treatments do not protect the bone marrow from chemotherapy-induced cytotoxic effects, but they do impart individual risks for adverse reactions.
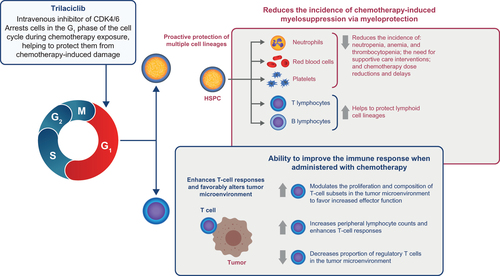
Trilaciclib (COSELAFootnotei), a first-in-class breakthrough therapy, is currently the only therapy that protects multiple hematopoietic lineages simultaneouslyCitation13,Citation14. Trilaciclib transiently arrests HSPCs in the G1 phase of the cell cycle by inhibiting the activity of cyclin-dependent kinases 4 and 6, thus protecting HSPCs from damage by cytotoxic chemotherapy ()Citation14. Three individual Phase II randomized clinical trials have shown that trilaciclib improves multiple clinically meaningful myelosuppression endpoints, which cannot currently be addressed by a single existing interventionCitation14–16. In February 2021, trilaciclib was approved by the United States Food and Drug Administration (FDA) to decrease the incidence of chemotherapy-induced myelosuppression in adult patients when administered prior to a platinum/etoposide-containing or topotecan-containing regimen in the setting of extensive-stage small cell lung cancer (ES-SCLC)Citation17, after receiving FDA priority review and breakthrough therapy designations for this indication in 2019. In March 2021, the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN GuidelinesFootnoteii) added trilaciclib as a prophylactic option to manage chemotherapy-induced myelosuppression in patients with ES-SCLC, as per its indication, to its Guidelines for Small Cell Lung Cancer and Hematopoietic Growth FactorsCitation12,Citation18. The high unmet need for a new treatment that provides multilineage myeloprotection from cytotoxic damage is reflected in the priority review granted by the FDA and the rapid inclusion of trilaciclib in the NCCN GuidelinesCitation12,Citation17,Citation18. As such, it is important to provide timely information for health care decision-makers that helps them to understand the economic implication of administering trilaciclib to patients with ES-SCLC prior to chemotherapy. The objective of this study, therefore, was to assess the cost-effectiveness of administering trilaciclib prior to chemotherapy versus chemotherapy alone for patients with ES-SCLC, from a United States payer perspective.
Methods
Overview
We conducted an economic assessment of trilaciclib, using a trial-based cost-effectiveness analysis (CEA) approach based on clinical data from the pivotal Phase II trial, with cost data applied from literature (). Given that trilaciclib was FDA-approved in February 2021, there were insufficient real-world data available on the use of trilaciclib at the time of analysis. Therefore, this initial economic evaluation was derived from available clinical trial data comparing the use of trilaciclib prior to first-line chemotherapy versus the use of the same chemotherapy regimen without trilaciclib. Of the three individual, Phase II randomized trials, G1T28-05 (NCT03041311)Citation15 was the pivotal trial; therefore data from this trial served as the foundation for the economic evaluation. A further two studies of trilaciclib in patients with ES-SCLC (G1T28-02 [NCT02499770]Citation14 and G1T28-03 [NCT02514447]Citation16) were considered proof-of-concept studies; therefore, data from these trials were used only to strengthen certain estimates (frequency of AEs [G1T28-05 and G1T28-02 combined] and reduction in the use of prophylactic G-CSF [G1T28-05, G1T28-02, and G1T28-03 combined]) ().
Table 1. Main parameters of the economic evaluation.
Table 2. Model parameters and assumptions.
G1T28-05 was a randomized, double-blind, placebo-controlled Phase II study of trilaciclib or placebo administered prior to treatment with etoposide, carboplatin, and atezolizumab (E/P/A) in patients with newly diagnosed ES-SCLC. A total of 107 patients were randomized to receive trilaciclib (n = 54) or a placebo (n = 53) prior to administration of E/P/A. Patients were treated in an induction phase and a maintenance phase. During induction, carboplatin (area under the concentration–time curve [AUC] 5 mg/mL/min) and atezolizumab (1,200 mg) were administered on day 1, and etoposide (100 mg/m2) and either trilaciclib (240 mg/m2) or placebo on days 1, 2, and 3 of a 21-day cycle for a maximum of four cycles. During maintenance, patients received atezolizumab monotherapy on day 1 of every 21-day cycle. Chemotherapy, trilaciclib, or placebo were not administered during the maintenance phase.
The effects of trilaciclib on multilineage myeloprotection and health-related quality of life (HRQoL) were collected during the induction phase. The primary endpoints were the duration of severe neutropenia in cycle 1 and the occurrence of severe neutropenia during the induction treatment period, whereby severe neutropenia was defined as absolute neutrophil count <0.5 × 109 cells per liter. Compared with placebo, administration of trilaciclib prior to E/P/A resulted in statistically significant decreases in the duration of severe neutropenia in cycle 1 (0 vs 4 days, p < .0001) and occurrence of severe neutropenia (49.1% vs 1.9%, p < .0001)Citation15.
Population, reference, and comparator
Consistent with the pivotal Phase II clinical trial, the population of interest was patients with ES-SCLC who received first-line treatment with chemotherapy (E/P/A). This analysis compared two treatment strategies: using trilaciclib prior to E/P/A versus the same chemotherapy regimen without trilaciclib.
Time horizon
The economic evaluation used a 12-week time horizon, consistent with the duration of the induction phase of the pivotal Phase II G1T28-05 trial, during which all the relevant endpoints were observed. Extending the time horizon beyond 12 weeks would add uncertainty by introducing additional assumptions. In the G1T28-05 trial, most patients completed four cycles of chemotherapy (44 [84.6%] patients in the trilaciclib group and 48 [90.6%] patients in the placebo group)Citation15; therefore, our analysis assumed that all patients received four cycles of chemotherapy. This is also in line with NCCN Guidelines for Small Cell Lung Cancer, which recommend four cycles of therapy, but allow that up to six cycles may be received based on response and tolerability after four cyclesCitation18.
Study design
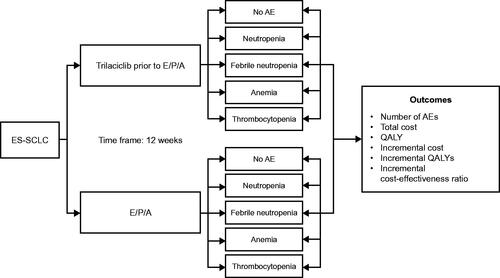
In this analysis, patients received E/P/A either with or without prior administration of trilaciclib (). During the 12 weeks of treatment, patients could experience any of four myelosuppressive hematologic AEs: neutropenia, febrile neutropenia, anemia, and thrombocytopenia. Further, patients could experience more than one AE and/or multiple episodes of the same AE during therapy.
Figure 2. Study schematic to estimate the value of trilaciclib for patients with ES-SCLC receiving first-line chemotherapy. Abbreviations. AE, adverse event; CEA, cost-effectiveness analysis; E/P/A, etoposide, carboplatin and atezolizumab; ES-SCLC, extensive-stage small cell lung cancer; QALY, quality-adjusted life year.
Outcomes
Health and economic outcomes (the number of myelosuppressive AEs, costs, and quality-adjusted life-years [QALYs]) were estimated and compared for each treatment strategy, with the calculation of the incremental cost and incremental QALYs between the two treatment strategies (; ).
No discount on costs and benefits was applied given the short time horizon. It was assumed that trilaciclib did not impact response to the underlying ES-SCLC treatment or mortality, as no difference in progression-free survival (hazard ratio [HR] of trilaciclib vs placebo = 0.83, 95% CI: 0.55–12.4) or overall survival (HR = 0.92, 95% CI: 0.57–1.49) was observed in the pivotal Phase II trial of trilaciclibCitation15. Therefore, mortality outcomes were assumed to be the same across treatment strategies and were not included in the analysis.
Inputs
Clinical inputs
Clinical inputs related to myelosuppressive hematologic AEs were derived from the pivotal Phase II clinical trial of trilaciclib (G1T28-05)Citation15. The analysis considered grade 3 or 4 AEs only, as lower-grade events were anticipated to have a minimal impact on clinical and economic outcomes from the payer perspective. Event rates represented the percentage of patients who experienced at least one AE. The frequency of a given AE represented the average number of events per patient during the treatment period and was calculated as the total number of events divided by the number of patients who had at least one event in each treatment strategy. As AE frequencies were similar between the two clinical trials of trilaciclib prior to first-line chemotherapy for ES-SCLC (G1T28-05 and G1T28-02), the data were pooled to generate a larger sample size. Thus, the weighted average of the pooled data was used to estimate the frequency of each AE (). An exploratory analysis was also conducted using AE frequency data from G1T28-05 only.
Inputs related to the prophylactic G-CSF use
The analysis assumed that 26% of patients in the E/P/A treatment strategy received prophylactic G-CSF to avoid potential neutropenia events, based on a market research study conducted by G1 Therapeutics, Inc. In the deterministic analysis, it was assumed that patients who were assigned to trilaciclib therapy had a 50% reduction in G-CSF prophylaxis (i.e. 13% of patients used G-CSF prophylactically in the trilaciclib treatment strategy)Citation19. Prophylactic G-CSF costs were calculated using a weighted average of the wholesale acquisition cost (WAC) of commonly used G-CSF brands and biosimilars from approved product labelsCitation20. Total cost per cycle was calculated based on the WAC per unit, dosing information, and cost weighting using estimated market shares. The drug cost was then added to the weighted administrative cost to generate the average cost for G-CSF. The average cost associated with the prophylactic use of G-CSF per cycle, including administration costs, was estimated to be $5,733Citation20,Citation21. The number of prophylactic G-CSF cycles was calculated on the basis of a weighted average of the mean number of cycles from the two most commonly used G-CSFs – filgrastim and pegfilgrastimCitation22.
Treatment cost inputs
Therapy costs for E/P/A included treatment acquisition costs and administration costs. Acquisition costs were calculated by combining published WAC costs for each product within the respective treatment regimens and per the recommended dosing schedule. Administration costs were sourced from the literatureCitation26. It was assumed that all patients completed the recommended four treatment cyclesCitation20. The WAC cost for trilaciclib was $1,417 per 300-mg vial, or $2,834 per doseCitation24. The total cost of trilaciclib per course of chemotherapy was calculated by multiplying the cost per dose of trilaciclib ($2,834) by the number of doses required per cycle (three doses per cycle), then multiplying by the number of cycles in each chemotherapy regimen (four cycles). Health care resource utilization required for the administration of trilaciclib was not considered in the analysis because trilaciclib is a 30-min intravenous therapy administered within 4 h prior to chemotherapy, so it is given during the same visit as chemotherapy administration. Based on the timing of administration of trilaciclib, and per expert input, it was assumed that trilaciclib is administered concurrently with premedication and does not require additional chair time in the infusion setting. In the base-case analysis, we assumed no administration cost of trilaciclib; however, an exploratory scenario analysis including an administration cost of trilaciclib per Current Procedural Terminology (CPT) codes ($77.86; Medicare national fee schedule for CPT 96365) was conducted.
AE management cost inputs
The management costs for neutropenia, thrombocytopenia, and anemia were obtained from the retrospective claims-based analyses conducted by Wong et al.Citation10 This retrospective matched cohort study assessed the incremental health care costs associated with AEs in adult patients with cancer (breast, digestive organs and peritoneum, genitourinary organs (including bladder and ovary and other uterine adnexa), lung, lymphatic and hematopoietic tissue, and skin) in the United States from 2006 to 2015Citation10. The management costs for febrile neutropenia were obtained from the retrospective claims-based analyses conducted by Weycker et al.Citation23 This was a retrospective cohort study that assessed the clinical and economic risks and consequences of febrile neutropenia among patients with metastatic cancer (breast, colon/rectum, lung, ovaries, and prostate) in the United States from 2007 to 2011Citation23. All costs were adjusted for inflation to 2021 USD using the medical care component of the Consumer Price Index, according to the United States Bureau of Labor Statistics as of March 2021Citation9.
Health utility inputs
Clinical trials of trilaciclib included exploratory patient-reported outcome endpoints to assess the effects of trilaciclib on HRQoL, based on the validated Functional Assessment of Cancer Therapy–General (FACT-G), FACT–Lung (FACT-L), and FACT–Anemia (FACT-An) questionnairesCitation15,Citation16,Citation19,Citation27–30. FACT-G is a 27-item questionnaire designed to measure four domains of HRQoL in patients with cancer: physical, social, emotional, and functional well-being. FACT-L meets a growing need for disease-specific HRQoL questionnaires that address the general and unique concerns of patients diagnosed with lung cancer, and FACT-An, which supplements the core FACT-G questionnaire, was developed to assess specific HRQoL concerns related to anemia and fatigue in patients with cancer. Health utility inputs were derived from the FACT-G survey data, specifically in the pivotal Phase II clinical trial of trilaciclibCitation15. FACT-G survey responses were mapped to EuroQol 5-Dimension (EQ-5D) utility weights using a published algorithm developed by Teckle et al. to estimate utility score for each treatment strategyCitation25. Health utility for each treatment strategy was calculated based on baseline utility (average utility among all patients at day 1, cycle 1) and treatment-specific midpoint percentage change in utility between the first and last cycle. The resulting utility weights for trilaciclib prior to chemotherapy and placebo prior to chemotherapy were multiplied by the treatment duration to estimate the QALYs for each treatment strategy ().
Sensitivity analyses
One-way sensitivity analyses (OWSAs) were conducted by systematically varying one parameter at a time. This allowed for an evaluation of key outcomes with changes in a single parameter and helped to determine the main driver of the results. For AE rates and frequencies, two approaches were used. In the first approach, the underlying AE rate or AE frequency was varied directly (±5%) whereas observed risk reduction with trilaciclib was kept constant. In the second approach, the underlying AE rate and AE frequency were kept the same and the relative risk reduction ratio was varied for patients on trilaciclib (±5%)Citation31. AE management costs were analyzed by applying a ± 10% change to each parameter (). To understand the impact of trilaciclib pricing on model results, the WAC for trilaciclib was varied ±10%, and a tornado diagram was generated for an incremental cost.
Probabilistic sensitivity analyses (PSAs) were performed to account for multivariate uncertainty in the model. The uncertainty in each parameter was characterized using probability distributions and analyzed using simulations of up to 1,000 iterations. Normal distributions were applied to AE rate and frequency parameters. Beta distributions were applied to utility weights, whereas gamma distributions were applied to costs ()Citation32. Cholesky decomposition was also applied to correlated parameters. A correlation coefficient of 0.7 was used to represent a moderate-to-high correlation for AE rates and frequenciesCitation33, and an incremental cost-effectiveness plane was generated.
Results
Deterministic (base-case) results
Results from the deterministic analysis are presented in . Administration of trilaciclib prior to chemotherapy was associated with fewer myelosuppressive events, respectively, compared with administration of chemotherapy alone (82% reduction in neutropenia (0.3 vs 1.5), 75% reduction in febrile neutropenia (0.02 vs 0.1), 43% reduction in anemia (0.3 vs 0.5), and 96% reduction in thrombocytopenia (0.03 vs 0.7). As a result of fewer AEs, use of trilaciclib was associated with a reduced cost of AE management ($13,833 with trilaciclib vs $64,139 without). Of the $50,307 cost saving from AE management, neutropenia and thrombocytopenia were the major cost drivers ($26,442 saving from neutropenia and $17,472 from thrombocytopenia). Additionally, administration of trilaciclib was associated with a reduced cost of G-CSF prophylaxis ($2,541 with trilaciclib vs $5,082 without). Overall, trilaciclib was associated with a total cost saving of $18,840 per patient. There was a marginal gain in QALYs of 0.005 for patients who received trilaciclib prior to chemotherapy compared with those who received chemotherapy alone.
Table 3. Deterministic (base-case) results for first-line treatment of patients with ES-SCLC.
Sensitivity analysis results
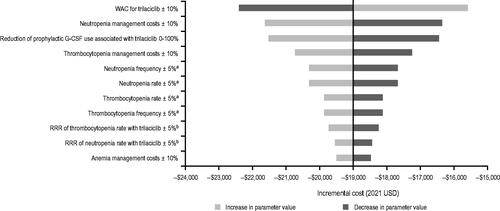
In the OWSA, the use of trilaciclib resulted in cost savings ranging from $16,196 to $21,484 (; Supplemental Table S1). The WAC price of trilaciclib and costs of managing neutropenia and thrombocytopenia had the biggest impact on cost savings, followed by AE rates and frequencies of neutropenia and thrombocytopenia.
Figure 3. Incremental costs estimated from OWSAs. aUnderlying AE event rate and episode frequency were applied to both trilaciclib prior to E/P/A and E/P/A alone. bRRR of trilaciclib prior to E/P/A vs E/P/A alone. Abbreviations. AE, adverse event; E/P/A, etoposide, carboplatin, and atezolizumab; OWSA, one-way sensitivity analysis; RRR, relative risk reduction; USD, United States dollars.
The base-case analysis assumed no administration cost for trilaciclib. An exploratory scenario analysis that included an administration cost for trilaciclib based on CPT code 96935, showed consistent results (cost savings $17,905; incremental QALY 0.005).
In the PSA, the mean cost saving was $18,999 (standard deviation [SD]: $8,596; 95% CI: $3,371, $37,034) from 1,000 iterations (). The incremental cost was negative (i.e. cost-saving) for 99.0% of the iterations. The mean incremental QALY was 0.006 (SD: 0.03; 95% CI: −0.052, 0.068). Overall, 99.6% of the PSA iterations showed cost savings, 58.2% of the iterations showed both cost savings and an improvement in QALYs, and 41.4% of the iterations showed cost savings and a decrease in QALY (). The cost-effectiveness acceptability curve indicated that the use of trilaciclib prior to first-line chemotherapy in patients with ES-SCLC had a 97.6–99.6% chance of being cost-effective when the WTP threshold increased from $0 to $200,000 per QALY, that is, the higher threshold used by the Institute for Clinical and Economic Review to assess the cost-effectiveness of health interventionsCitation34 ().
Figure 4. Cost-effectiveness plane (A) and cost-effectiveness acceptability curve (B) for the PSA. Abbreviations. E/P/A, etoposide, carboplatin, and atezolizumab; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life year; USD, United States dollars; WTP, willingness-to-pay threshold.
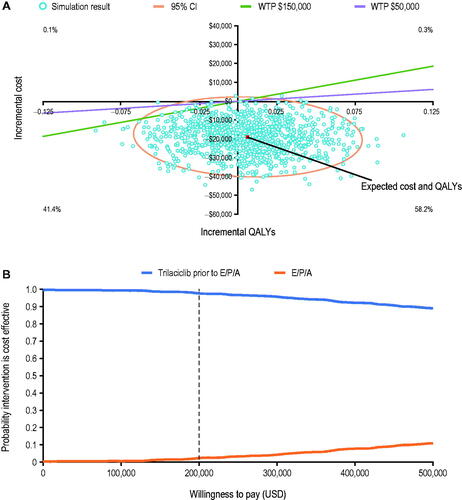
Table 4. PSA results.
Discussion
Current treatment strategies for the management of myelosuppressive hematologic AEs are specific to single hematopoietic lineagesCitation3,Citation6,Citation11,Citation12. Trilaciclib is the first and, to date, the only myeloprotective therapy that simultaneously protects multiple hematopoietic lineages from chemotherapy-induced damage, as evidenced in adult patients with ES-SCLCCitation13–16. Moreover, current supportive care interventions for chemotherapy-induced myelotoxicity are each associated with certain safety concerns, such as venous thromboembolism with erythropoiesis-stimulating agents, and bone pain or development of secondary hematologic malignancies with G-CSFsCitation35–37, among others; in this regard, trilaciclib may offer additional advantages over lineage-specific interventions. This is the first study to evaluate the costs and benefits of trilaciclib administration prior to first-line chemotherapy in patients with ES-SCLC. Although there have been numerous analyses of the economic impact of febrile neutropenia and the cost-effectiveness of G-CSFs across various tumor typesCitation38–48, the results of these studies are not directly comparable to the current analysis given that trilaciclib provides multilineage myeloprotection. As this is the first economic evaluation of a first-in-class therapy, real-world data on the use of trilaciclib have not yet accrued, and available data are limited to outcomes from the pivotal Phase II trial of trilaciclib in newly diagnosed patients with ES-SCLC. As such, the evaluation was adjusted to these circumstances and incorporated a trial-based CEA comparing the use of trilaciclib prior to chemotherapy with the same chemotherapy without trilaciclib. Historically, data from Phase III trials were typically used to support marketing authorization and reimbursement of new drugs. However, Phase II data are increasingly being used as primary evidence to support these purposesCitation49 and, by necessity, are subsequently used for economic evaluations like ours; the first one, to our knowledge, being the economic evaluation of another supportive care therapy (amifostine), for cytoprotection in patients with advanced head and neck cancer receiving chemoradiationCitation50.
The findings from the analysis suggest that administration of trilaciclib prior to chemotherapy reduces the incidence of myelosuppressive AEs. The results show a 78% reduction in the number of myelosuppressive AEs and associated costs. Specifically, administering trilaciclib prior to chemotherapy may result in an 82% reduction in grade 3/4 neutropenia, 75% reduction in febrile neutropenia, 43% reduction in anemia, and 96% reduction in thrombocytopenia. The acquisition cost of trilaciclib ($34,008) is offset by the reduction in chemotherapy-related AE management costs ($50,307). In addition, the administration of trilaciclib was associated with a reduced cost of G-CSF prophylaxis ($2,541). Together, the findings suggest that the use of trilaciclib is a cost-saving approach to the management of chemotherapy-induced myelosuppression. The value of PSAs in providing an unbiased measure of uncertainty in health economic models is widely recognized by economists and decision makersCitation51,Citation52. As such, it is notable that 99.6% of the PSA iterations in the CEA showed cost savings, and 58.2% had both cost savings and QALY improvement. An alternative approach would have been to conduct a trial-based bootstrapping analysis to test uncertainty relating to the clinical inputs from the trial data; however, bootstrapping cannot provide estimates for economic inputs relating to AE management, as these were derived from literature. Therefore, we performed a PSA, which allowed us to test the uncertainty around clinical, economic, and utility inputs.
QALY gain was small in the model (0.005), which may be partially related to the short time horizon (12 weeks, which is consistent with the recommended treatment duration of the underlying treatment combinations)Citation12,Citation18. In addition, utility weights were calculated on the basis of the published mapping algorithm between FACT-G and EQ-5DCitation25, given EQ-5D results were not collected in the trilaciclib pivotal Phase II trial (G1T28-05)Citation15. In this trial, administration of trilaciclib prior to chemotherapy resulted in longer times to deterioration in FACT-G (HR = 0.58), FACT-L (HR = 0.70), and FACT-An (HR = 0.58) domains compared with administration of chemotherapy alone. The benefits of trilaciclib in FACT-L and FACT-An were not reflected in the analysis, as the mapping algorithm was based on FACT-G. Results from the PSA suggested there was high uncertainty on the QALY results, with 41.4% of the iterations showing cost-savings and a decrease in QALY, and 58.2% of the iterations showing cost-savings and an improvement in QALY. Future studies are therefore recommended to further refine the study results.
AE rates (proportion of patients experiencing an AE) were based on the results from the pivotal Phase II trilaciclib clinical trial in patients with ES-SCLC receiving first-line chemotherapyCitation15, whereas the AE frequencies (number of AE episodes among patients who experienced more than one AE) were based on weighted averages from G1T28-05Citation15 and G1T28-02Citation14. Data from study G1T28-02 were not used for AE rate input because the G1T28-02 study included a different chemotherapy backbone (E/P in the G1T28-02 study vs E/P/A in the G1T28-05 study) and AE rates were considered different between the two trials. However, the AE frequency results were similar between the two trials and, therefore, were pooled to generate a more robust estimate based on larger sample size. Results from an exploratory analysis using AE frequency data from only the G1T28-05 clinical trial were consistent with those of the deterministic analysis (cost saving of $16,749; Supplemental Table S2). Frequency and rates of grade 3 or 4 AEs were non-primary endpoints in the trilaciclib clinical trials and therefore were not powered to detect statistically significant differences between treatment arms.
This analysis sheds light on the potential value of, and the need for, myeloprotective interventions, such as trilaciclib, in the management of multilineage myelosuppression. The clinical trial results of trilaciclib coupled with the cost-saving findings from this analysis warrant more research in other tumor types that are treated with myelosuppressive chemotherapy. Of note, there is a general paucity of recent data on the costs associated with myelosuppressive AEs, meaning that data for targeted, hormonal, and immunotherapies are limited except for in high-incidence cancers. Future research that examines shifts in cancer treatment practices and the impact on myelotoxicity-related AEs is therefore warranted. It should also be noted that this analysis did not include costs related to caregivers to estimate the overall economic impact of trilaciclib. Indirect costs (e.g. paid caregiver, caregiver work loss) account for 34–44% of the total cost of managing neutropenia, and more than 50% of the total cost of managing thrombocytopeniaCitation5. Future studies on the impact of trilaciclib on caregiver burden are recommended.
There were several key assumptions in this analysis. First, it was assumed that trilaciclib therapy does not affect the treatment response or the survival of the patient, as there was no statistically significant impact on overall survival in either of the two Phase II clinical trials conducted in the first-line setting. For G1T28-02, the HR for overall survival was 0.87 for trilaciclib versus placebo (80% CI: 0.61–1.24)Citation14 and in the pivotal G1T28-05 trial, the HR was 0.92 (95% CI: 0.57, 1.49)Citation15. Therefore, no impact on relative survival was applied for trilaciclib. Second, all patients were assumed to be treated over four cycles (21 days per cycle) without treatment interruptions, dose adjustments, or discontinuations based on data from the pivotal clinical trial, in which most patients received four chemotherapy cycles. To date, real-world data on the use of trilaciclib are limited and insufficiently powered to infer treatment patterns. However, we acknowledge that, in clinical practice, patients may die or switch treatments for clinical or safety reasons, and that analyses based on real-world data are a critical priority for the future. Indeed, we anticipate analyzing real-world data at both the patient level and at the chemotherapy session-level, once available. No direct data were available for the reduction of prophylactic G-CSF usage associated with trilaciclib. Inputs were obtained from a pooled analysis of clinical trial data from all three individual randomized clinical trials of trilaciclib that reported a 50% reduction in any G-CSF use in patients who received trilaciclib prior to chemotherapy compared with those who received chemotherapy aloneCitation19. This assumption was explored by varying the G-CSF use from 0% to 100%, which led to consistent results (cost savings ranging from $16,299 to $21,381). Third, for the utility weight inputs, AE-specific utility decrements were not used, but leveraged population estimates for each treatment strategy in the trial, which implicitly captured the frequency and severity of events experienced by patients in the trial. We considered that using disutility for each event based on clinical trial data would introduce further assumptions regarding the analysis of multiple and simultaneous events. Lastly, consistent with other economic evaluations in oncology, only AEs of grade 3 or higher were included in the analysis. Grade 1 and 2 AEs were assumed to have a negligible impact on health and economic outcomes from the payer perspective.
This model used WAC prices for the acquisition costs of E/P/A, G-CSF, and trilaciclib, which may have limitations for Medicare beneficiaries. The Average Selling Price (ASP), which is used for reimbursement of Medicare beneficiaries, was not available for trilaciclib at the time of analysis. The WAC is the only available public cost for trilaciclib for estimating costs for the Medicare population. We intend to re-estimate our results once a stable ASP is available. At that time, we may consider reporting ASP-based results for the Medicare population, WAC-based results for the commercially insured population, and an age-proportionate blended ASP/WAC rate reflecting the age distribution of cancer in the United States. The cost of prophylactic use of G-CSF was a small proportion of the total cost saving ($2,541 saving from prophylactic use of G-CSF, out of the total cost saving of $18,840). Therapeutic use of G-CSF was assumed to be included in the AE management cost and was not modeled separately. OWSA of varying trilaciclib price (±10% WAC price) yielded consistent results (cost savings varied from $15,439 to $22,241).
As well as identifying areas for future research, our analysis has some limitations. First, the AE rates were based on the results observed in the pivotal Phase II clinical trial, and it was assumed that all patients would complete four treatment cycles. We acknowledge that data from real-world practice may differ, and we intend to re-estimate our results once real-world data with sufficient statistical power are available. Second, patients were recruited to the Phase II trilaciclib trials from the United States, as well as Western and Eastern Europe – each with disparate health care delivery and financing systems. Therefore, health care resource utilization was not sufficiently captured in these trials to derive economic inputs for AE management. The deterministic estimates for the cost of AE management were based on data from large United States claims-based studies that include multiple oncology indications, not specifically for SCLC, and were assumed to be the same for grade 3 and grade 4 eventsCitation10,Citation23. These cost estimates were assessed in sensitivity analyses and the findings were consistent with those in the deterministic analysis. Third, our model did not include a survival analysis component; therefore, the impact of mortality on QALYs was not assessed. Because the primary objective of Phase II trilaciclib trials was to demonstrate multilineage myeloprotective effects and associated outcomes rather than antitumor efficacy, the studies were not designed or powered to show an effect on survival. Additional clinical data from ongoing Phase III trials of trilaciclib in patients with triple-negative breast cancer (NCT04799249) and colorectal cancer (NCT04607668), which include antitumor efficacy and QoL measures as primary or secondary endpoints, will be therefore important to allow the potential survival benefits associated with trilaciclib to be modeled for other conditions in the future. Finally, given that this is the first economic analysis of a first-in-class myeloprotection agent, it was not possible to validate the results against real-world data or against other studies analyzing the same problem; however, establishing cross validity and external validity will be a priority for future evaluations once appropriate data sources become available.
Conclusions
This is the first economic analysis to assess the value of treating patients with ES-SCLC with trilaciclib prior to first-line chemotherapy. Trilaciclib is a first-in-class intervention that provides multilineage protection of the bone marrow among ES-SCLC patients. Results from this study suggest that administration of trilaciclib prior to standard first-line chemotherapy can be a cost-saving approach to reduce the incidence of chemotherapy-induced myelosuppression compared with chemotherapy alone. Therefore, trilaciclib could provide both clinical and economic benefits for the treatment of patients with ES-SCLC.
Transparency
Declaration of funding
This work was supported by G1 Therapeutics, Inc.
Declaration of financial or other interests
IA and KM are equity shareholders in Matrix45, which is under contract with G1 Therapeutics, Inc. for services unrelated to this study and manuscript.
UO is an employee of ZS Associates.
BD was an employee of ZS Associates at the time of the study.
MC was an employee of G1 Therapeutics, Inc. at the time of the study and manuscript preparation. DM and HH are employees of G1 Therapeutics, Inc.
The peer reviewers on this manuscript have received an honorarium from JME for their review work and one of the reviewers was an author on one of the studies cited in this article. The other reviewers have no other relevant financial relationships to disclose.
Authors contributions
All authors were involved in the design of the study, the analysis of data, the interpretation of results, the drafting of the manuscript, and the final review.
Previous presentations
Data included in this manuscript were presented, in part, at the International Society of Pharmacoeconomics and Outcomes Research 2021 Annual Meeting (PCN48): Deniz B, et al. Value in Health. 2021;24(Suppl_1):S27 (https://doi.org/10.1016/j.jval.2021.04.140)
Supplemental Material: Interview with Authors
Download MS Word (26.6 KB)Supplemental Material
Download MS Word (24.1 KB)Acknowledgements
The authors thank Avijeet Chopra (employee of ZS Associates at the time of manuscript preparation) for his editorial and programming assistance. The authors also thank Dinesh Mishra and Amit Goyal (employees of ZS Associates) for their assistance with model programming. Editorial assistance was provided by Alligent Europe (part of Envision Pharma Group), funded by G1 Therapeutics, Inc. The authors thank the anonymous reviewers for their valuable suggestions during the manuscript review process.
Data availability statement
The data that support the findings of this study are available from the co-author, H.H., upon reasonable request.
Notes
i G1 Therapeutics, Inc., Research Triangle Park, NC, USA
ii NCCN. Clinical Practice Guidelines in Oncology. Small Cell Lung Cancer, version 3.2021. NCCN. Clinical Practice Guidelines in Oncology. Hematopoietic Growth Factors, version 4.2021.
References
- Epstein RS, Aapro MS, Basu Roy UK, et al. Patient burden and real-world management of chemotherapy-induced myelosuppression: results from an online survey of patients with solid tumors. Adv Ther. 2020;37(8):3606–3618.
- Bagnyukova TV, Serebriiskii IG, Zhou Y, et al. Chemotherapy and signaling: how can targeted therapies supercharge cytotoxic agents? Cancer Biol Ther. 2010;10(9):839–853.
- Kuter DJ. Managing thrombocytopenia associated with cancer chemotherapy. Oncology. 2015;29(4):282–294.
- Horn L, Mansfield AS, Szczęsna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229.
- Liou SY, Stephens JM, Carpiuc KT, et al. Economic burden of haematological adverse effects in cancer patients: a systematic review. Clin Drug Investig. 2007;27(6):381–396.
- Bryer E, Henry D. Chemotherapy-induced anemia: etiology, pathophysiology, and implications for contemporary practice. Int J Clin Transf Med. 2018;6:21–31.
- Tai E, Guy GP, Dunbar A, et al. Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract. 2017;13(6):e552–e561.
- Baugh CW, Faridi MK, Mueller EL, et al. Near-universal hospitalization of US emergency department patients with cancer and febrile neutropenia. PLoS One. 2019;14(5):e0216835.
- U.S. Bureau of Labor Statistics [Internet]. Consumer price index. Washington (DC): U.S. Bureau of Labor Statistics; 2020 [cited 2020 April 21]. Available from: https://www.bls.gov/cpi/tables/
- Wong W, Yim YM, Kim A, et al. Assessment of costs associated with adverse events in patients with cancer. PLoS One. 2018;13(4):e0196007.
- Gilreath JA, Rodgers GM. How I treat cancer-associated anemia. Blood. 2020;136(7):801–813.
- NCCN [Internet]. Clinical Practice Guidelines in Oncology. Hematopoietic Growth Factors, version 4.2021 [cited 2021 November 1]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/growthfactors.pdf
- He S, Roberts PJ, Sorrentino JA, et al. Transient CDK4/6 inhibition protects hematopoietic stem cells from chemotherapy-induced exhaustion. Sci Transl Med. 2017;9(387):eaal3986.
- Weiss JM, Csoszi T, Maglakelidze M, et al. Myelopreservation with the CDK4/6 inhibitor trilaciclib in patients with small-cell lung cancer receiving first-line chemotherapy: a phase Ib/randomized phase II trial. Ann Oncol. 2019;30(10):1613–1621.
- Daniel D, Kuchava V, Bondarenko I, et al. Trilaciclib prior to chemotherapy and atezolizumab in patients with newly diagnosed extensive-stage small cell lung cancer: a multicentre, randomised, double-blind, placebo-controlled phase II trial. Int J Cancer. 2021;148(10):2557–2570.
- Hart LL, Ferrarotto R, Andric ZG, et al. Myelopreservation with trilaciclib in patients receiving topotecan for small cell lung cancer: results from a randomized, double-blind, placebo-controlled phase II study. Adv Ther. 2021;38(1):350–365.
- G1 Therapeutics [Internet]. COSELA™ (trilaciclib). Full prescribing information. Research Triangle Park (NC): G1 Therapeutics; 2021 [cited 2021 March 3]. Available from: https://www.g1therapeutics.com/cosela/pi/
- NCCN [Internet]. Clinical Practice Guidelines in Oncology. Small Cell Lung Cancer, version 3.2021 [cited 2021 November 1]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf
- Weiss J, Goldschmidt J, Andric Z, et al. Effects of trilaciclib on chemotherapy-induced myelosuppression and patient-reported outcomes in patients with extensive-stage small cell lung cancer: pooled results from three phase II randomized, double-blind, placebo-controlled studies. Clin Lung Cancer. 2021;22(5):449–460.
- Drugs.com [Internet]. Drug price information from Drugs.com; c2000-2021. 2019 [cited 2019 December 31]. Available from: https://www.drugs.com/price-guide/
- Eldar-Lissai A, Cosler LE, Culakova E, et al. Economic analysis of prophylactic pegfilgrastim in adult cancer patients receiving chemotherapy. Value Health. 2008;11(2):172–179.
- Naeim A, Henk HJ, Becker L, et al. Pegfilgrastim prophylaxis is associated with a lower risk of hospitalization of cancer patients than filgrastim prophylaxis: a retrospective United States claims analysis of granulocyte colony-stimulating factors (G-CSF). BMC Cancer. 2013;13:11.
- Weycker D, Li X, Edelsberg J, et al. Risk and consequences of chemotherapy-induced febrile neutropenia in patients with metastatic solid tumors. J Oncol Pract. 2015;11(1):47–54.
- International Business Machines [Internet]. IBM Watson Health Micromedex® Red Book; 2021 [cited 2019 December 31]. Available from: https://www.ibm.com/uk-en/products/micromedex-red-book
- Teckle P, McTaggart-Cowan H, Van der Hoek K, et al. Mapping the FACT-G cancer-specific quality of life instrument to the EQ-5D and SF-6D. Health Qual Life Outcomes. 2013;11:203.
- Duh MS, Hackshaw MD, Ivanova JI, et al. Costs associated with intravenous cancer therapy administration in patients with metastatic soft tissue sarcoma in a US population. Sarcoma. 2013;2013:947413.
- Cella D, Eton DT, Fairclough DL, et al. What is a clinically meaningful change on the functional assessment of cancer Therapy-Lung (FACT-L) questionnaire? Results from Eastern cooperative oncology group (ECOG) study 5592. J Clin Epidemiol. 2002;55(3):285–295.
- Cella D, Eton DT, Lai JS, et al. Combining anchor and distribution-based methods to derive minimal clinically important differences on the functional assessment of cancer therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24(6):547–561.
- Yost KJ, Eton DT. Combining distribution- and anchor-based approaches to determine minimally important differences: the FACIT experience. Eval Health Prof. 2005;28(2):172–191.
- Butt Z, Webster K, Eisenstein AR, et al. Quality of life in lung cancer: the validity and cross-cultural applicability of the functional assessment of cancer Therapy-Lung Scale. Hematol Oncol Clin North Am. 2005;19(2):389–420.
- Vreman RA, Geenen JW, Knies S, et al. The application and implications of novel deterministic sensitivity analysis methods. Pharmacoeconomics. 2021;39(1):1–17.
- Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. In: Handbooks in health economic evaluation. Vol. 1. Oxford (UK): Oxford University Press; 2006.
- Hinkle DE, Wiersma W, Jurs SG. Applied statistics for the behavioral sciences. 5th ed. Boston (MA): Houghton Mifflin; 2002.
- Institute for Clinical and Economic Review [Internet]. 2020-2023 value assessment framework. Boston (MA): ICER; 2020 [cited 2020 January 31]. Available from: https://34eyj51jerf417itp82ufdoe-wpengine.netdna-ssl.com/wp-content/uploads/2021/03/ICER_2020_2023_VAF_013120-4-2.pdf
- Bennett CL, Silver SM, Djulbegovic B, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA. 2008;299(8):914–924.
- Lambertini M, Del Mastro L, Bellodi A, et al. The five “Ws” for bone pain due to the administration of granulocyte-colony stimulating factors (G-CSFs). Crit Rev Oncol Hematol. 2014;89(1):112–128.
- Bennett CL, Evens AM, Andritsos LA, et al. Haematological malignancies developing in previously healthy individuals who received haematopoietic growth factors: report from the research on adverse drug events and reports (RADAR) project. Br J Haematol. 2006;135(5):642–650.
- Bennett CL, Calhoun EA. Evaluating the total costs of chemotherapy-induced febrile neutropenia: results from a pilot study with community oncology cancer patients. Oncologist. 2007;12(4):478–483.
- Calhoun EA, Schumock GT, McKoy JM, et al. Granulocyte colony–stimulating factor for chemotherapy-induced neutropenia in patients with small cell lung cancer: the 40% rule revisited. Pharmacoeconomics. 2005;23(8):767–775.
- Lyman GH, Lalla A, Barron RL, et al. Cost-effectiveness of pegfilgrastim versus filgrastim primary prophylaxis in women with early-stage breast cancer receiving chemotherapy in the United States. Clin Ther. 2009;31(5):1092–1104.
- Fust K, Li X, Maschio M, et al. Cost-Effectiveness analysis of prophylaxis treatment strategies to reduce the incidence of febrile neutropenia in patients with Early-Stage breast cancer or Non-Hodgkin lymphoma. Pharmacoeconomics. 2017;35(4):425–438.
- Liu Z, Doan QV, Malin J, et al. The economic value of primary prophylaxis using pegfilgrastim compared with filgrastim in patients with breast cancer in the UK. Appl Health Econ Health Policy. 2009;7(3):193–205.
- Hill G, Barron R, Fust K, et al. Primary vs secondary prophylaxis with pegfilgrastim for the reduction of febrile neutropenia risk in patients receiving chemotherapy for non-Hodgkin’s lymphoma: cost-effectiveness analyses. J Med Econ. 2014;17(1):32–42.
- Lee EK, Wong WW, Trudeau ME, et al. Cost-effectiveness of prophylactic granulocyte colony-stimulating factor for febrile neutropenia in breast cancer patients receiving FEC-D. Breast Cancer Res Treat. 2015;150(1):169–180.
- Ramsey SD, Liu Z, Boer R, et al. Cost-effectiveness of primary versus secondary prophylaxis with pegfilgrastim in women with early-stage breast cancer receiving chemotherapy. Value Health. 2009;12(2):217–225.
- Aarts MJ, Grutters JP, Peters FP, et al. Cost effectiveness of primary pegfilgrastim prophylaxis in patients with breast cancer at risk of febrile neutropenia. J Clin Oncol. 2013;31(34):4283–4289.
- Whyte S, Cooper KL, Stevenson MD, et al. Cost-effectiveness of granulocyte colony-stimulating factor prophylaxis for febrile neutropenia in breast cancer in the United Kingdom. Value Health. 2011;14(4):465–474.
- Bojke L, Sculpher M, Stephens R, et al. Cost effectiveness of increasing the dose intensity of chemotherapy with granulocyte colony-stimulating factor in small-cell lung cancer: based on data from the medical research council LU19 trial. Pharmacoeconomics. 2006;24(5):443–452.
- Li YYR, Mai H, Trudeau ME, et al. Reimbursement recommendations for cancer drugs supported by phase II evidence in Canada. Curr Oncol. 2020;27(5):e495–e500.
- Bennett CL, Lane D, Stinson T, et al. Economic analysis of amifostine as adjunctive support for patients with advanced head and neck cancer: preliminary results from a randomized phase II clinical trial from Germany. Cancer Invest. 2001;19(2):107–113.
- Briggs AH, Weinstein MC, Fenwick EA, et al. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM modeling good research practices task force working group-6. Med Decis Making. 2012;32(5):722–732.
- Hatswell AJ, Bullement A, Briggs A, et al. Probabilistic sensitivity analysis in Cost-Effectiveness models: determining model convergence in cohort models. Pharmacoeconomics. 2018;36(12):1421–1426.