Abstract
Aims
This study compared the aggregate duration of treatment administration of approved eculizumab and ravulizumab treatment regimens and resultant productivity implications for patients with atypical hemolytic uremic syndrome (aHUS) and their caregivers.
Methods
The aggregate duration of treatment administration (which includes waiting time for medication preparation and time for infusion, recovery, and travel to and from the clinic) was determined for a hypothetical population of patients with aHUS treated with eculizumab (10 mg/mL) or ravulizumab (10 or 100 mg/mL), in the clinic or at home, for 1 year, in Germany, Italy, the UK, and the US. The data for US patients treated in the clinic was used to extend a previously published cost-minimization model (CMM) to estimate the annual lost productivity associated with treatment administration and to compare the overall annual treatment costs for hypothetical adult and pediatric patients in the US.
Results
The aggregate duration of treatment administration associated with ravulizumab 10 mg/mL and 100 mg/mL was reduced by 44–52% and 69–74%, respectively, compared with eculizumab 10 mg/mL, across all four countries. Using the CMM, the adult and pediatric US patient lost productivity costs due to treatment were reduced by 56–60% and 73–76% with ravulizumab 10 mg/mL and 100 mg/mL, respectively, compared with eculizumab 10 mg/mL, and overall discounted annual treatment costs (direct and lost productivity costs owing to treatment) were reduced for ravulizumab (10 mg/mL and 100 mg/mL) vs eculizumab 10 mg/mL for adult and pediatric patients.
Limitations
This study was based on hypothetical patients, and assumptions were made regarding caregiver involvement, patient characteristics, and treatment patterns.
Conclusions
Compared with eculizumab, ravulizumab reduces the lost productivity costs associated with treatment. This reduction in costs is greater with the ravulizumab 100 mg/mL formulation, compared with ravulizumab 10 mg/mL, owing to shorter infusion times with this more concentrated formulation.
PLAIN LANGUAGE SUMMARY
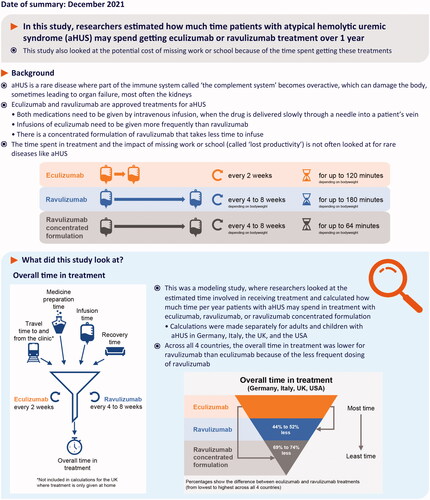

Introduction
Many diseases impose an extraordinary burden on patients and caregivers due to a decline in well-being and living standards. Patient and caregiver financial and societal burden has been measured for cancers and diseases of aging such as Alzheimer’s diseaseCitation1–3. However, it is infrequently measured and poorly understood in rare diseasesCitation4,Citation5.
Atypical hemolytic uremic syndrome (aHUS) is a rare disease in which uncontrolled complement activation leads to thrombotic microangiopathyCitation6–8. Without appropriate targeted treatment, aHUS can lead to accumulating morbidity, including loss of kidney function and mortalityCitation7,Citation9,Citation10. The terminal complement inhibitors eculizumab and ravulizumab are the only drug treatments for patients with aHUS approved by the US Food and Drug Administration and the European Medicines AgencyCitation11–14.
Eculizumab was approved in 2011 for the treatment of patients with aHUS and has since transformed the progression of this diseaseCitation15–20. Eculizumab is highly effective in treating aHUS and has a favorable safety profile, however, it requires intravenous infusions every 2 weeks in patients with a bodyweight of ≥10 kg, which can impose a treatment burden on patients and caregivers and is associated with high treatment costsCitation11,Citation21,Citation22. Ravulizumab was designed via targeted modification of eculizumab to increase the elimination half-life while retaining the safety and efficacy profile of eculizumab, thus enabling a reduced dosing frequency (every 4 weeks for patients with a bodyweight of <20 kg and every 8 weeks for patients with a bodyweight of ≥20 kg)Citation14,Citation23–26. The 100 mg/mL formulation of ravulizumab, approved by the US Food and Drug Administration and European Medicines Agency in 2020, can be delivered in a smaller volume and hence requires a shorter infusion time compared with the ravulizumab 10 mg/mL formulationCitation13,Citation14. This newer formulation of ravulizumab offers further potential to reduce the treatment burden for patients, caregivers, and healthcare providers.
Like many therapies, eculizumab and ravulizumab treatment can impose economic burdens on patients and society owing to both medical cost and lost work productivity resulting from time spent during treatment (including travel, preparation, infusion, and recovery time), and minimization of these burdens is important for patients undergoing such treatments. The aim of this study was to demonstrate the potential magnitude of savings in treatment time and associated lost productivity costs with ravulizumab compared with eculizumab treatment over 1 year, based on a hypothetical population of patients. The first part of this study compared the aggregate duration of treatment administration across four countries – Germany, Italy, the UK, and the US – over 1 year of treatment with approved eculizumab and ravulizumab dosing regimens for patients with aHUS. These countries represent a selection of large markets in which both eculizumab and ravulizumab are approved. Italy and the UK represent example countries wherein only in-clinic or only at-home infusion options are available, respectively, whereas Germany and the US represent countries where both of these options are available.
In the second part of this study, the per-patient annual cost of treatment and lost productivity associated with treatment for adult and pediatric patients with aHUS in the US was compared for the approved eculizumab and ravulizumab dosing regimens, using an extension of a cost-minimization model (CMM) previously published by Wang et alCitation27. The previous CMM was used to evaluate, from a US payer perspective, the lifetime economic consequences of eculizumab and ravulizumab treatment for patients with aHUS, and showed that ravulizumab 10 mg/mL provided reductions in direct medical costs of 32% and 35% for adults and children, respectively, compared with eculizumab 10 mg/mLCitation27. The model described here extends the previously described model, which was developed from a health payer perspective, to incorporate patient and caregiver lost productivity costs and the option of treatment with the concentrated formulation of ravulizumab (100 mg/mL), which requires a shorter infusion time compared with ravulizumab 10 mg/mL.
Methods
Aggregate duration of treatment administration in a hypothetical population
The aggregate duration of treatment administration was defined as the waiting time for medication preparation (taken from product monographs), infusion time, recovery time, and travel time to and from the clinic (for in-clinic infusions only), reflecting the time-related treatment burden. The aggregate duration of treatment administration was calculated for a hypothetical population of 100 patients with aHUS, treated with eculizumab (10 mg/mL) or ravulizumab (10 mg/mL or 100 mg/mL), at home or in the clinic, for 1 year, in four countries – Germany, Italy, the UK, and the US. The hypothetical patient group was evenly distributed across four weight categories: ≥10 to <20 kg, ≥20 to <40 kg, ≥40 to <60 kg, and ≥60 kg. Typical values for the calculation of the aggregate duration of treatment administration for each regimen studied were obtained from Alexion affiliates in each of the four countries (see Supplementary Table S1).
Table 1. Breakdown of costs for eculizumab 10 mg/mL, ravulizumab 10 mg/mL, and ravulizumab 100 mg/mL treatment in (a) adult and (b) pediatric patients with aHUS annualized over a lifetime, and (c) pediatric patients with aHUS annualized from ages 6 to 18 years; all results derived using a decision-tree framework for cost minimization.
It was assumed that all treatments were administered over 1 year according to prescribed product dosing schedules, without discontinuationsCitation11,Citation14. The infusion time was assumed to vary according to dose (dependent on patient weight as per prescribing information) and was assumed to be shorter for ravulizumab 100 mg/mL compared with ravulizumab 10 mg/mL (Supplementary Table S1). For in-clinic infusions, a travel time of 120 min was assumed for a round trip to the clinic and parking (held constant to allow fair comparison between different treatment regimens). It was assumed that caregivers travel with the patient to the clinic and remain for the entire duration of treatment. Using weight as a proxy for age, a percentage of patients were assumed to be accompanied by a caregiver in each of the four countries (Supplementary Table S2).
Table 2. Cost differences for total on-treatment costs (treatment plus lost productivity costs) for ravulizumab 10 mg/mL and ravulizumab 100 mg/mL vs eculizumab 10 mg/mL in (a) adult and (b) pediatric patients with aHUS annualized over a lifetime and (c) pediatric patients annualized from ages 6 to 18 years; all results derived using a decision-tree framework for cost minimization.
Cost-minimization model (CMM)
The average annual, per patient, lost productivity costs due to treatment, for adult and pediatric patients with aHUS receiving eculizumab (10 mg/mL) or ravulizumab (10 mg/mL or 100 mg/mL), were compared using a CMM based on the model previously described by Wang et alCitation27. The CMM described by Wang et al. used a decision-tree approach to simulate transitions between the following health states: on-treatment, discontinued treatment, and relapse (with subsequent re-initiation of treatment)Citation27. The characteristics of hypothetical adult and pediatric patients were based on clinical trials, and treatment patterns were derived from eculizumab studies with long-term follow-up. In this study, it was assumed that the patient populations comprised complement inhibitor treatment-naïve patients only, and the CMM was extended to include: (1) a treatment option of ravulizumab 100 mg/mL; and (2) the lost productivity costs for patients and caregivers, associated with eculizumab or ravulizumab treatment.
Taken from the four-country study described in the previous section, the aggregate duration of treatment administration in a hypothetical population of 100 US patients with aHUS treated in the clinic for 1 year with eculizumab (10 mg/mL) or ravulizumab (10 or 100 mg/mL) was transformed into hours per patient over the relevant treatment cycle (e.g. 2-week or 8-week cycle, as per US prescribing information). This data transformation allowed alignment with the time of the defined model cycle. Using a normalized baseline wage of $20/h, these hours per patient and caregiver were transformed into costs of lost productivity per treatment cycle. Lost productivity costs were applied equally to both pediatric and adult patients, as it was assumed that leisure and scholastic time hold the same value as earning wagesCitation28. A 3.0% discount rate was applied to the average annual costs.
Treatment costs associated with being on-treatment (drugs, administration, and meningococcal vaccination), discontinuation (monitoring), and relapses (acute hospital events and management) were included in the CMM (Supplementary Table S3), in addition to the lost productivity costs associated with eculizumab or ravulizumab treatment multiplied by the number of treatment administration cycles per calendar year. Post-relapse costs included the cost of restarting treatment with eculizumab or ravulizumab following relapse and the treatment costs from the point of this reinitiation. Based on eculizumab clinical trial outcomes with long-term follow-up, adult and pediatric patients were assumed to first discontinue treatment after 2.3 and 1.3 years, respectively. Patients who receive treatment with eculizumab or ravulizumab are required to be vaccinated against meningococcal infection (as per product prescribing information) and the rate of meningococcal infections in patients with aHUS treated with eculizumab or ravlizumab have been reported to be lowCitation23–26. It was therefore assumed that the risk of meningococcal infection would be low and hence the cost of complications of meningococcal infections were not included in the model.
The model was run separately for onset during adulthood (mean age 42 years) and during childhood (mean age 6 years). To determine average annual costs through a patient’s lifetime (age 42 through 100 years for adults and age 6 through 100 years for pediatric patients), total costs were divided by life-years after accounting for mortality. The average annual costs for pediatric patients were also determined based on ages 6 through 18 years only. The proportion of patients who were assumed to be accompanied by a caregiver during treatment is provided in Supplementary Table S2 for US patients.
Results
Duration of treatment administration in four countries
In all four countries, the aggregate duration of treatment administration, reflecting the time-related treatment administration burden, for 100 patients over 1 year was shortest for those treated with ravulizumab 100 mg/mL and longest for those treated with eculizumab 10 mg/mL: at home and in a clinic, range 3,558–6,664 h for ravulizumab 100 mg/mL; 7,291–10,895 h for ravulizumab 10 mg/mL; and 13,873–21,870 h for eculizumab 10 mg/mL ().
Figure 1. The aggregate duration of treatment administration per 100 patients with aHUS treated for 1 year with eculizumab 10 mg/mL, ravulizumab 10 mg/mL, or ravulizumab 100 mg/mL in (A) Germany, (B) Italy, (C) the UK, and (D) the USA.
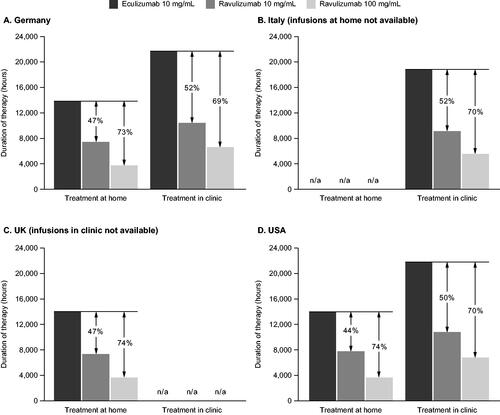
Compared with eculizumab 10 mg/mL, treatment with ravulizumab 10 mg/mL and 100 mg/mL reduced the aggregate duration of treatment administration by 44–52% and 69–74%, respectively, across all four countries, in a clinic or at home. For example, in the US, for patients treated in the clinic, there was an estimated 70% reduction in aggregate duration of treatment administration with ravulizumab 100 mg/mL compared with eculizumab 10 mg/mL (6,649 and 21,870 h, respectively, for 100 patients treated over 1 year, ).
Extended CMM (US patients)
Adult patients – annual per patient cost of lost productivity associated with treatment
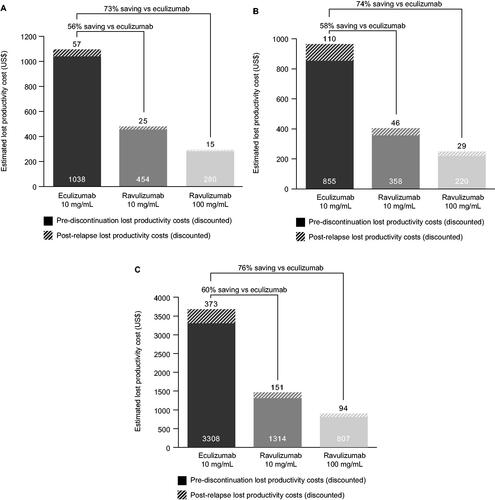
The average annual treatment-associated lost productivity cost (pre-discontinuation plus post-relapse) per adult patient with aHUS treated with eculizumab 10 mg/mL was estimated to be US$1,095 (discounted) and US$2,281 (undiscounted) (, ). A 56% saving in lost productivity costs (discounted and undiscounted) was estimated for those treated with ravulizumab 10 mg/mL and a 73% saving (discounted and undiscounted) for those treated with ravulizumab 100 mg/mL, compared with eculizumab 10 mg/mL-treated patients (, ).
Figure 2. Annual discounted lost productivity costs, derived from a cost minimization model, for patients with aHUS treated with eculizumab 10 mg/mL, ravulizumab 10 mg/mL, or ravulizumab 100 mg/mL for (A) adults and (B) pediatric patients annualized costs over a lifetime and (C) pediatric patients annualized from 6 to 18 years.
Adult patients – total annual per patient treatment costs (treatment plus lost productivity)
When costs were discounted, ravulizumab 10 mg/mL and 100 mg/mL were associated with annual total treatment cost savings of US$101,370 (32.5%) and US$101,735 (32.6%), compared with eculizumab 10 mg/mL (total costs: US$210,372, US$210,007, and US$311,741, respectively) (). The undiscounted total treatment costs of ravulizumab 10 mg/mL and 100 mg/mL were associated with cost savings of US$212,197 (32.7%) and US$212,958 (32.8%), respectively, compared with eculizumab 10 mg/mL (total costs: US$436,810, US$436,049, and US$649,007 respectively) (). The largest proportion of total costs for all therapies was the on-treatment costs (treatment plus lost productivity costs, ).
Pediatric patients – annual per patient cost of lost productivity associated with treatment
The average annual treatment-associated lost productivity cost over the lifetime of a pediatric patient with aHUS treated with eculizumab 10 mg/mL was estimated to be US$964 (discounted) and US$2,416 (undiscounted) (, ). Compared with eculizumab 10 mg/mL, a 58% (discounted) or 57% (undiscounted) saving in lost productivity costs was estimated for those treated with ravulizumab 10 mg/mL and a 74% (discounted) or 73% (undiscounted) saving for those treated with ravulizumab 100 mg/mL (, ).
From age 6 to 18 years, the average annual treatment-associated lost productivity cost per pediatric patient with aHUS treated with eculizumab 10 mg/mL was estimated to be US$3,681 (discounted) and US$4,293 (undiscounted) (, ), which is 236% and 88% higher (for discounted and undiscounted costs, respectively) than the estimated annual cost over a lifetime per adult patient. Compared with eculizumab 10 mg/mL, a 60% saving in lost productivity costs (both discounted and undiscounted) was estimated for those treated with ravulizumab 10 mg/mL, and a 76% and 75% saving (for discounted and undiscounted costs, respectively) was estimated for those treated with ravulizumab 100 mg/mL (, ).
Pediatric patients – total annual per patient treatment costs
When costs were discounted, ravulizumab 10 mg/mL and 100 mg/mL were associated with annual total treatment cost savings of US$68,318 (35.7%) and US$68,594 (35.8%), compared with eculizumab 10 mg/mL (total costs: US$123,057, US$122,781, and US$191,374, respectively) (). The undiscounted total treatment costs of ravulizumab 10 mg/mL and 100 mg/mL treatment were associated with annual cost savings of US$202,978 (34.2%) and US$203,732 (34.4%), respectively, compared with eculizumab 10 mg/mL (total costs: US$389,862, US$389,109, and US$592,840, respectively) (). As seen in adult patients, the largest proportion of total costs for all therapies was the on-treatment costs ().
From age 6 to 18 years, ravulizumab 10 mg/mL and 100 mg/mL were associated with annual total treatment discounted cost savings of US$156,230 (39.0%) and US$157,099 (39.2%), compared with eculizumab 10 mg/mL (total costs: US$244,824, US$243,955, and US$401,053, respectively), and the undiscounted total treatment costs of ravulizumab 10 mg/mL and 100 mg/mL treatment were associated with annual cost savings of US$195,484 (40.0%) and US$196,504 (40.3%), respectively, compared with eculizumab 10 mg/mL (total costs: US$292,628, US$291,608, and US$488,112, respectively) ().
Discussion
This study aimed to compare the aggregate duration of treatment administration and resultant lost productivity and treatment costs for adult and pediatric patients with aHUS (and their caregivers) treated with either eculizumab or one of two formulations of ravulizumab (10 mg/mL or 100 mg/mL). The choice of inputs for this analysis was guided by applying two principles: measuring all-important time expenditures and valuing time in a way that is consistent across therapies and regimensCitation29.
Treatment choice can have a substantial impact on socio-economic and financial consequences for both patients and caregivers. A published discrete choice experiment estimated utility values for key aHUS-related attributes in general population samples across five countries (Australia, Canada, Netherlands, Sweden, and the UK)Citation30. In this study, treatments administered every 8 weeks (the dosing schedule for ravulizumab) were associated with significant utility increases compared with treatments administered every 2 weeks (the dosing schedule for eculizumab).
In our study, treatment with ravulizumab (either 10 mg/mL or 100 mg/mL) was estimated to substantially reduce the aggregate duration of treatment administration and associated treatment administration burden over 1 year for patients with aHUS and their caregivers, across four countries, compared with eculizumab. The ravulizumab 100 mg/mL formulation requires less infusion time than the 10 mg/mL formulation (e.g. for US patients weighing ≥60 kg, infusion time for ravulizumab 100 mg/mL is reduced by 75% compared with ravulizumab 10 mg/mL). Ravulizumab 100 mg/mL, administered by intravenous infusion in a clinic or at home, was estimated to result in a lower aggregate duration of treatment administration over 1 year, compared with ravulizumab 10 mg/mL and eculizumab 10 mg/mL. While administration of eculizumab or ravulizumab at home was estimated to have a shorter aggregate duration of treatment compared with administration in a clinic (in Germany and the US), the percent savings in treatment time with ravulizumab versus eculizumab remained approximately the same for both locations. In clinics and hospitals, quicker infusions may reduce chair time and potentially relieve healthcare resources that are in high demand and provide economic benefits to the healthcare system.
In a CMM using indicated dosing schedules and several assumptions, the average annual costs of lost productivity owing to treatment were quantified for all currently available eculizumab and ravulizumab treatment regimens for adult and pediatric patients with aHUS in the US. Ravulizumab 100 mg/mL was estimated to achieve a 73–76% saving in the annual lost productivity costs associated with treatment compared with eculizumab 10 mg/mL. This cost-saving was more pronounced in pediatric patients compared with adult patients, as the total lifetime lost productivity costs were greater for pediatric vs adult patients.
For children, missing school due to illness can have lifelong consequences. The value of lost productivity was therefore applied equally regardless of weight/age category, and this aligns with the approach advocated by many researchersCitation28,Citation31. It is also expected that patients who start treatment as children will have more years on treatment than those who start treatment as adults, which will impact lifetime treatment costs. Lost productivity costs during treatment of patients with aHUS were projected to be greater for pediatric patients compared with adults, owing to greater caregiver involvement up to the age of 18 years. While the total lost productivity costs for pediatric patients over a lifetime were greater than for adult patients, across all treatments, the annual lost productivity costs for pediatric patients were actually lower over a lifetime, as the total lifetime costs were averaged over 94 years for pediatric patients and over 58 years for adults. Furthermore, pediatric patients were assumed to first discontinue treatment earlier than adults. In order to show the impact of greater caregiver involvement during the treatment of pediatric patients, the annualized lost productivity costs were calculated for pediatric patients from age 6 to 18 years and were shown to be more than double the adult annualized costs (discounted).
Overall treatment costs (direct costs and lost productivity costs) were reduced with ravulizumab treatment compared with eculizumab treatment in adult and pediatric patients with aHUS, and the greatest reduction was estimated for treatment with the 100 mg/mL formulation of ravulizumab.
This study had several limitations. As this study was based on a hypothetical population of patients with aHUS, assumptions were necessary regarding the level of caregiver support. For the extended CMM assumptions were also made with regard to patient characteristics and treatment patterns (which were based on data from published phase 3 trials, NCT02949129 and NCT03131219)Citation23,Citation25,Citation26. Also, the human–capital approach employed in this study, which counts any hour not worked during a working day as an hour lost, overestimates lost productivity costs relative to the friction–cost approach, which only counts hours lost as those hours not worked until another employee takes over the duties/tasks of the absent employeeCitation32. Finally, the assumed travel time for treatment was set to reflect a mean value and was applied universally; however, if more precise data on distributions of travel times were available and different assumptions were applied, this could change the absolute values of lost productivity. In some countries, patients can be treated with eculizumab or ravulizumab at home and, while not accounted for in this study, this could be applied to the model for country-specific modeling of treatment costs. Extensive studies with real-world patients are required to more fully understand the burden of an aggregate duration of treatment administration.
There is considerable evidence to show that lost productivity due to illness and time spent in treatment matters to patientsCitation33,Citation34, caregiversCitation33,Citation35,Citation36, employersCitation37, and societyCitation38. The Second US Panel on Cost-Effectiveness recommended that economic evaluations include a societal perspective that includes productivity costsCitation38. However, despite a recommendation to quantify costs of caregiver time in assessments of healthcare costs, relatively few studies have included informal care in the assessment of disease burdenCitation39. A complete description of the economic burden of illness should account for such “spillover effects” of diseases and treatments on the welfare of family members/caregiversCitation40. Cancers, and diseases of aging such as Alzheimer’s disease, have well documented spillover effects on families, both in terms of caregiver economic burden and declines in caregiver well-beingCitation1,Citation2; however, spillover effects are less well documented in rare diseases. When a child is afflicted with a rare disease, there are large spillover effects on parents/caregivers. For example, approximately 34% of parents caring for children with cystic fibrosis reported evidence of clinical depression, and a similar proportion reported vocational impactsCitation41. Assessing the magnitude of informal care is arguably of high relevance for rare diseases that often manifest in children who may require decades of such care.
Modern treatments for diseases such as aHUS have changed patient outcomes from low survival to chronic conditions with an expectation of much greater longevity. The evolution of complement inhibitor therapies has positively transformed the lives of patients with aHUS, translating to gains for patients, caregivers, employers, and society at largeCitation16,Citation42–44. In meta-analyses of clinical trials and real-life data studies, eculizumab and ravulizumab were found to be similar in terms of their protective effect in patients with aHUSCitation42,Citation45. Compared with eculizumab, the savings in treatment-associated lost work productivity offered by both ravulizumab formulations reported here are important factors to consider in the treatment of patients with aHUS.
Conclusion
In patients with aHUS, ravulizumab reduces the aggregate duration of treatment administration and associated lost productivity costs compared with eculizumab. This study showed that the recently approved ravulizumab 100 mg/mL formulation could provide further benefits in this regard.
Transparency
Declaration of funding
This study was supported by Alexion, AstraZeneca Rare Disease.
Declaration of financial/other relationships
ARL is a paid consultant for Alexion, AstraZeneca Rare Disease. PC is an employee of and owns stock/options in Alexion, AstraZeneca Rare Disease. KJ is an employee of Broadstreet HEOR and a paid consultant of Alexion, AstraZeneca Rare Disease. YW is an employee of and owns stock/options in Alexion, AstraZeneca Rare Disease. EP is an employee of Broadstreet HEOR and a paid consultant of Alexion, AstraZeneca Rare Disease. IT is an employee of and owns stock/options in Alexion, AstraZeneca Rare Disease. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (32 KB)Acknowledgements
The medical writing support was provided by Oxford PharmaGenesis and was funded by Alexion, AstraZeneca Rare Disease.
Data availability statement
Owing to the proprietary nature of this research, the models generated and supporting data are not publicly available.
References
- Junkins CC, Kent E, Litzelman K, et al. Cancer across the ages: a narrative review of caregiver burden for patients of all ages. J Psychosoc Oncol. 2020;38(6):782–798.
- Yu H, Wang X, He R, et al. Measuring the caregiver burden of caring for community-residing people with Alzheimer's disease. PLOS One. 2015;10(7):e0132168.
- Wimo A, Reed CC, Dodel R, et al. The GERAS study: a prospective observational study of costs and resource use in community dwellers with Alzheimer's disease in three European countries – study design and baseline findings. JAD. 2013;36(2):385–399.
- White W. A rare disease patient/caregiver perspective on fair pricing and access to gene-based therapies. Gene Ther. 2019;27(10–11):474–481.
- Linertová R, García-Pérez L, Gorostiza I. Cost-of-illness in rare diseases. Adv Exp Med Biol. 2017;1031:283–297.
- Campistol JM, Arias M, Ariceta G, et al. An update for atypical haemolytic uraemic syndrome: diagnosis and treatment. A consensus document. Nefrologia. 2015;35(5):421–447.
- Fakhouri F, Zuber J, Frémeaux-Bacchi V, et al. Haemolytic uraemic syndrome. Lancet. 2017;390(10095):681–696.
- George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371(7):654–666.
- Sellier-Leclerc AL, Fremeaux-Bacchi V, Dragon-Durey MA, et al. Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2007;18(8):2392–2400.
- Fremeaux-Bacchi V, Fakhouri F, Garnier A, et al. Genetics and outcome of atypical hemolytic uremic syndrome: a Nationwide French Series Comparing Children and Adults. CJASN. 2013;8(4):554–562.
- US Food and Drug Administration. Eculizumab (SOLIRIS) Prescribing Information, 2020. [cited June 2021]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125166s434lbl.pdf
- European Medicines Agency. Eculizumab (SOLIRIS). 2020. [cited June 2021]. https://www.ema.europa.eu/en/documents/product-information/soliris-epar-product-information_en.pdf
- European Medicines Agency. Ravulizumab (ULTOMIRIS). [cited June 2021]. 2021. https://www.ema.europa.eu/en/documents/product-information/ultomiris-epar-product-information_en.pdf
- US Food and Drug Administration. Ravulizumab (ULTOMIRIS) Prescribing Information, 2021. [cited June 2021]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761108s012lbl.pdf
- Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic–uremic syndrome. N Engl J Med. 2013;368(23):2169–2181.
- Zuber J, Frimat M, Caillard S, et al. Use of highly individualized complement blockade has revolutionized clinical outcomes after kidney transplantation and renal epidemiology of atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2019;30(12):2449–2463.
- Fakhouri F, Hourmant M, Campistol JM, et al. Terminal complement inhibitor eculizumab in adult patients with atypical hemolytic uremic syndrome: a single-arm, open-label trial. Am J Kidney Dis. 2016;68(1):84–93.
- Greenbaum LA, Fila M, Ardissino G, et al. Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int. 2016;89(3):701–711.
- Rondeau E, Cataland SR, Al-Dakkak I, et al. Eculizumab safety: five-year experience from the global atypical hemolytic uremic syndrome registry. Kidney Int Rep. 2019;4(11):1568–1576.
- Licht C, Greenbaum LA, Muus P, et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int. 2015;87(5):1061–1073.
- Ariceta G. Optimal duration of treatment with eculizumab in atypical hemolytic uremic syndrome (aHUS)-a question to be addressed in a scientific way. Pediatr Nephrol. 2019;34(5):943–949.
- Yüksel S, Evrengül H, Özçakar ZB, et al. First-line, early and long-term eculizumab therapy in atypical hemolytic uremic syndrome: a case series in pediatric patients. Paediatr Drugs. 2016;18(6):413–420.
- Rondeau E, Scully M, Ariceta G, et al. The long-acting C5 inhibitor, ravulizumab, is effective and safe in adult patients with atypical hemolytic uremic syndrome naive to complement inhibitor treatment. Kidney Int. 2020;97(6):1287–1296.
- Barbour T, Scully M, Ariceta G, et al. Long-term efficacy and safety of the long-acting complement C5 inhibitor ravulizumab for the treatment of atypical hemolytic uremic syndrome in adults. Kidney Int Rep. 2021;6(6):1603–1613.
- Tanaka K, Adams B, Aris AM, et al. The long-acting C5 inhibitor, ravulizumab, is efficacious and safe in pediatric patients with atypical hemolytic uremic syndrome previously treated with eculizumab. Pediatr Nephrol. 2021;36(4):889–898.
- Ariceta G, Dixon BP, Kim SH, et al. The long-acting C5 inhibitor, ravulizumab, is effective and safe in pediatric patients with atypical hemolytic uremic syndrome naïve to complement inhibitor treatment. Kidney Int. 2021;100(1):225–237.
- Wang Y, Johnston K, Popoff E, et al. A US cost-minimization model comparing ravulizumab versus eculizumab for the treatment of atypical hemolytic uremic syndrome. J Med Econ. 2020;23(12):1503–1515.
- Verbooy K, Hoefman R, van Exel J, et al. Time is money: investigating the value of leisure time and unpaid work. Value Health. 2018;21(12):1428–1436.
- Russell LB. Completing costs: patients' time. Med Care. 2009;47(7 Suppl 1):S89–S93.
- Williams K, Aggio D, Chen P, et al. Utility values associated with atypical hemolytic uremic syndrome-related attributes: a discrete choice experiment in five countries. Pharmacoeconomics. 2021;39(8):901–912.
- Brouwer WB, Koopmanschap MA, Rutten FF. Productivity costs in cost-effectiveness analysis: numerator or denominator: a further discussion. Health Econ. 1997;6(5):511–514.
- van den Hout WB. The value of productivity: human-capital versus friction-cost method. Ann Rheum Dis. 2010;69(Suppl 1):i89–91.
- Abrams HR, Leeds HS, Russell HV, et al. Factors influencing family burden in pediatric hematology/oncology encounters. J Patient Cent Res Rev. 2019;6(4):243–251.
- Tai B-WB, Bae YH, Le QA. A systematic review of health economic evaluation studies using the patient's perspective. Value Health. 2016;19(6):903–908.
- de Meijer C, Brouwer W, Koopmanschap M, et al. The value of informal care-a further investigation of the feasibility of contingent valuation in informal caregivers. Health Econ. 2010;19(7):755–771.
- van Exel NJ, Brouwer WB, van den Berg B, et al. What really matters: an inquiry into the relative importance of dimensions of informal caregiver burden. Clin Rehabil. 2004;18(6):683–693.
- Heffernan M, Meit M, Powers M, et al. Business leaders' attitudes about value of employee and community health. J Public Health Manag Pract. 2020;26(5):493–496.
- Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103.
- Grosse SD, Pike J, Soelaeman R, et al. Quantifying family spillover effects in economic evaluations: measurement and valuation of informal care time. Pharmacoeconomics. 2019;37(4):461–473.
- Basu A, Meltzer D. Implications of spillover effects within the family for medical cost-effectiveness analysis. J Health Econ. 2005;24(4):751–773.
- Neri L, Lucidi V, Catastini P, et al. Caregiver burden and vocational participation among parents of adolescents with CF. Pediatr Pulmonol. 2016;51(3):243–252.
- Bernuy-Guevara C, Chehade H, Muller YD, et al. The inhibition of complement system in formal and emerging indications: results from parallel one-stage pairwise and network meta-analyses of clinical trials and real-life data studies. Biomedicines. 2020;8(9):355.
- Raina R, Grewal MK, Radhakrishnan Y, et al. Optimal management of atypical hemolytic uremic disease: challenges and solutions. Int J Nephrol Renovasc Dis. 2019;12:183–204.
- Menne J, Delmas Y, Fakhouri F, et al. Outcomes in patients with atypical hemolytic uremic syndrome treated with eculizumab in a long-term observational study. BMC Nephrol. 2019;20(1):125.
- Tomazos IH, Cataland S, Chen P, et al. Comparative efficacy of ravulizumab and eculizumab in the treatment of atypical hemolytic uremic syndrome: an indirect comparison using clinical trial data. Clin Nephrol. 2021. DOI:10.5414/CN110516.
