Abstract
Aims
To assess the cost-effectiveness of carbetocin versus oxytocin for the prevention of postpartum hemorrhage (PPH) following vaginal birth from the perspective of the UK National Health Service (NHS).
Materials and methods
A decision tree model was designed to analyze the cost per PPH event avoided associated with utilizing carbetocin versus oxytocin for prophylactic treatment of PPH in women following vaginal birth from a UK perspective. It modelled the potential for women to require an additional uterotonic after prophylaxis, and to still experience a PPH event and receive associated treatment. Inpatient recovery and follow-up periods post-PPH were also included in the model. Costs associated with drug acquisition and administration, PPH management (i.e. additional staffing and possible operating theater and high dependency unit utilization), inpatient hospitalization, and follow-up visits were all considered. Adverse event management costs were not included. Resource utilization varied depending on the severity of the PPH event (as defined by the amount of blood lost). PPH events avoided were estimated. In an exploratory analysis, quality adjusted life years (QALYs) were estimated as well.
Results
In the deterministic base case, costs were £55 lower and PPH events were 0.0342 lower per woman with carbetocin use compared to oxytocin use. Across the cohort of 100 women the reduction in PPH events led to the largest cost savings (£4,233 saved) out of all cost categories, with total cost savings of £5,495. Carbetocin utilization amongst the entire cohort led to 3.42 avoided PPH events compared to oxytocin utilization, comprised of 3.03 fewer mild/moderate PPH events and 0.39 fewer severe PPH events. Carbetocin utilization led to 0.0001 additional QALYs per woman.
Conclusion
Carbetocin utilization leads to lower prophylactic treatment costs and less PPH events versus oxytocin when utilized for the prevention of PPH following vaginal birth in the UK.
Introduction
Postpartum hemorrhage (PPH), defined by the World Health Organization (WHO) as blood loss of more than 500 ml within 24 h of deliveryCitation1, is the most commonly occurring form of obstetric hemorrhageCitation2,Citation3. Further stratification exists, with a blood loss of ≥1,000 ml designated “severe PPH” by the WHOCitation1 and “major PPH” by the Royal College of Obstetricians and GynecologistsCitation2.
Reported incidence of PPH varies in the literature (for example from 3.2% in the USCitation4 to 8.3% in AustraliaCitation5) however, findings show that incidence is increasing year over year in many developed countriesCitation4,Citation6–9. Uterine atony, a condition where the uterus fails to contract after delivery, is the primary cause of 58–90% of PPH eventsCitation10–12 and increasing rates of uterine atony are the largest driver of increasing PPH incidenceCitation8.
Management of PPH is associated with long-term morbidity, mental health issues, and substantial costs. A study of French women that took place over a 13-year period found that 41.2% of women who had a severe PPH experienced long-term repercussionsCitation13. Of these women, 7.4% reported a persistent fear of death and 20.6% reported that they had decided against another pregnancy due to fear of PPH recurrenceCitation13. In addition, a separate study reported complications as severe as acute renal failure, acute respiratory failure, sepsis, and death for women experiencing PPHCitation10. Maternal death affects developing nations much more severely than developed regions; the WHO estimates that developing regions accounted for 99% of all maternal deaths in 2015Citation14. Furthermore, while 8.0% of maternal deaths (an estimated 1,200 women) were attributable to PPH in the developed world between 2003 and 2009, 19.7% (479,000 women) of maternal deaths in the developing world were found to be caused by PPHCitation3. Administration of uterotonic drugs, which stimulate uterine contractions, are considered the first line of treatment for PPHCitation2,Citation15. However, if this fails, invasive treatments, such as a hysterectomy, arterial embolization, arterial ligation, and uterine suture, may be necessary to control bleeding and avoid maternal deathCitation12. Invasive treatments are associated with further morbidity including increased risk of complications in subsequent pregnancies and infertilityCitation16,Citation17.
Due to all of these factors, the cost of managing PPH events can greatly outweigh the cost of preventative treatment. Severe PPH can increase the time spent in the hospital by over 2 days in a UK setting and requires additional specialized staff to manageCitation18. A UK cost-effectiveness model found that management of a woman who had lost 1,000–1,499 ml of blood was estimated to cost £1,782 per event, while managing a woman who had lost 1,500 ml or more cost £3,507Citation19. The main cost drivers were “other costs” (including additional inpatient stay in a high dependency unit, operating theater costs, case discussion, and consultant follow-up) and staff time, while prophylactic uterotonic use comprised only 0.5–1.3% of the total cost of PPH managementCitation19.
In addition to their use for treatment of PPH, uterotonics are the gold standard of care for the prevention of PPH events and are given as part of the Active Management of the Third Stage of Labor (AMTSL), which includes interventions given after delivery of the babyCitation1. A recent study that surveyed midwives across four European nations found that 93% use uterotonics during AMTSL, with 89% using oxytocinCitation20. In the UK, the National Institute for Health and Care Excellence (NICE) recommends 10 IU of oxytocin be given intramuscularly to women having a vaginal birth either with the birth of the anterior shoulder or after delivery but before the cord is clampedCitation21. However, a single administration is not always sufficient to prevent progression to PPH and 13.5% of all women require additional uterotonics following oxytocinCitation22.
Carbetocin (PABALFootnotei 100 micrograms/ml solution for injection, Ferring) is a synthetic oxytocin analogue that has been modified to increase both half-life, duration of action, and heat stabilityCitation23,Citation24. A Cochrane network meta-analysis (NMA) of 196 studies found that ergometrine plus oxytocin, misoprostol plus oxytocin, and carbetocin were the most effective uterotonics for the prevention of PPH (blood loss ≥500 ml)Citation22. Carbetocin has been found to be dominant versus oxytocin for the prevention of PPH at caesarean section in the base case of a UK cost-effectiveness modelCitation19.
The objective of this study is to assess the cost per PPH event avoided attributable to the utilization of carbetocin monotherapy versus oxytocin monotherapy for the prevention of PPH following vaginal birth from the perspective of the UK National Health Service (NHS). An exploratory analysis was also performed to ascertain incremental QALYS in the same setting.
Methods
Model design
A decision tree model () was designed to analyze the cost per PPH event avoided associated with utilizing carbetocin versus oxytocin for prophylactic treatment of PPH in women following vaginal birth. The perspective of interest was that of the UK NHS and Personal Social Services. A targeted literature review was conducted prior to model construction to inform the model structure and inform input values. Only direct costs (including drug acquisition and administration, staffing, medical equipment, and inpatient stay) were included. A Cochrane systematic review and network meta-analysis of uterotonics in the prevention of PPH was used to inform the inclusion of adverse events in the analysisCitation25. The primary adverse events analyzed were vomiting, hypertension and fever and it reported risk ratios for carbetocin versus oxytocin, based on the network meta-analysis, of 0.89 (95% confidence interval 0.55–1.42), 0.85 (95% confidence interval 0.15–4.77), and 0.86 (95% confidence interval 0.22–.35), respectivelyCitation25. In regard to these findings it concluded that “Carbetocin has similar risk for side-effects compared with oxytocin although the quality evidence was very low for vomiting and for fever, and was low for hypertension”Citation25. Based on this conclusion no adverse event management costs were included in the model. The analysis had a time horizon of 30 days in order to model the period starting from uterotonic prophylaxis and ending after a follow-up visit.
Figure 1. Model diagram. *Mild/moderate PPH: blood loss of ≥500 ml and <1,000 ml. **Severe PPH: blood loss ≥1,000 ml.
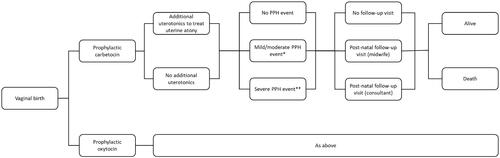
The model analyzed a hypothetical cohort of 100 women having a vaginal birth. The women’s ages were distributed according to data from the UK Office for National StatisticsCitation26. Maternal mortality due to PPH, which had been adjusted for age, was included in the model structureCitation27. Women may die at the end of the model structure, after their follow-up visit. Neither costs nor health outcomes have been discounted, due to the short model time horizon. Microsoft ExcelFootnoteii 2016 was used to develop the model.
Treatment pathway
A 100 µg prophylactic dose of carbetocin given intramuscularly was compared with a 10 IU bolus of oxytocin. Prophylactic treatment was administered during AMSTL in accordance with NICE guidelinesCitation21. Initial prophylactic treatment may not be enough to prevent a PPH event, so a percentage of women may require administration of additional uterotonic drugs that prevent PPH in some but not all cases. The percentage of women that required an additional uterotonic after their first dose varied according to their prophylactic treatment. Options included an oxytocin infusion (either 10 IU over 2 h or 10 IU or 20 IU or 30 IU or 40 IU all given over 4 h); misoprostol (600 µg), ergometrine (500 µg); or a combination of ergometrine and oxytocin 1 ml amp. The cost of a day ward nurse administering additional uterotonics was accounted for as well, with different treatments requiring different lengths of time.
After initial prophylaxis and possible additional treatment with uterotonics, a proportion of women, defined by their initial uterotonic, experienced a PPH. PPH events were classified as either mild/moderate (500–999 ml blood loss) or severe (≥1,000 ml blood loss)Citation1. Additional staff costs (corresponding to time spent by an anesthetist, obstetrician, midwife, and junior doctor) were accrued by women who experienced a PPH, with women who experienced a severe PPH event requiring both more staff and more time to be treated. Furthermore, women experiencing severe PPH events accrued costs associated with an operating theater and high dependency unit, which women who experienced a mild/moderate PPH event did not accrue. Women who experienced a PPH event of either severity were further treated with either ergometrine and oxytocin (1 ml amp), oxytocin (8 doses of 5 IU), carboprost (6 doses of 250 µg), ergometrine (7 doses of 500 ml), misoprostol (500 µg), or carbetocin (300 ml). Carbetocin is only licensed for prophylactic use, specifically for the prevention of postpartum hemorrhage due to uterine atony, in the UK; however, the source for this data, a survey of midwives, revealed that, in practice, it may be considered as an option for PPH treatment (though only one of 25 UK midwives surveyed reported this)Citation20. PPH treatment utilization rates varied based on whether the women experienced a mild/moderate or severe PPH event.
A transfusion was given to a proportion of women, again defined by prophylactic treatment, regardless of whether they had a PPH event. Women experiencing mild/moderate PPH required the same amount of blood as women who did not experience a PPH, while women experiencing severe PPH utilized a much greater amount. For women who did not experience PPH, the time spent in recovery post-delivery was dependent on whether they required a transfusion (4 h) or did not (30 min). Time spent in recovery following PPH was accounted for in the additional time and staffing requirements already discussed. All women were further hospitalized for a period determined by whether they experienced a PPH and how severe the event was.
A follow-up visit of 15 min, conducted by a midwife, was attended by all women. Those who had experienced a PPH event had the possibility of a second 15-min follow-up conducted by an obstetrician. Follow-up visits occurred within 30 days of delivery. A mortality rate due to PPH, adjusted to account for the age distribution of the model population, was includedCitation28.
Clinical effectiveness
Clinical data regarding the effectiveness of carbetocin and oxytocin was sourced from a Cochrane systematic review and NMA examining uterotonics for the prevention of PPHCitation22. Uterotonic effectiveness data from 196 trials across 53 countries, including 140 trials focused on vaginal birth, was collected and analyzed. Oxytocin was the most utilized treatment, being used in 137 trial arms as either an intervention or comparator, compared to carbetocin which was a treatment of interest in 33 trial arms across all included trials. High-, middle-, and low-income nations were all well represented, and 95.4% of trials were performed in a hospital setting. While model inputs were derived from the findings of the NMA as a whole, one included trial of particular interest and weight is the CHAMPION trial, which examined the effectiveness of carbetocin versus oxytocin in 135,559 women having a vaginal birth across 10 nations, including developing regions and the UKCitation22,Citation29. The primary outcomes of the review were the prevention of PPH ≥500 ml and prevention of PPH ≥1,000 mlCitation22. Secondary outcome data such as use of additional uterotonics and blood transfusion rates were also utilized in the model. All values taken from this review were specific to whether the woman utilized carbetocin or oxytocin as their initial prophylactic therapy (). Among women who utilized carbetocin, 8.78% experienced a PPH event (6.17% experiencing a mild/moderate event and 2.61% experiencing a severe event), compared with 12.20% of those who utilized oxytocin (9.20% mild/moderate and 3.00% severe)Citation22.
Table 1. Clinical inputs.
Resource use and unit cost estimates for year 2019
UK-specific uterotonic treatment utilization rates following a PPH event, dependent on the severity of the event, were sourced from a survey of midwives across France, Italy, the Netherlands, and the UK ()Citation18. Values regarding the staffing needs during a PPH event (e.g. the specialty of the staff and the length of time spent treating the woman) as well as the length of hospital stay following a PPH event were taken from the same survey () and are also UK-specific and stratified by PPH severityCitation18,Citation20. The utilization rates and specific dosing for the administration of additional uterotonics were sourced from a Cochrane systematic review focusing on carbetocin use in preventing PPH ()Citation30. Expert opinion, derived from an advisory board containing one UK doctor and one UK midwife, was the source for the length of time needed to administer additional uterotonics (10 min for oxytocin infusions and 5 min for all other treatments) and suggested that a day ward nurse would perform this. Blood transfusion utilization (0.03 units for women not experiencing PPH and women experiencing mild/moderate PPH and 0.36 for women experiencing severe PPH) was provided by the North Bristol NHS Trust Postpartum Haemorrhage Study and the cost per unit of blood was taken from NHS dataCitation31.
Table 2. Uterotonic treatment utilization inputs.
Table 3. Resource utilization inputs.
Drug acquisition costs for uterotonics () were derived from the Monthly Index of Medical Specialties (MIMS)Citation32 and administration costs (i.e. necessary saline and fluid administration apparatus) were provided by a Ferring-sponsored advisory boardCitation19. This advisory board was also the source for the additional cost of an operating theater and a 24-h high dependency unit stay for women experiencing severe PPHCitation19. NHS healthcare resource group (HRG) code NZ27Z (non-elective inpatient excess bed day) was used to acquire the cost accrued per day in the hospital (all costs were inflated to 2019 Great British Pounds [GBP] using the Personal Social Services Research Unit (PSSRU) Inflation Indices)Citation33,Citation34. Staffing costs per hour were provided by the PSSRUCitation34. The type of staff on hand for recovery (a nurse team manager), the staff to woman ratio (2 to 1), and recovery time for women who did not experience a PPH event (240 min for women who received an infusion and 30 min for those who did not) were based on assumptions utilized in a previous UK cost-effectiveness model examining prophylactic carbetocin use in women having a C-section birth, as were the proportion of women who accrue costs for a follow-up visit and the duration of the visitsCitation19.
Table 4. Cost inputs.
Utilities
Due to a lack of published literature, a study reporting the disutilities of gastrointestinal bleedings in patients with immune thrombocytopenia was used as a proxyCitation35. Patients with “platelets >50 × 109/L and no bleeding” were assumed to have similar utilities to women not experiencing PPH, while patients with “platelets >50 × 109/L and bleeding managed in outpatient care” and “platelets >50 × 109/L and gastrointestinal/other bleeding” were assumed to be similar to women experiencing a mild/moderate PPH event and a severe PPH event, respectively ()Citation35. PPH event-specific utilities assume a length of 7 days for a mild/moderate PPH event and 12 days for a severe PPH event based on a previously published PPH cost-effectiveness modelCitation19. The length assigned to severe PPH is an average of two values, 10 days for “major PPH (1,000–1,500 ml blood loss)” and 14 days for “massive PPH (>1,500 ml blood loss)”. All values represent EQ-5D-3L utilities.
Table 5. Utility inputs (LeeCitation35).
Sensitivity analysis
A one-way sensitivity analysis was performed in order to understand the variables which most impact the incremental cost. The results of this analysis were reported via a tornado diagram. A probabilistic sensitivity analysis was also conducted to explore uncertainty, and 2,000 iterations were simulated (). Results of each run were plotted according to incremental cost and incremental PPH events and the mean costs and PPH events across all runs were reported as well.
Table 6. Distribution used for variables within each parameter category in the PSA.
Results
Deterministic base case results
Carbetocin was found to be less costly and led to less PPH events versus oxytocin when used for the prevention of PPH following vaginal birth, leading to savings of £55 and 0.0342 less PPH events per woman (). Across the entire cohort, women utilizing carbetocin experienced cost savings of £5,495 compared with oxytocin, of which £4,233 was attributable to PPH event management. The largest cost savings were associated with the reduction of severe PPH events (savings of £2,938), followed by recovery (savings of £2,543). The costliest phase of treatment was recovery, largely driven by the cost of inpatient stay. Savings in the recovery phase of treatment can be attributed to the decrease in severe PPH events associated with the use of carbetocin for prevention of PPH; more specifically due to an associated reduction in hospital length of stay of 0.03 days.
Table 7. Base case cost, PPH events and QALY results following vaginal birth.
These cost savings are attributable to fewer PPH events resulting from the prophylactic use of carbetocin compared to oxytocin use. Compared to women receiving prophylactic oxytocin, women receiving carbetocin experienced a decrease of 3.03 mild/moderate PPH events and 0.39 severe PPH events among the cohort.
The exploratory analysis found that QALYs were similar between both groups, as the reduction of both mild/moderate and severe PPH events led to a marginal increase of 0.0001 QALYs per woman attributable to carbetocin utilization.
Sensitivity analysis
One-way sensitivity analysis
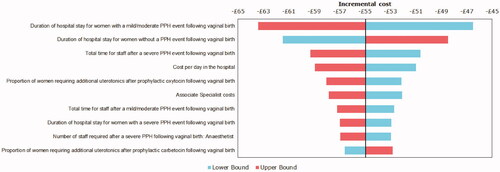
The impact of a one-way sensitivity analysis on incremental treatment costs are presented in a tornado diagram (). The two most sensitive variables were duration of hospital stay for women with a mild/moderate PPH event, followed by the duration of hospital stay for women who did not experience a PPH event. These were followed by the total staff time needed to manage a severe PPH event, the cost per day in the hospital, and the proportion of women on prophylactic oxytocin who required additional uterotonics.
Probabilistic sensitivity analysis
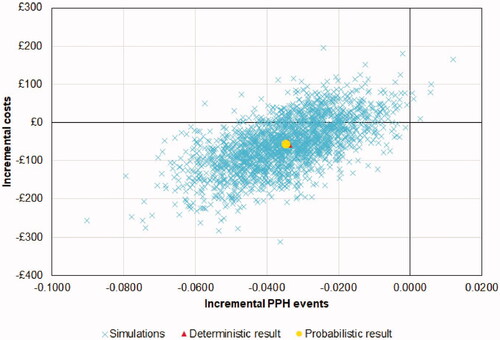
A probabilistic sensitivity analysis (PSA) was also developed and the results of 2,000 runs are plotted in . Carbetocin was found to provide mean cost savings of £57 and resulted in a mean reduction of 0.0348 PPH events per woman. The average probabilistic analysis results are similar to those of the deterministic analysis, demonstrating the robustness of the base case results. Carbetocin was found to be less costly and more effective in 79.5% of all runs.
Discussion
This decision tree model was developed to examine the cost per PPH event avoided of carbetocin versus oxytocin when used as a prophylactic treatment for PPH following vaginal birth from the perspective of the UK healthcare system.
In the deterministic base case analysis, carbetocin was found to provide a cost savings of £55 and led to 0.0342 less PPH events. Cost savings were driven by a reduction in costs attributable to severe (≥1,000 ml blood loss) PPH events, which were decreased by £2,938 across the cohort of 100 women due to carbetocin utilization. Prophylactic treatment costs were higher in the carbetocin population, due to carbetocin’s higher acquisition cost; however, this was offset by incremental savings in PPH events, additional uterotonic usage, recovery, and follow-up costs. Carbetocin utilization amongst the entire cohort led to 3.42 avoided PPH events compared to oxytocin utilization. A reduction of 3.03 mild/moderate PPH events and 0.39 severe PPH events specifically led to cost savings of £1,285 and £2,938, respectively. In an exploratory analysis carbetocin usage also led to an increase of 0.0001 QALYs per woman when compared to women using oxytocin. The low QALY gain is likely attributable to several factors. PPH events have a low mortality risk in the UK, especially compared to developing countries, meaning that very few deaths were preventedCitation3. Additionally. mild/moderate PPH events have a much lower disutility associated with them compared to severe PPH events, which also explains the low QALY gain due to carbetocin useCitation35. Finally, PPH events occur over a short time frame, and while PPH events are known to lead to long-term morbidity, there is no published data on the long-term disutilitiesCitation10,Citation13. A one-way sensitivity analysis found the key cost drivers to be the duration of hospital stay for women with mild/moderate PPH, followed by the duration of hospital stay for women who did not experience a PPH event. Hospitalization largely drove the cost of recovery, which was the costliest phase of treatment. A probabilistic sensitivity analysis found the mean cost savings due to carbetocin usage compared to oxytocin across all 2,000 runs to be £57 per woman and a mean 0.0348 PPH events were prevented per woman. The similarity between the deterministic base case and the probabilistic sensitivity analysis demonstrates the robustness of the base case results.
Our findings align with previous analyses about the cost-effectiveness of carbetocin as a prophylactic treatment for women having a caesarean section birth. A cost-effectiveness model from the perspective of the UK NHS reported that carbetocin was cost-saving versus oxytocin for the prevention of PPH following caesarean section and led to a reduction of PPH events; the analysis did not report the QALYs associated with the base case results but did report that carbetocin was cost-effective at a threshold of £20,000 per QALY in 70.5% of scenarios in their probabilistic sensitivity analysisCitation19. The authors also report that the cost savings are mostly driven by the reduction in PPH events, followed by reduction of time spent in recovery, which agrees with our findings. Another model was constructed by Gallos et al. as part of the systematic review and NMA that informed the clinical efficacy data used in our modelCitation36. When examining the scenario most comparable to that reported in our analysis, vaginal births without adverse events, carbetocin was found to be more costly (incremental costs of £6.41 per woman) but more effective (0.36 less mild/moderate PPH events and 0.000036 less severe PPH events per woman) than oxytocin alone. The differences between our findings may be driven by the inclusion of additional surgical procedures, including a balloon tamponade, in Gallos et al.Citation36, as well as the fact that our study included costs post-PPH event such as recovery and a follow-up visit. Furthermore, we used additional sources beyond the NMA to inform clinical inputs including a midwife survey and a carbetocin specific NMACitation18,Citation20,Citation30.
Though the current model focused on a UK perspective there is no reason to believe that this information is not generalizable to other countries. Developing nations in particular may find the results of particular interest given that these countries account for 99% of all maternal deathsCitation14. Of these maternal deaths, 19.7% can be attributed directly to PPH, which highlights the significance of this disease areaCitation3. While costs and mortality may vary greatly between the UK and developing nations, the source of efficacy data, a Cochrane systematic review, included reported results from developed and developing nations around the world and as such the reported PPH events avoided are likely of relevance to nations of any resource-levelCitation22. One included study, the CHAMPION trial, specifically examined women having a vaginal birth and included nations of varying resource levels (Argentina, Egypt, India, Kenya, Nigeria, Singapore, South Africa, Thailand, Uganda, and the UK)Citation22,Citation29.
Furthermore, published literature reporting the cost-effectiveness of carbetocin compared to oxytocin from the perspective of developing nations aligns with our findings as well. Studies focusing on an EcuadorianCitation37 and MalaysianCitation38 perspective found carbetocin was associated with cost savings compared with oxytocin for the prevention of PPH following caesarean section, with the latter specifically highlighting the reduction in use of additional uterotonics and reduced staffing utilization as key cost-saving elements. A Peruvian study also focused on women having a caesarean section birth, similarly reported that carbetocin use was cost effective and below the expected ICER threshold for PeruCitation39. Like the Malaysian study it highlighted that the reduction in the use of additional uterotonics, as well as the reduction of PPH events themselves, led to cost-savings associated with carbetocinCitation39.
Several key aspects which differentiate carbetocin from oxytocin were not captured in our model. The model assumed a single 10 IU bolus of oxytocin, according to NICE guidelinesCitation21; however, there is known to be variation in oxytocin dosing and administration methods, including multiple injections or an infusionCitation40. These dosing methods account for oxytocin’s duration of action, which is approximately 30 minCitation41. Carbetocin, however, has a duration of action of approximately 119 ± 69 min following intramuscular dosing, meaning that it may be administered in a single dose, which removes the need for extra monitoring or staff skilled in managing infusionsCitation42. Another uncaptured advantage of carbetocin is that vials are stable at 30 °C and 75% humidity for up to 3 yearsCitation23, which limits the need for access to a refrigerator. In contrast, oxytocin requires cold chain storage facilities, which may be unfeasible in developing countries or for mothers giving birth outside of a hospital setting.
Our study examined the cost per PPH event avoided of carbetocin use in women having a vaginal birth in the UK for the entire journey from prophylactic treatment until follow-up and combined robust efficacy data with clinician reported resource utilization information. Efficacy data was sourced from a robust source; a Cochrane NMA which examined 196 randomized clinical trials including 135,559 women across 53 countriesCitation22. A possible limitation is that studies included in the NMA had some methodological differences, including oxytocin dosing and administration. However, as noted earlier, oxytocin administration is variable in practice as well, and synthesizing this data provides a value that represents this varianceCitation40. This same review concluded that “Carbetocin has similar risk for side-effects compared with oxytocin although the quality evidence was very low for vomiting and for fever, and was low for hypertension”Citation25. Adverse events and their associated management costs were not included based on these findings, however future evidence may permit the inclusion of these events and costs. One limitation of the model is the use of expert opinion for some values which do not have published sources. This includes the type of staff necessary and the staff time required to administer additional uterotonics. Furthermore, there is no published data on the QALY decrement associated with PPH events, necessitating the use of a proxy (gastrointestinal bleeding in patients with immune thrombocytopenia), hence the exploratory nature of these analyses. Studies have previously reported that PPH events can lead to long term effects on physical and mental health, which are not captured given that the model utilized proxy utilities and only applied them to women for a short period post-birthCitation13. The volume of blood loss during PPH can also reach levels where treatment and management strays from the severe PPH pathway assigned in the model. At this stage, additional costs such as Factor VII utilization, intensive care unit admission, and possibly even litigation-related expenses may be accrued; however, no literature exists to inform modeling such events, and so they were not included. Several inputs were sourced from a survey of midwives, which provided healthcare resource utilization for PPH as reported by healthcare providers who work with it most directly in practice; however, as with any survey, these values may be subject to responder bias and may not be representative of all maternity settings within the UKCitation18,Citation20.
Conclusion
PPH is the most commonly occurring form of obstetric hemorrhage and has been increasing in prevalence over time in many developed and developing countries, driven by an increase in uterine atony, which causes almost 80% of PPH eventsCitation10. Uterotonics are the standard of care for the prevention of PPH events following vaginal birth and oxytocin is the most widely used among them. Carbetocin is a synthetic oxytocin analogue; when compared with oxytocin for PPH prophylaxis following vaginal birth, carbetocin was found to cost-effective from a UK NHS perspective, leading to savings of £55 and 0.0342 less PPH events per woman in the deterministic base case. In addition, per an exploratory analysis, it led to a marginal increase of 0.0001 QALYs per woman. Carbetocin may provide a less costly alternative while preventing more PPH events following vaginal birth.
Transparency
Declaration of funding
This study and manuscript were funded by Ferring Pharmaceuticals, Saint-Prex, Switzerland.
Declaration of financial/other interests
FLA and YLYS are employed by Ferring Pharmaceuticals.
SM is an employee of BresMed Netherlands, which received consultancy fees from Ferring Pharmaceuticals in connection with this study.
MG is an employee of Genesis Research, which received consultancy fees from Ferring Pharmaceuticals in connection with this study.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
MG performed the primary analysis and wrote the manuscript.
SM was involved in the development of the economic model and reviewed the manuscript.
FLA and YLYS were involved in the model development, data collection, interpretation of the analyses and reviewed the manuscript.
Acknowledgements
We acknowledge the contributions from Julie Perroud and Pierre-Emmanuel Puig, both formerly employees of Ferring Pharmaceuticals.
Notes
i PABAL is a registered trademark of Ferring Pharmaceuticals, Saint-Prex, Switzerland.
ii Microsoft Excel is a registered trademark of Microsoft Corporation, Redmond, WA, USA.
References
- WHO. WHO recommendations; uterotonics for the prevention of postpartum haemorrhage. Geneva: World Health Organisation; 2018.
- Mavrides E, Allard S, Chandraharan E, et al. Prevention and management of postpartum haemorrhage. BJOG. 2016;124:e106–e149.
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(6):e323-33–e333.
- Reale SC, Easter SR, Xu X, et al. Trends in postpartum hemorrhage in the United States from 2010 to 2014. Anesth Analg. 2020;130(5):e119–e122.
- Ford JB, Patterson JA, Seeho SK, et al. Trends and outcomes of postpartum haemorrhage, 2003-2011. BMC Pregnancy Childbirth. 2015;15:334.
- Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: United States, 1994-2006. Am J Obstet Gynecol. 2010;202(4):353.e1-6–353.e6.
- Knight M, Callaghan WM, Berg C, et al. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the international postpartum hemorrhage collaborative Group. BMC Pregnancy Childbirth. 2009;9:55.
- Lutomski JE, Byrne BM, Devane D, et al. Increasing trends in atonic postpartum haemorrhage in Ireland: an 11-year population-based cohort study. BJOG. 2012;119(3):306–314.
- Mehrabadi A, Hutcheon JA, Lee L, et al. Epidemiological investigation of a temporal increase in atonic postpartum haemorrhage: a population-based retrospective cohort study. BJOG. 2013;120(7):853–862.
- Bateman BT, Berman MF, Riley LE, et al. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Anal. 2010;110(5):1368–1373.
- Carroli G, Cuesta C, Abalos E, et al. Epidemiology of postpartum haemorrhage: a systematic review. Best Pract Res Clin Obstet Gynaecol. 2008;22(6):999–1012.
- Kayem G, Dupont C, Bouvier-Colle MH, et al. Invasive therapies for primary postpartum haemorrhage: a population-based study in France. BJOG. 2016;123(4):598–605.
- Sentilhes L, Gromez A, Clavier E, et al. Long-term psychological impact of severe postpartum hemorrhage. Acta Obstet Gynecol Scand. 2011;90(6):615–620.
- WHO, UNICEF, UNFPA, et al. Trends in maternal mortality: 1990 to 2015: estimates by WHO, UNICEF, UNFPA, world bank group and the united nationas population division. Geneva: World Health Organisation; 2015.
- WHO. WHO recommendations for the prevention and treatment of postpartum haemorrhage. Geneva: World Health Organisation; 2012.
- Rasheed SM, Amin MM, Abd Ellah AH, et al. Reproductive performance after conservative surgical treatment of postpartum hemorrhage. Int J Gynaecol Obstet. 2014;124(3):248–252.
- Sathe NA, Likis FE, Young JL, et al. Procedures and uterine-sparing surgeries for managing postpartum hemorrhage: a systematic review. Obstet Gynecol Surv. 2016;71(2):99–113.
- Hollier-Hann G, Richardson J, von Wilamowitz-Moellendorff CM, et al. Healthcare resource use for the management of postpartum haemorrhage in France, Italy, The Netherlands, and the UK. Value in Health. 2019;22(Supplement 3):S635.
- van der Nelson HA, Draycott T, Siassakos D, et al. Carbetocin versus oxytocin for prevention of post-partum haemorrhage at caesarean section in the United Kingdom: an economic impact analysis. Eur J Obstet Gynecol Reprod Biol. 2017;210:286–291.
- Richardson J, Hollier-Hann G, Kelly K, et al. A study of the healthcare resource use for the management of postpartum haemorrhage in France, Italy, The Netherlands, and the UK. Eur J Obstet Gynecol Reprod Biol. 2021;268:92–99.
- NICE. Care in third stage of labour: National Institute for Health and Care Excellence; 2021. [updated 2021 Apr 20; cited 2021 Oct 15]. Available from: https://pathways.nice.org.uk/pathways/intrapartum-care/care-in-third-stage-of-labour.
- Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database Syst Rev. 2018;2018(12):CD011689.
- Malm M, Madsen I, Kjellstrom J. Development and stability of a heat-stable formulation of carbetocin for the prevention of postpartum haemorrhage for use in low and middle-income countries. J Pept Sci. 2018;24(6):e3082.
- Rath W. Prevention of postpartum haemorrhage with the oxytocin analogue carbetocin. Eur J Obstet Gynecol Reprod Biol. 2009;147(1):15–20.
- Gallos ID, Williams HM, Price MJ, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database Syst Rev. 2018;4:CD011689.
- Office for National Statistics. Dataset: births by parents' characteristics. England and Wales: Office for National Statistics; 2017. [cited 2019 May 1]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/datasets/birthsbyparentscharacteristics.
- Knight M, Nair M, Tuffnell D. Saving lives, improving mothers’ care lessons learned to inform maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2013–15. Oxford: National Perinatal Epidemiology Unit; 2017.
- Knight M, Bunch K, Tuffnell D, et al. Saving lives, improving mother's care: lessons learned to inform maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2014–16. Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2018.
- Widmer M, Piaggio G, Nguyen TMH, et al. Heat-Stable carbetocin versus oxytocin to prevent hemorrhage after vaginal Birth. N Engl J Med. 2018;379(8):743–752.
- Su LL, Chong YS, Samuel M. Carbetocin for preventing postpartum haemorrhage. Cochrane Database Syst Rev. 2012;15(2):CD005457.
- National Health Service. Department of Health Reference Costs 2018–19. 2019. [cited 2019 Mar]. Available from: https://improvement.nhs.uk/resources/reference-costs/.
- Monthly Index of Medical Specialities. Drug costs. MIMS; 2020. [cited 2020 Jan]. Available from: http://www.mims.co.uk.
- National Health Service. Department of Health Reference Costs 2017–18. 2018. [cited 2019 Mar]. Available from: https://improvement.nhs.uk/resources/reference-costs/.
- Curtis L. Unit Costs of Health and Social Care 2019. 2019. [cited 2020 Jan]. Available from: https://www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2019/.
- Lee D, Thornton P, Hirst A, et al. Cost effectiveness of romiplostim for the treatment of chronic immune thrombocytopenia in Ireland. Appl Health Econ Health Policy. 2013;11(5):457–469.
- Gallos ID, Williams H, Price M, et al. Uterotonic drugs to prevent postpartum haemorrhage: a network meta-analysis. Health Technol Assess. 2019;23(9):1–356.
- Henríquez-Trujillo AR, Lucio-Romero RA, Bermúdez-Gallegos K. Analysis of the cost-effectiveness of carbetocin for the prevention of hemorrhage following cesarean delivery in Ecuador. J Comp Eff Res. 2017;6(6):529–536.
- Voon HY, Shafie AA, Bujang MA, et al. Cost effectiveness analysis of carbetocin during cesarean section in a high volume maternity unit. J Obstet Gynaecol Res. 2018;44(1):109–116.
- Caceda SI, Ramos RR, Saborido CM. Pharmacoeconomic study comparing carbetocin with oxytocin for the prevention of hemorrhage following cesarean delivery in Lima, Peru. J Comp Eff Res. 2018;7(1):49–55.
- Al Wattar BH, Tamblyn JA, Parry-Smith W, et al. Management of obstetric postpartum hemorrhage: a national service evaluation of current practice in the UK. RMHP. 2017;10:1–6. 01/17
- Embrey MP. Simultaneous intramuscular injection of oxytocin and ergometrine: a tocographic study. Br Med J. 1961;1(5241):1737–1738.
- Hunter DJ, Schulz P, Wassenaar W. Effect of carbetocin, a long-acting oxytocin analog on the postpartum uterus. Clin Pharmacol Ther. 1992;52(1):60–67.