Abstract
Aims
Ocular toxicities are common adverse events (AEs) associated with anticancer agents. There is a paucity of data documenting their impact on patient care. This study assessed the clinical and economic burden of corneal AEs and related symptoms (collectively termed corneal AEs) in patients receiving multiple myeloma (MM) treatment.
Materials and Methods
Adults with a newly diagnosed MM (MM cohort) were identified from PharMetrics Plus, a US insurance claims database. Incidence, outpatient (OP) care, emergency department (ED) visits, hospitalizations, and costs were assessed for corneal AEs of interest: keratopathy/keratitis, blurred vision/decreased acuity, dry eye, eye pain, and photophobia. Incidence of new corneal AEs, healthcare resource utilization (HCRU), corneal AE-related HCRU, and costs were assessed and benchmarked against a hematology cohort of patients.
Results
The MM cohort included 2,120 patients with a median follow-up of 734.5 days. Overall, 11.7% of patients in the MM cohort and 7.4% in the hematology cohort had ≥1 corneal AE of interest. In the MM cohort, dry eye (6.1%), blurred vision/decreased acuity (3.4%), and keratopathy/keratitis (2.5%) were the most frequent. The overall median corneal AE-related per-patient-per-month (PPPM) cost was $27, predominantly contributed by OP care (median $19 PPPM). During follow-up, 4.8% of patients visited the ED, 3.6% were hospitalized, and 42.5% of patients visited an ophthalmologist/optometrist (∼1.69 visits/year). Costs of these visits were negligible (median PPPM $19) compared to total all-cause costs (median PPPM $17,286).
Limitations
The results can only be generalized to commercially insured and Medicare Advantage patients. Claims-based diagnosis of corneal AEs may underestimate true incidences.
Conclusions
Corneal AEs were observed in ∼12% of patients in the MM cohort, the most common were keratopathy/keratitis, dry eye, and blurred vision. Most of them required only OP care. The clinical and economic burden for treating corneal AEs was low when compared with total all-cause or MM-related PPPM costs.
Introduction
Traditional cytotoxic chemotherapy, steroids, and immunotherapies used to treat cancers may cause a variety of eye-related toxicities, such as dryness, keratopathy/keratitis, and blurred visionCitation1–3. More recently, molecularly targeted therapies and immunotherapies that inhibit multiple pathways responsible for growth and survival of cancer cells emerged as a new cancer treatment strategy. However, despite their more specific anticancer nature, these therapies can lead to both on-target and off-target effects. The eye and its adnexa can be susceptible to some novel anticancer therapies, as many of the critical signaling molecules that drive cancer growth are also expressed in ocular tissuesCitation1. With increased use of novel anticancer therapies, ophthalmologic side-effects become increasingly commonCitation2. Yet, given the novelty of many of these agents and the complexity of ocular pathology, oncologists may lack familiarity with the potential ophthalmologic adverse effects of targeted anticancer therapy.
Multiple myeloma (MM) is a type of hematologic cancer predominantly affecting older populations (median age at first MM diagnosis in the United States is 69 years)Citation4. Almost all patients with MM experience relapse and require multiple lines of treatment (LOT)Citation5. Adverse events (AEs), commonly reported by patients receiving combination therapy for MM, can particularly impact quality-of-life and increase burden on the healthcare systemCitation6–8. Ophthalmologic side-effects have been reported with some approved treatments for MMCitation9–12. While the cost burden of MM has been studied previouslyCitation13, the cost of specifically managing treatment-related front-of-the-eye conditions is unknown in hematologic cancers, including MM. Therefore, the aim of this retrospective cohort study was to assess the incidence and clinical and economic burden of managing corneal AEs and related symptoms in clinical practice among patients receiving treatment for MM and among patients with a diagnosed hematologic malignancy.
Methods
Study overview
This was a retrospective database study designed to assess costs, healthcare resource utilization (HCRU), and clinical burden of patients, with MM experiencing corneal AEs and related symptoms of interest. A cohort of adults with MM was identified from a large US insurance claims database, and all-cause as well as MM-related healthcare costs were assessed. Incidence, outpatient care, emergency department visits, hospitalizations, and costs were also assessed for five specific corneal AEs of interest (keratopathy/keratitis, blurred vision/decreased acuity, dry eye, eye pain, and photophobia), collectively termed corneal AEs hereafter. These corneal AEs were identified prior to any database analyses through discussions with regulatory authorities and eye care professionals and by cross-walking from Medical Dictionary for Regulatory Activities (MedDRA) “corneal disorders” codes to International Classification of Diseases (ICD) codes confirmed by clinical expertsCitation14. A larger, separate cohort of hematology patients was identified from the same database to enable comparison of the incidence and costs of corneal AEs with those obtained for the MM sample.
Data sources
Data were extracted from the IQVIA PharMetrics Plus database, which contains adjudicated health insurance claims data from commercially insured plans for >100 million patients in the United States. Data from 2006 onward are available for 90% of US hospitals and 80% of US doctors. Patients enrolled into the database are representative of the US national, commercially insured population in age and gender.
Participants
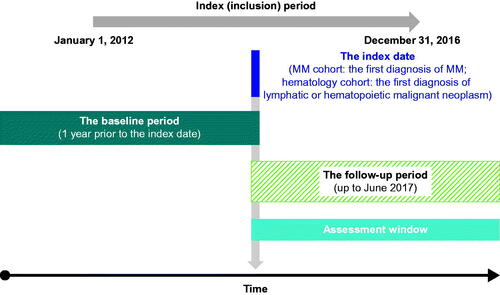
The overall evaluation period was from 1 January 2011 to 31 December 2017. The index period, defined as the first date the patient received a novel MM therapy, was from 1 January 2012 to 31 December 2016 (). Two samples were included to address the study objectives: adults with newly diagnosed MM (the “MM cohort”) and adults with a lymphatic or hematopoietic malignant neoplasm (the “hematology cohort”).
The MM cohort
Patients were included in the incident MM cohort if they had at least two MM diagnoses (ICD-9 203.0x and ICD-10 C90.0x) ≥30 days apart during the index period, were aged ≥18 years at the time of initial diagnosis (index date), received at least one MM treatment of interest (see Appendix 1 for details) after the incident MM diagnosis, and were continuously enrolled for pharmacy and medical benefits for ≥12 months prior to and after the index date.
Patients who had an MM diagnosis or received a treatment of interest within 12 months prior to the index date had invalid dates for their birth year, gender, or health plan enrollment, or had ≥2 diagnoses for other lymphomas and/or leukemias ≥30 days apart during the study period were excluded.
The hematology cohort
Patients were included in the hematology cohort if they had ≥2 diagnoses of a lymphatic or hematopoietic malignant neoplasm (codes ICD-9 200–209 and ICD-10 C81–C96) from any settings (outpatient and/or inpatient) ≥30 days apart during the index period, if they were aged ≥18 years at index date, if they had ≥2 claims of chemotherapy or targeted therapy within 1 year of index date, and if they were continuously enrolled for pharmacy and medical benefits for ≥12 months after initial diagnosis. Patients with invalid dates for their birth year, gender, or health plan enrollment were excluded.
Outcomes
Incidence of corneal AEs
Patients without any ICD-9/10 diagnosis codes or treatments indicative of diagnosis of corneal AEs in the baseline period (defined as up to 12 months prior to the index period for both cohorts) but who had incidence of corneal AEs during any line of therapy were assessed. Corneal AEs were identified by cross-walking from MedDRA “corneal disorders” codes to ICD codes (ICD-9 or ICD-10). The incidence of any corneal AE as well as individual corneal AEs are reported as a percentage of patients with incident corneal AEs (patients without corneal AE in the baseline period but who had an incidence in the follow-up period) among all patients, irrespective of the baseline presence of corneal AEs. This study specifically investigated the incidence of keratopathy/keratitis, blurred vison/decreased acuity, and dry eye, as well as eye pain and photophobia. The incidence of corneal AE was assessed by LOT (1, 2, 3, and 4+).
Corneal AE-related HCRU and costs
Corneal AE-related HCRU and costs were assessed by identifying medical claims related to corneal AEs and cross-walking from MedDRA “corneal disorders” codes to ICD codes (ICD-9 or ICD-10) specifically for outpatient visits, emergency department visits, inpatient admissions (primary admission or discharge diagnosis), and ophthalmic prescriptions filled in at a pharmacy. Corneal AE-related HCRU were assessed for the MM cohort and within 12 months after the index period for the hematology cohort.
HCRU and costs related to ophthalmological/optometrist visits
Prescriber specialties were identified from medical claims. The HCRU and costs related to ophthalmologist or optometrist visits were assessed, including regimens based on novel MM therapies (approved after 2015 and through 2017, the end of the evaluation period of this study), for the MM cohort only. Novel therapies covered in the analyses included carfilzomib, pomalidomide, panobinostat, daratumumab, ixazomib, and elotuzumab.
Data analyses
All analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC). Descriptive analyses were performed, and HCRU and costs (in US dollars) are reported on a per-patient-per-month (PPPM) basis. For any annualized data, events may or may not have lasted the entire year. Mean, median, standard deviation (SD), and interquartile range (IQR) were generated as measures of central tendency and variance for continuous variables. For categorical variables, frequencies and percentages are presented. Unadjusted resource utilization costs were reported as descriptive statistics (mean, median, and SD).
Results
Study participants
Within the index period, 20,698 and 194,403 patients were identified for potential inclusion into the MM and hematology cohorts, respectively. Following eligibility screening, 2,120 (10.2%) and 26,923 (13.8%) patients were included in the final MM and hematology cohorts, respectively (see Appendix 1 for participant attrition).
Of the 2,120 patients included in the MM cohort, 248 (11.7%) had ≥1 incident of corneal AEs after initial MM treatment. Of the 26,923 patients included in the hematology cohort (12 months post-index period), 1989 (7.4%) had ≥1 incident of corneal AEs of interest. Baseline characteristics of patients with ≥1 incident of corneal AEs in the MM cohort (n = 248) and the hematology cohort 12-months post-index period (n = 1989) are summarized in and Appendix 2, respectively. In the MM cohort, mean (SD) age was 60.9 (9.93) years and exactly half were female (). In the hematology cohort (12-months post-index period), patients with ≥1 incident corneal AE had a mean (SD) age of 57.3 (13.87) years and 52.1% were female (Appendix 2). In the MM cohort with ≥1 incident of corneal AE, keratopathy/keratitis, blurred vision/decreased acuity, or photophobia occurred more frequently in individuals with a higher overall comorbidity burden (Charlson Comorbidity Index 2.23–2.54; data not shown) than dry eye or eye pain (Charlson Comorbidity Index 1.91–1.93; data not shown). Other demographic characteristics were similar among patients in the two cohorts (; Appendix 2).
Table 1. Baseline demographics and characteristics of the MM cohort by corneal AEs of interest (n = 2,120).
Incidence of corneal AEs
While not common, dry eye (n = 129, 6.1%), blurred vision/decreased acuity (n = 73, 3.4%), and keratopathy/keratitis (n = 52, 2.5%) were the most frequently observed corneal AEs in the MM cohort across all LOTs (). Most incidents of corneal AEs were diagnosed in the outpatient setting by an ophthalmologist or optometrist (69.5% for all five corneal AEs combined). Only five patients (0.2%) had photophobia, and none were diagnosed by an ophthalmologist or optometrists (data not shown). The overall incident rates of corneal AE were similar across LOTs, ranging from 4.7% in LOT2 to 6.4% in LOT4+.
Table 2. Overall rate of incident corneal AEs of interest in the MM cohort by LOT.
As in the MM cohort, although not common, dry eye (n = 1,022, 3.8%), blurred vision/decreased acuity (n = 599, 2.2%), and keratopathy/keratitis (n = 343, 1.3%) were the most frequently observed corneal events in the hematology cohort during the 12-month post-index period (Appendix 3). Also, similarly to the MM cohort, most corneal events in the hematology cohort were diagnosed in the outpatient setting by an ophthalmologist or optometrist (63.25%; data not shown).
Corneal AE-related HCRU
Of the 248 patients with a corneal AE in the MM cohort, 97 (39.1%) had at least one ophthalmic prescription filled in a pharmacy (). Only nine (3.6%) and 12 (4.8%) patients were ever hospitalized or visited an emergency department, respectively, due to a corneal AE. In contrast, nearly all patients with an event (n = 238, 96.0%) visited a physician office or had an outpatient visit, and 171 (69.0%) visited an ophthalmologist or optometrist. The remaining 4% of patients with an event were either hospitalized or visited an emergency department. The median number of PPPM visits to an ophthalmologist or optometrist was 0.1. Most patients with eye pain (n = 18/23, 78.3%) and a relatively high proportion of patients with keratopathy/keratitis (n = 21/52; 40.4%) or dry eye (n = 50/129; 38.8%) had an ophthalmic prescription. However, there were no apparent differences in the average number of monthly outpatient visits, including ophthalmologist or optometrist visits, among patients with various corneal AEs.
Table 3. Healthcare resource utilization* (per-patient-per-month†) for corneal AEs of interest in the MM cohort (n = 2,120).
Of the 1989 patients with a corneal AE in the hematology cohort, 871 (43.8%) patients had at least one ophthalmic prescription filled in a pharmacy (Appendix 4). Only 128 (6.4%) and 138 (6.9%) patients were ever hospitalized or visited an emergency department, respectively, due to a corneal AE. In contrast, nearly all patients (n = 1,868, 93.9%) visited a physician office or had an outpatient visit, and 1,255 (63.1%) patients visited an ophthalmologist or optometrist. The median number of PPPM visits to an ophthalmologist or optometrist was 0.1, the same as for the MM cohort. The remaining 6.1% of patients with an event were either hospitalized or visited an emergency department.
When comparing the five corneal events in the hematology cohort (Appendix 4), more than half (n = 207, 60.3%) of the patients with keratopathy/keratitis or with eye pain (n = 104, 59.1%) had an ophthalmic prescription filled in a pharmacy. Similarly, a large proportion of patients with keratopathy/keratitis (n = 265, 77.3%) or dry eye (n = 793, 77.6%) visited an ophthalmologist or optometrist.
Corneal AE-related healthcare costs
For the 248 patients with a corneal AE in the MM cohort, the overall median corneal AE-related PPPM cost was $27 (). This cost represented <1% of median all-cause costs during the follow-up period (median total all-cause PPPM cost $17,286, median MM-related PPPM cost $13,851; ).
Table 4. Healthcare costs* (median per-patient-per-month) for each corneal AEs of interest in the MM cohort (n = 2,120).
Table 5. All-cause, MM-related, and corneal AEs-related healthcare costs* (median per-patient-per-month) in the MM cohort.
These corneal AE-related costs were predominantly driven by outpatient care (median PPPM cost $19) and prescriptions (median PPPM cost $16) (). Emergency department visits (median PPPM cost $66) and hospitalizations (median PPM cost $16) were rare. Blurred vision/decreased acuity was associated with the highest PPPM cost (median PPPM $41) compared with the other corneal AEs. However, it should be noted that data were from relatively small numbers of incident corneal AE cases (23–129 patients, depending on the type of corneal AE), so outliers may have impacted these data.
Overall costs for managing corneal AEs were lower in the hematology cohort (median PPPM cost $20) compared with the MM cohort described above, but costs across both cohorts were dominated by outpatient care (median PPPM cost $13; Appendix 5). As in the MM cohort, emergency department visits (median PPPM $17) and hospitalizations (median PPM cost $10) were uncommon, and blurred vision/decreased acuity was associated with the highest PPPM cost (median $30) compared with the other four corneal AEs of interest.
HCRU and costs related to ophthalmological visits
Of the total 2,120 patients in the MM cohort, 901 (42.5%) had at least one visit to an ophthalmologist or optometrist during the follow-up period. For patients who ever visited an ophthalmologist or optometrist, visit frequency was low (0.14 PPPM, or approximately 1.69 visits/year). Patients who received novel MM treatments were numerically more likely to visit an ophthalmologist or optometrist compared with patients not receiving the newer treatments (45.9% vs 41.4%, respectively). However, patients receiving newer treatments had numerically lower costs for their visits to an ophthalmologist or optometrist compared with patients not receiving newer treatments (median PPPM $12 vs $14, respectively). Moreover, ophthalmologist or optometrist visit costs (median PPPM cost $14) accounted for only a small portion of the all-cause costs (median PPPM cost $17,286).
Discussion
In this retrospective cohort study examining the specific burden of corneal AEs of interest for patients with MM, the cost burden of managing corneal AEs in these individuals was found to be less than 1% of total all-cause or MM-related costs. Inpatient and emergency department visits for corneal AEs were infrequent; most patients were managed in outpatient settings only.
In patients with MM who received active treatment, corneal AEs were not uncommon, and their collective incidence was similar across LOTs. This was similar for patients with various types of hematologic tumors. Typically, for both patient populations, corneal AEs were diagnosed and managed in an outpatient setting. The most frequent corneal AEs observed in both populations were dry eye and blurred vision/decreased acuity. The incidence remained largely constant across baseline demographic characteristics, comorbid conditions, and LOTs. These findings are consistent with previous reports of manageable corneal events with approved targeted therapies, such as lenalidomide, pomalidomide, ixazomib, and belantamab mafodotinCitation1,Citation2,Citation9–12,Citation15.
Previous studies have shown the cost burden of newly diagnosed MM patients is high, and primarily driven by costs of outpatient servicesCitation13. However, the specific costs of AEs in these patients have not been well studied. In the present study, median all-cause PPPM cost was $17,286 and median MM-related PPPM cost was $13,851 for the MM cohort, which are comparable to previous studiesCitation13. Comparatively, median PPPM cost for corneal AEs was $27, predominantly diagnosed in outpatient care. This suggests that the cost of corneal AEs is substantially lower than total all-cause or MM-related costs. However, collaboration between hematologist/oncologists and eye care professionals managing these corneal AEs will inform treatment decisions, which could impact cost.
This study utilizing healthcare insurance claims data provides insights not reported in the existing literature on the real-world clinical and economic burden of managing corneal AEs in MM patients in the US, particularly in patients receiving novel therapies emerging between 2015 and 2017. Specifically, this study assessed the incidence and quantified the cost burden associated with the corneal AEs of interest. However, some limitations inherent to administrative claims data exist. Firstly, the use of a 12-month washout period to identify newly treated patients (a proxy to identify newly diagnosed patients) may have resulted in diagnostic misclassification as some of these patients may not have been newly diagnosed and/or newly treated due to the lack of complete patient histories. Additionally, the PharMetrics Plus database does not provide information on systemic factors that could affect care, including benefit and formulary design.
Secondly, although the PharMetrics Plus database contains a representative sample of commercially insured and Medicare Advantage patients, it does not include information from patients who do not participate in commercial plans (e.g. uninsured patients, Medicaid, and those covered by non-commercial Medicare). Therefore, although the study results may be generalizable to the <65 years age group, they may not be to the general population with MM or to the ≥65 years age group of Medicare patients, since those populations may be under-represented in the database. Additionally, because the prevalence of MM is higher in patients ≥65 years of age, patients with MM in our study were younger than a general MM sample. Furthermore, since older patients are at higher risk of dry eye syndrome, excluding these older patients from our analysis may have led to an under-reporting of corneal AEs.
Finally, other relational information regarding comorbidities is not available in retrospective claims databases. Causality cannot be established since the ocular conditions of interest observed and reported in this study may be MM disease-related or therapy-related. Mild or moderate symptoms associated with these ophthalmic conditions may not be billed or coded in claims, and thus the actual incidences of these corneal AEs may have been underestimated in the study. Development of a validated algorithm to guide patient selection and identification of corneal AEs of interest from a claims database could help to confirm these findings in future studies and allow for cross-validation with medical chart reviews to capture more detailed patient histories.
Conclusions
This real-world data analysis demonstrated that there was a small proportion of incident corneal AEs in patients receiving treatment for MM, with the most common being keratopathy/keratitis, dry eye, and blurred vision. Most of these corneal AEs required only outpatient care, while emergency department visits or hospitalizations were rare. The clinical and economic burden for treating these events was very low when compared with total all-cause PPPM costs or MM-related PPPM costs. As the current treatment landscape includes newer treatments, better understanding of these targeted treatments and their level of resource utilization is important. Therefore, further research and real-world evidence are warranted to assess the relationship between corneal AEs and MM treatment and to explore costs associated with specific treatments.
Transparency
Declaration of Funding
This study was funded by GlaxoSmithKline [208295].
Declaration of financial/other relationships
FW, SF, EMM, JW, and LS are all GSK employees and have stocks and/or shares in GSK. C-CC and KS are employees of IQVIA, which receives consulting fees/research funding from GSK. SN was an employee of IQVIA at the time of study design and initiation. DMK has received consulting fees from GSK and Triphase Accelerator US Corporation, has a management role in Calm Water Therapeutics LLC, and has stock and/or shares in Eyeon Therapeutics, Inc. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Ethics approval and consent to participate
This study complied with all applicable laws regarding participant privacy. During this study, there was no direct contact with any of the study participants or primary collection of individual human data. Study results were presented in a tabular form and aggregate analyses did not include subject identifiers. Therefore, informed consent, ethics committee approval, or IRB approval were not required for this study.
Acknowledgements
GSK contributed to the study design, implementation, data collection, interpretation, and analysis. Medical writing support was provided by Crystal Kraft, PhD, of Fishawack Indicia Ltd, UK, funded by GSK.
Data availability statement
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to www.clinicalstudydatarequest.com. To access data for other types of GSK sponsored research, for study documents without patient-level data, and for clinical studies not listed, please submit an enquiry via the website.
References
- Renouf DJ, Velazquez-Martin JP, Simpson R, et al. Ocular toxicity of targeted therapies. J Clin Oncol. 2012;30(26):3277–3286.
- Fu C, Gombos DS, Lee J, et al. Ocular toxicities associated with targeted anticancer agents: an analysis of clinical data with management suggestions. Oncotarget. 2017;8(35):58709–58727.
- Schmid KE, Kornek GV, Scheithauer W, et al. Update on ocular complications of systemic cancer chemotherapy. Surv Ophthalmol. 2006;51(1):19–40.
- Gandhi UH, Cornell RF, Lakshman A, et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia. 2019;33(9):2266–2275.
- Robak P, Drozdz I, Szemraj J, et al. Drug resistance in multiple myeloma. Cancer Treat Rev. 2018;70:199–208.
- Palumbo A, Mateos MV, Bringhen S, et al. Practical management of adverse events in multiple myeloma: can therapy be attenuated in older patients? Blood Rev. 2011;25(4):181–191.
- SEER Cancer Statistics. Cancer Stat Facts: Myeloma. 2020; [cited 2020 July 23]. Available from: https://seer.cancer.gov/statfacts/html/mulmy.html.
- Delforge M, Dhawan R, Robinson D, Jr, et al. Health-related quality of life in elderly, newly diagnosed multiple myeloma patients treated with VMP vs. MP: results from the VISTA trial. Eur J Haematol. 2012;89(1):16–27.
- Celgene Europe BV. Imnovid 1 mg hard capsules [package insert]. 2013; [cited 2021 April 8]. Available from: https://www.ema.europa.eu/en/documents/product-information/imnovid-epar-product-information_en.pdf.
- Celgene Corporation. REVLIMID [package insert]. 2019; [cited 2021 April 8]. Available from: http://www.celgene.com/content/uploads/revlimid-pi.pdf.
- GlaxoSmithKline. BLENREP [package insert]. 2020; [cited 2021 April 8]. Available from: https://gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Blenrep/pdf/BLENREP-PI-MG.PDF.
- Takeda Pharmaceutical Company Ltd. NINLARO capsules [package insert] 2015; [cited 2021 April 8]. Available from: https://www.ninlaro.com/prescribing-information.pdf.
- Fonseca R, Abouzaid S, Bonafede M, et al. Trends in overall survival and costs of multiple myeloma, 2000–2014. Leukemia. 2017;31(9):1915–1921.
- WHO. Classification of Diseases (ICD). 2020; [cited 2021 April 8]. Available from: https://www.who.int/classifications/icd/en/.
- Lonial S, Lee HC, Badros A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020;21(2):207–221.
Appendix 1.
Selection of study participants (attrition) for the MM cohort (A) and the hematology cohort (B)
Appendix 2.
Baseline demographics and characteristics of the hematology cohort (12 months post-index period; n = 26,293)
Appendix 3.
Rate of incident corneal AEs of interest in the hematology cohort (during the 12-month post-index period)
Appendix 4.
Healthcare resource utilization (per-patient-per-month) for corneal AEs of interest in the hematology cohort (n = 26,293)
Appendix 5.
Healthcare costs (per-patient-per-month) for corneal AEs of interest in the hematology cohort (n = 26,293)