Abstract
Aims
Estimate the clinical and economic benefits of lenzilumab plus standard of care (SOC) compared with SOC alone in the treatment of patients hospitalized with COVID-19 pneumonia from the United States (US) hospital perspective.
Materials and methods
A per-patient cost calculator was developed to report the clinical and economic benefits associated with adding lenzilumab to SOC in newly hospitalized COVID-19 patients over 28 days. Clinical inputs were based on the LIVE-AIR trial, including failure to achieve survival without ventilation (SWOV), mortality, time to recovery, intensive care unit (ICU) admission, and invasive mechanical ventilation (IMV) use. Base case costs included the anticipated list price of lenzilumab, drug administration, and hospital resource costs based on the level of care required. A scenario analysis examined projected one-year rehospitalization costs.
Results
In the base case and all scenarios, lenzilumab plus SOC improved all specified clinical outcomes relative to SOC alone. Lenzilumab plus SOC resulted in estimated cost savings of $3,190 per patient in a population aged <85 years with C-reactive protein (CRP) levels <150 mg/L and receiving remdesivir (base case). Per-patient cost savings were observed in the following scenarios: (1) aged <85 years with CRP <150 mg/L, with or without remdesivir ($1,858); (2) Black and African American patients with CRP <150 mg/L ($13,154); and (3) Black and African American patients from the full population, regardless of CRP level ($2,763). In the full modified intent-to-treat population, an additional cost of $4,952 per patient was estimated. When adding rehospitalization costs to the index hospitalization, a total per-patient cost savings of $5,154 was estimated.
Conclusions
The results highlight the clinical benefits for SWOV, ventilator use, time to recovery, mortality, time in ICU, and time on IMV, in addition to an economic benefit from the US hospital perspective associated with adding lenzilumab to SOC for COVID-19 patients.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has had a substantial cumulative societal and economic impact in the United States (US) and worldwideCitation1–3. As of July 5, 2021, over 33 million cases and 603,000 deaths were reported in the USCitation4. Data from the US Centers for Disease Control and Prevention (CDC) indicate that 8% of all individuals who developed COVID-19 between August 2020 and March 2021 required hospitalizationCitation5. Unfortunately, the need for hospitalizations remains despite approximately 68% of the ≥12-year-old US population having been fully vaccinatedCitation6, with forecasts for November 2021 estimating that 4,700–5,000 new confirmed COVID-19 hospital admissions will continue to occur each day in the USCitation7. Furthermore, based on data from a large subset of US hospitals, for those patients hospitalized with COVID-19 between August 2020 and March 2021, 44.6% of patients required intensive care unit (ICU) admission and 12.5% required invasive mechanical ventilation (IMV)Citation8. Thus, there continues to be an urgent unmet need for effective therapies to prevent IMV and/or death for patients who are hospitalized with COVID-19.
The overall healthcare cost burden associated with the treatment of COVID-19 is substantialCitation9–11. From the hospital perspective, providing care to patients with COVID-19 is associated with average per-patient costs ranging from a low of $14,325 USD for patients who do not require ICU admission to a high of $78,245 USD for patients who require both ICU admission and IMVCitation10. Given the detrimental health outcomes and high economic burden among hospitalized patients with COVID-19, particularly those who require IMV, there remains a significant unmet need for cost-effective treatment optionsCitation12–14.
Many adverse clinical outcomes among patients with COVID-19 are the result of an immunopathological process called cytokine stormCitation15,Citation16. This process is, in a significant part, mediated by a granulocyte-macrophage colony-stimulating factor (GM-CSF), which activates and mobilizes myeloid cells, leading to dysregulated production of inflammatory cytokines and elevation of inflammatory markers (including C-reactive protein [CRP])Citation15,Citation17. In patients with COVID-19, levels of GM-CSF–secreting T-cells and the extent of inflammatory cytokine production are directly correlated with lung tissue injury, disease severity, and ICU admissionCitation16,Citation18–20. Therefore, GM-CSF has been identified as an important therapeutic target in COVID-19Citation20.
Lenzilumab is a novel HumaneeredFootnotei anti-human GM-CSF monoclonal antibody that directly binds GM-CSF, thereby preventing its downstream signalingCitation15. The Phase 3 LIVE-AIR trial (NCT04351152) is a randomized, double-blind, placebo-controlled study designed to evaluate early intervention with lenzilumab compared with placebo, both in combination with standard of care (SOC), in newly hospitalized patients with COVID-19 pneumonia who have an oxygen saturation (SpO2) ≤94% on room air or require supplemental oxygen but have not progressed to IMVCitation15. Preliminary results of the LIVE-AIR trial showed that lenzilumab significantly improved the likelihood of achieving survival without ventilation (SWOV) (sometimes referred to as ventilator-free survival) by day 28 compared with placeboCitation15. SWOV is a robust composite endpoint used in many of the recent COVID-19 studies that are less prone to favor treatments with discordant effects on survival and days free of ventilation while avoiding the need for sample sizes approaching those of mortality trials to enable timely availability of study resultsCitation21. Lenzilumab plus SOC was most efficacious as measured by SWOV in the groups of patients aged <85 years with CRP levels <150 mg/L, and patients aged <85 years with CRP levels <150 mg/L receiving remdesivir, compared with SOC aloneCitation22. C-reactive protein is an important marker of systemic inflammation and elevated levels of CRP are associated with poor clinical outcomes among patients with severe COVID-19Citation15,Citation22–24; therefore, preliminary findings from the LIVE-AIR trial suggest that lenzilumab may be particularly effective when used as an early intervention in hospitalized patients.
Results from a post hoc analysis of the LIVE-AIR trial also suggest that Black and African American patients may exhibit the greatest response to lenzilumab, with a nearly 9-fold increase in SWOV among patients with CRP levels <150 mg/LCitation25. This is notable as Black and African American persons have a 3-fold greater risk of hospitalization and 2-fold greater risk of death from COVID-19Citation26. The hyper-vulnerability in this population may be attributed to the low vaccination rates, the high prevalence of chronic illness (e.g. diabetes, hypertension, obesity), and the social determinants of health (e.g. socioeconomic status, healthcare access), all of which result in a higher risk of infection, hospitalization, and deathCitation26–29. Despite the disproportional incidence of COVID-19 in Black and African American persons, racial minority groups are typically underrepresented in COVID-19 clinical trialsCitation30. However, Black and African Americans were well represented in LIVE-AIR, representing 14.8% of the trial population, which closely aligns with real-world demographics from the latest US Census (13.4%)Citation25,Citation31.
Although clinical evidence indicates that lenzilumab is effective at improving SWOV in hospitalized patients with COVID-19Citation15, its overall clinical and economic value from the hospital decision-maker perspective has not been previously characterized. The purpose of this study was to report the clinical benefits from the LIVE-AIR trial in a way that is relevant to hospital decision-makers and to use the clinical trial data to estimate the per-patient costs to highlight the economic benefit of lenzilumab plus SOC compared with SOC alone in the treatment of hospitalized COVID-19 patients from the US hospital perspective. This analysis was conducted ex-ante (i.e. before regulatory approval of lenzilumab and before an established US list price was set).
Methods
Calculator structure
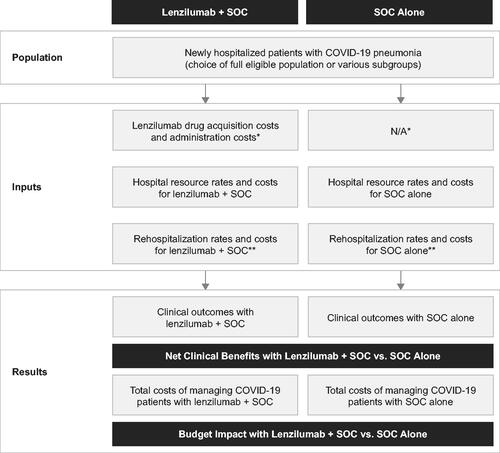
A per-patient cost calculator was developed in MicrosoftFootnoteii Excel to report the clinical benefits and estimated per-patient costs of adding lenzilumab to SOC versus SOC alone for newly hospitalized patients with COVID-19 pneumonia from a US hospital payer perspective. The calculator was developed with a 28-day time horizon for the index COVID-19 hospitalization (base case) and a 1-year time horizon for long-term costs (scenario). A 28-day time horizon for the index hospitalization was selected to align with data censoring in the LIVE-AIR trial (28 days following enrollment)Citation15. The calculator structure is presented in .
Figure 1. Calculator structure. Abbreviations. N/A, not applicable; SOC, standard of care. *It was assumed that the use of SOC drugs would not be affected by the concomitant use of lenzilumab, resulting in no cost differences for SOC drugs between both groups. This assumption was supported by the balanced use of remdesivir and corticosteroids in both treatment arms of the LIVE-AIR trialCitation15. **Rehospitalization costs are optional in the calculator. They were not included in the base case analysis but were included for scenario analysis #5.
Target population
Patient eligibility for the calculator was based on inclusion criteria for the LIVE-AIR trial. In brief, eligible patients were newly hospitalized with COVID-19 pneumonia, with SpO2 ≤94% on room air and/or requiring supplemental oxygen, but not on IMVCitation15. As described previously, data from the LIVE-AIR trial indicated that patients aged <85 years with CRP <150 mg/L particularly benefited from lenzilumab, and patients who received concomitant remdesivir also experienced a greater benefit than patients who did notCitation22. Furthermore, US clinical practice guidelines recommend remdesivir for the treatment of hospitalized patients with COVID-19 who require supplemental oxygen but not IMVCitation32. Therefore, a population that only included patients aged <85 years with CRP <150 mg/L and who were receiving remdesivir was deemed the most informative for hospital decision-makers and was selected for the base case. This base case population is also in line with the population being evaluated in the ongoing ACTIV-5/BET-B trial that aims to further elucidate the efficacy of lenzilumab in this patient populationCitation33. Scenario analyses were also conducted in patients aged <85 years with CRP <150 mg/L (with or without remdesivir) (scenario #1), in the full LIVE-AIR modified intent-to-treat (mITT) populationFootnoteiii (scenario #2), in Black and African American patients with CRP <150 mg/L (with or without remdesivir) (scenario #3), and in Black and African American patients from the full mITT population (scenario #4). Data for the mITT population, the pre-specified primary analysis population, from the LIVE-AIR trial were used for all analysesCitation34.
Treatment efficacy
Inputs for treatment efficacy of lenzilumab plus SOC versus SOC alone were obtained from the LIVE-AIR trialCitation34. Treatment efficacy inputs for the base case and scenario analyses are presented in and include failure to achieve SWOV, mortality, time to recovery, time in ICU, and time on IMV. Time to recoveryFootnoteiv was used as a proxy for a patient’s length of stay during the index hospitalization.
Table 1. Treatment efficacy inputs from LIVE-AIR trial dataCitation34a.
Clinical outcomes
Treatment efficacy from the LIVE-AIR trial was then used to report clinical outcomes in a way that is relevant to a hospital decision-maker, including the number needed to treat (NNT) for one patient to achieve SWOV, NNT for one life saved, reduction in IMV use, and the number of bed days, ICU days, and IMV days saved.
Per-patient cost calculator
Costs considered in the base case included drug acquisition and administration costs for lenzilumab, and hospital resource costs based on the level of care required during the index hospitalization. Adverse event (AE) management was not considered because the incidence of grade ≥3 AEs was similar in both arms of the LIVE-AIR trial (all differences for individual AEs between arms were less than 3%), and the lenzilumab arm had a lower overall incidence of grade ≥3 AEs (26.7% versus 32.7% for the safety populations of the lenzilumab and placebo arms, respectively)Citation15.
Drug acquisition and administration costs
A targeted literature review was conducted to support the parameterization of costing inputs informing the calculator. All costs were reported in 2021 USD, with adjustments for inflation made based on the US Bureau of Labor Statistics Consumer Price Index for medical care, when appropriateCitation35. The drug acquisition cost for lenzilumab was $10,000 per patient for the entire treatment course, which represented the anticipated US list priceCitation36. The treatment course for lenzilumab consisted of three 1-hour intravenous infusions, each administered 8 h apartCitation15. In the absence of a list price for lenzilumab, this anticipated cost was guided by the cost of a treatment course for a commercially available monoclonal antibody for COVID-19 that targets interleukin-6, which is downstream of GM-CSF in the hyperinflammatory immune response pathwayCitation37,Citation38. It was assumed that the administration cost to the hospital for each infusion was $34.87, for a total administration cost of $104.61. This cost was calculated based on the pharmacy labor and nonlabor (supplies) costs associated with administration of a monoclonal antibody over 60 min in a hospital-based setting, as reported by Schmier and colleaguesCitation39. Additional details on administration cost calculations are provided in the Supplementary Appendix. Drug costs for SOC, including remdesivir and/or corticosteroids, were assumed to be captured in hospital resource use costs; they were not included as separate inputs in the calculator because it was assumed that lenzilumab would have no impact on the utilization or cost of background therapies. This assumption was supported by balanced remdesivir and corticosteroid use in both arms of the LIVE-AIR trialCitation15.
Hospital resource use and costs
Based on a study by Di Fusco and colleaguesCitation10 that examined the economic burden of hospitalized patients with COVID-19 in the US, patients were divided into four levels of care required: no ICU and no IMV, ICU but no IMV, IMV but no ICU, and both ICU and IMV. Inputs for daily hospital resource costs were then obtained by dividing the mean total hospital costs for each level of care by the corresponding mean length of stay as reported by Di Fusco and colleaguesCitation10. It was assumed that these daily costs incorporated all costs associated with SOC, including any remdesivir/corticosteroid use.
Inputs for hospital resource use included the proportion of patients and time to recovery for each level of care informed by data from the LIVE-AIR trialCitation34. Costs and resource use for each level of care are presented in .
The cost calculator then calculated hospital resource costs per patient as a weighted average based on the four levels of care required. First, the average time to recovery for each level of care was multiplied by the corresponding daily hospital resource use cost to obtain the average total hospital resource use cost for each level of care. Next, the weighted average cost per patient was calculated as the sum product of average total hospital resource use costs for each level of care and the corresponding proportion of patients requiring each level of care. These calculations were performed independently for the lenzilumab plus SOC and SOC alone arms to obtain separate weighted average costs for the two treatment options. Additional details on hospital resource cost calculations are provided in the Supplementary Appendix.
Table 2. Hospital resource costs and use inputs from LIVE-AIR trial dataa.
Rehospitalization costs
The calculator also included the flexibility to explore the addition of rehospitalization costs within one year of the index hospitalization (scenario analysis #5). Although long-term rehospitalization data were not captured in the LIVE-AIR trial, it is expected that differences in long-term costs may be observed for patients who required an IMV compared with those who did not. As such, scenario analysis #5 explored the inclusion of projected long-term rehospitalizations costs within one year of the index COVID-19 hospitalization (in addition to the index hospitalization).
Given the paucity of rehospitalization data specific to COVID-19 pneumonia, it was assumed that rates from a study of acute respiratory distress syndrome (ARDS) patients by Wu and colleaguesCitation40 could be used as a proxy. The study included annual rehospitalization rates for ARDS patients who received mechanical ventilation in their index hospitalization and non-ARDS hospitalized controlsCitation40. Thus, the rehospitalization rate of 53.2% for ARDS patients in the study by Wu and colleaguesCitation40 was assumed for IMV patients in the calculator and the rehospitalization rate of 12.9% for non-ARDS patients in this study was assumed for non-IMV patients in the calculator.
Rehospitalization costs were also derived from Wu and colleaguesCitation40 who reported total annual healthcare resource costs of $82,749 for ARDS patients and $22,670 for non-ARDS patients. As a breakdown of costs was not provided by the authors, it was assumed that 76% of the annual costs from Wu and colleaguesCitation40 were from hospital readmissions, based on the estimate reported in a study of acute lung injury survivors by Ruhl and colleaguesCitation41. Therefore, it was assumed that if a patient was rehospitalized within one year of the index hospitalization, the cost would be $77,502 and $21,232 for IMV and non-IMV patients, respectively, after also adjusting for inflation.
It is important to note that for the projected long-term costs (scenario #5), only patients who survived the index hospitalization were eligible for rehospitalization. For IMV patients, this was calculated as the proportion with failure to achieve SWOV minus mortality after 28 days. For non-IMV patients, this was calculated as 100% minus the proportion with failure to achieve SWOV after 28 days. For additional details on the calculations used to inform long-term rehospitalization rate and cost estimates, see the Supplementary Appendix.
Scenario analyses
As mentioned previously, the base case analysis included patients aged <85 years with CRP <150 mg/L, and who were receiving remdesivir. Two scenario analyses were conducted to examine the cost per patient of lenzilumab in broader patient populations. Scenario analysis #1 included patients aged <85 years with CRP <150 mg/L, with or without remdesivir. The full LIVE-AIR mITT population was included in scenario analysis #2. Two scenario analyses were also conducted to examine the cost per patient in narrower patient populations. Scenario analysis #3 included Black and African American patients with CRP <150 mg/L, with or without remdesivir. Black and African American patients from the full mITT population were included in scenario analysis #4. Lastly, scenario analysis #5 added rehospitalization costs to the base case analysis to evaluate the combined per-patient costs to the hospital for the index hospitalization and projected one-year long-term care costs.
Sensitivity analyses
Sensitivity analyses were conducted to determine the key drivers of the per-patient costs in the current analysis. The following inputs were included for sensitivity analyses: daily hospital costs, patient distributions in each level of care (lenzilumab plus SOC arm only), lenzilumab drug costs (lenzilumab plus SOC arm only), and time to recovery (both SOC and lenzilumab plus SOC arms). Inputs for daily hospital costs and patient distributions were adjusted by ±25%, and lenzilumab drug cost inputs were adjusted by +10% and −30%, in the absence of data to inform variance. Daily hospital costs for each level of care were adjusted simultaneously. For patient distributions, the proportion of patients requiring both ICU and IMV was adjusted first and then the three other levels of care were reweighted, maintaining the proportionality between the levels of care prior to adjustment. The decision to use the ICU and IMV group for adjustment in the sensitivity analysis was based on the assumption that this group was the largest driver of costs in the calculator, as a result of the increased cost of care per day and the longer length of stay. Time to recovery inputs was adjusted based on the mean length of stay reported in Di Fusco and colleaguesCitation10. Briefly, the mean length of stay for each level of care from Di Fusco and colleaguesCitation10 was used to inform the time to recovery for the SOC alone arm. The risk ratios from the LIVE-AIR trial (time to recovery for lenzilumab plus SOC divided by time to recovery for SOC alone) were then calculated for each level of care and applied to the respective mean length of stay with SOC alone reported by Di Fusco and colleaguesCitation10 to calculate the adjusted time to recovery input for the lenzilumab plus SOC arm for each level of care.
Results
Base case and scenario analyses
In the base case and all scenario analyses, treatment with lenzilumab plus SOC improved all specified clinical outcomes over SOC alone (). In the base case, the NNT findings showed that every sixth treated patient avoided IMV or death if the cohort was treated with lenzilumab plus SOC rather than SOC alone. Across the scenario analyses, this NNT ranged from a low of 4 in the Black and African American with CRP <150 mg/L subgroups up to 15 in the full LIVE-AIR mITT population. Lenzilumab also provided a benefit in terms of mortality, with an NNT of 10 to save one life in the base case (ranging from 8 to 23 across the scenario analyses). Ventilator use was also reduced by 15.5% (ranging from 6.5% to 26.8%) with the addition of lenzilumab to SOC compared with SOC alone and there were 3.90 IMV days saved (ranging from 1.85 up to 5.50 days). Finally, lenzilumab plus SOC improved the time to recovery, saving 2.40 bed days (ranging from 0.99 up to 4.92 days) as well as reducing time in ICU by 2.97 ICU days (ranging from 1.21 up to 5.06 days).
Table 3. Estimated clinical benefits of lenzilumab plus SOC over SOC alone per treated patient.
In addition to improved clinical outcomes, adding lenzilumab to SOC was also estimated to produce cost savings of $3,190 per patient in the base case analysis (). Scenario analysis #1 (aged <85 years with CRP <150 mg/L, with or without remdesivir) also estimated cost savings of $1,858 per patient. In scenario analysis #2 (full LIVE-AIR mITT population), the addition of lenzilumab to SOC was estimated to result in an additional cost per patient of $4,952 over SOC alone. Scenario analysis #3 (Black and African American patients with CRP <150 mg/L, with or without remdesivir) and scenario analysis #4 (Black and African American patients from the full mITT population) estimated cost savings of $13,154 and $2,763 per patient, respectively. In addition to the index hospitalization, rehospitalization costs were included for scenario analysis #5 (with base case parameters for all other inputs); the addition of rehospitalization costs yielded further cost savings of $1,964 per patient in addition to the base case ($3,190), for total per-patient cost savings of $5,154.
Table 4. The estimated economic impact of lenzilumab plus SOC versus SOC alone per treated patient.
Sensitivity analyses
Inputs for hospital resource costs and patient distributions were adjusted by ±25%, lenzilumab drug cost inputs were adjusted by +10% and −30%, and time to recovery inputs was adjusted based on previously reported mean length of stay by the level of careCitation10 (with base case parameters for all other inputs) in sensitivity analyses to determine the key cost drivers. While most of the sensitivity analyses resulted in per-patient cost savings for lenzilumab plus SOC compared with SOC alone, there was variability relative to the base case results ().
Table 5. Sensitivity analyses.
Varying the hospital resource costs resulted in an additional cost of $134 per patient with lower resource use costs and cost savings of $6,513 per patient with higher resource use costs, a decrease and increase in cost savings of 104.2% relative to the base case, respectively. When decreasing the patient distribution in the IMV and ICU level of care for the lenzilumab plus SOC arm, the cost savings increased to $5,085 per patient, an increase in cost savings of 59.4% relative to the base. In contrast, increasing the patient distribution in the IMV and ICU decreased the cost savings by 59.4% relative to the base case to cost savings of $1,294 per patient. Altering the lenzilumab drug costs produced costs savings ranging from $6,190 per patient with lower drug costs to $2,190 per patient with higher drug costs, an increase of 94.1% and a decrease of 31.4% in cost savings relative to the base case, respectively. Finally, adjusting the time to recovery to align better with the previously reported mean length of stay resulted in an additional cost per patient of $2,409, representing a 175.5% decrease in cost savings relative to the base case.
Discussion
The present analysis evaluated the clinical benefits and per-patient costs of adding lenzilumab to SOC for the treatment of COVID-19 pneumonia from a US hospital perspective. The overall goal was to provide evidence that may assist hospital decision-makers considering the use of lenzilumab as an option in the treatment of this condition that urgently requires new efficacious therapies.
The addition of lenzilumab to SOC was found to improve all specified clinical outcomes in the base case and scenario analyses, including SWOV, mortality, time to recovery, ICU use, and IMV use. SWOV is an important outcome among patients with COVID-19 from the hospital perspective, as avoiding IMV greatly reduces the average length of a patient’s hospital stayCitation42. For all five analysis populations explored from the LIVE-AIR trial, the average time to recovery for patients with IMV was more than double that of patients without IMVCitation34. In the present analysis, the addition of lenzilumab to SOC resulted in a 15.5% absolute reduction in the probability of requiring IMV compared with SOC alone among patients aged <85 years with CRP <150 mg/L who were receiving remdesivir. The average number of bed days, ICU days, and IMV days were also reduced among patients who received lenzilumab in this patient population. Further, the NNT findings showed that every sixth patient avoided IMV or death if the cohort was treated with lenzilumab plus SOC rather than SOC alone. Lenzilumab also provided a benefit in terms of mortality, with an NNT of 10 to save one life. Therefore, for every 100 patients in this population, the results of this analysis suggest that 10 lives could be saved by treating with lenzilumab plus SOC in place of SOC alone.
Lenzilumab was also associated with per-patient cost savings from the hospital perspective in the base case analysis. Although the addition of lenzilumab to SOC was associated with increases in drug acquisition and administration costs compared with SOC alone, these additional costs were more than offset by the reduction in costs for hospital resource use among patients treated with lenzilumab. This led to a net per-patient cost savings of $3,190 with lenzilumab plus SOC compared with SOC alone among patients aged <85 years with CRP <150 mg/L who were receiving remdesivir.
In all scenario analyses conducted in both broader and narrower patient populations than that used for the base case analysis, the addition of lenzilumab to SOC improved all clinical outcomes of interest compared with SOC alone. The relative clinical benefits were smaller in scenarios considering the broader populations (i.e. <85 years with CRP <150 mg/L with or without concomitant remdesivir and full mITT populations) than those observed in the base case population but greater in the subgroup analysis for Black and African American patients with CRP <150 mg/L. For the overall Black and African American population, the relative clinical benefit compared with the base case population varied across clinical outcomes.
In terms of economic impact, the addition of lenzilumab to SOC among patients aged <85 years with CRP <150 mg/L with or without concomitant remdesivir was still associated with a cost savings of $1,858, although this was smaller than the net savings in the base case population. When assessed in the full LIVE-AIR mITT population, the economic impact of adding lenzilumab to SOC was $4,952 per patient. Taken together, the results of these analyses show that adding lenzilumab to SOC for patients aged <85 years with CRP <150 mg/L, regardless of remdesivir use, may provide clinical and economic benefits for US hospitals. In a broader population of patients with COVID-19 pneumonia without consideration of age or CRP criteria, adding lenzilumab to SOC also improved clinical outcomes but with an increased cost per patient.
Among patients hospitalized with COVID-19, elevated levels of CRP at the time of admission are positively correlated with disease severity and are associated with adverse clinical outcomesCitation15,Citation23,Citation24,Citation43,Citation44. Further, CRP levels >100 mg/L at the time of admission are among the strongest independent predictors of critical illnessCitation23,Citation24, and patients with CRP levels >150 mg/L are considered at high risk for escalation of respiratory support (i.e. need for non-invasive ventilation or intubation) or deathCitation45. Given that lenzilumab resulted in particularly favorable clinical outcomes and cost savings among patients aged <85 years with CRP levels <150 mg/L, irrespective of whether all patients were receiving remdesivir, it appears to be most effective and provide the best economic value when used as an early intervention. Since testing for CRP levels is widely accessible and inexpensive in the US hospital settingCitation46, it may be a valuable and feasible approach to identify patients hospitalized with COVID-19 who may benefit most from treatment with lenzilumab.
Despite the Black and African American population being disproportionately affected by COVID-19, clinical trials frequently underrepresent this populationCitation26. As a result, there is a gap in knowledge regarding differences in disease severity, outcomes, and treatments across racial populationsCitation30. Results from a retrospective analysis of the LIVE-AIR trial suggest that Black and African American patients, in particular those with a CRP level <150 mg/L, demonstrate the greatest response to lenzilumab treatmentCitation25. In line with this, use of lenzilumab in Black and African American patients with CRP levels <150 mg/L with or without concomitant remdesivir resulted in cost savings per patient of $13,154, and a cost savings of $2,763 when all Black and African American patients from the LIVE-AIR mITT population were assessed. While findings from the Black and African American population in the LIVE-AIR trial are limited by the small sample size, it should be noted that the distribution of Black and African American patients within the clinical trial is proportional to the US ethnic distribution and thus, were well represented in the study overallCitation25,Citation31.
Notably, the long-term health impacts of COVID-19 are not well characterized because of the novelty of the disease. However, the US CDC is monitoring several post-COVID conditions, referred to as long COVID, that may persist for weeks or months after initial infection and may impact most or all body systemsCitation47. Further, evidence from other recent outbreaks of conditions caused by similar coronaviruses, including severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS), suggests that adverse respiratory, cardiovascular, and mental health outcomes may persist for months or years after resolution of the initial diseaseCitation48–52. In terms of the present study, research into the long-term impact of COVID-19 would provide greater accuracy in the calculator’s economic estimations by reducing the number of assumptions required for rehospitalization costs. Despite these limitations, a scenario analysis was conducted to assess potential long-term cost savings with lenzilumab by incorporating rehospitalizations during the one year after initial hospitalization into the analysis. The results indicated that adding lenzilumab to SOC may result in total cost savings of $5,154 associated with a reduction in IMV use during the index hospitalization. However, since this scenario required assumptions from both ARDS and acute lung injury survivor populations, and data beyond 60 days are not available from the LIVE-AIR trial, there remains a need for longer-term data collection for patients treated with lenzilumab to assess if there is an impact on long-term rehospitalizations and additional outcomes including long COVID.
The sensitivity analyses suggested that results were robust to changes reflecting a reasonable level of uncertainty (±25%) in key inputs including hospital resource costs, patient distributions to different levels of care, and to variations (+10%, −30%) in the anticipated drug cost for lenzilumab. Based on these analyses, hospital resource costs had the greatest impact on the results of the calculator. Increasing the hospital resource costs by 25% for the lenzilumab plus SOC arm resulted in a 104.2% increase in cost savings relative to the base case. By comparison, varying lenzilumab drug costs and patient distributions to different levels of care had less impact on the cost savings associated with adding lenzilumab to SOC. Inputs for time to recovery were adjusted based on values reported by Di Fusco and colleaguesCitation10, as previously described. Adjusting the time to recovery had a large effect on the cost per patient, resulting in a decrease in cost savings of 175.5% (i.e. no longer cost savings). As such, time to recovery appears to be a major driver in the current per-patient cost calculator.
Moreover, it should be noted that the use of the costing data from Di Fusco and colleaguesCitation10 is associated with several limitations. First, as the cost per day was calculated as the mean total hospital costs divided by the mean length of stay, any variations in the costs per day overtime were not able to be assessed and, as such, it was assumed that all hospital days (within the respective level of care) were associated with the same cost. Second, as noted by Di Fusco and colleaguesCitation10, while data from the Premier Health Database COVID-19 Database is obtained from a large proportion of hospitals throughout the US, populations of hospitalized COVID-19 patients in geographic divisions with less representation may not be captured, thus decreasing generalizability to the entire US population. Finally, while the use of IMV outside the ICU is not typical clinical practice, this level of care was still represented by 4.5% of the study population, perhaps representing resource constraints at the time of data collection (1 April to 31 October 2020) as a result of the pandemic.
As with the extrapolation of any clinical trial results, this study had several limitations. The analysis used data from the LIVE-AIR Phase 3 clinical trial, which may be impacted by selection bias and, consequently, results from the trial may not be fully generalizable to a real-world US hospital population. In addition, some of the subgroups from the LIVE-AIR trial that were explored in the per-patient cost-calculator were limited by small sample sizes, in particular, the Black and African American subgroups noted above and will require additional validation with results from the upcoming ACTIV-5/BET-B trial. Patient data from LIVE-AIR were censored after 28 days following trial enrollment. This is noteworthy because patients with COVID-19 who are critically ill and/or who require IMV typically have extended time to recovery, sometimes beyond 28 daysCitation24,Citation53,Citation54. Therefore, the model may underestimate the time to recovery for patients who require IMV by only allowing up to 28 days of hospital resource use. Consequently, cost savings associated with the reduction in the proportion of patients who require IMV may be even greater than suggested by the results of the current analysis. It is also important to note that the current analyses were conducted ex-ante and, as such, were conducted prior to regulatory approval of lenzilumab and before an established US list price was set. As a result, the drug acquisition cost for lenzilumab was set at $10,000 per patient for the entire treatment course to represent the anticipated list price in the US. Finally, while the US Food and Drug Administration (FDA) recently (September 9th, 2021) declined the request for emergency use authorization (EUA) of lenzilumab to treat newly hospitalized COVID-19 patients based on the data from the LIVE-AIR trial concluding additional clinical data are requiredCitation55, the FDA has invited Humanigen to submit any supplemental data as they become available. It is also anticipated that the ongoing ACTIV-5/BET-B trial, which completed enrollment of over 400 patients in the primary analysis population, may provide additional efficacy and safety data sufficient to further support the use of lenzilumab for the treatment of hospitalized COVID-19 patientsCitation33,Citation56.
Although approximately 68% of individuals ≥12 years of age in the US are now fully vaccinated as of November 7th, 2021Citation6, there will remain an ongoing need for effective treatments for patients hospitalized with COVID-19 due to vaccine hesitancyCitation57,Citation58, waning immunityCitation59–61, emerging variantsCitation62, and a lack of vaccines for certain population groups. Lenzilumab is a particularly promising treatment option as its mechanism of action functions by blocking the action of GM-CSF, which is produced in response to infection. As such, as was reported in the COVID-19 Therapeutics Strategy published by the European Commission, its efficacy is not anticipated to be affected by the new SARS-CoV-2 variantsCitation63.
Overall, the results of this analysis highlight the clinical benefits for SWOV, ventilator use, time to recovery, mortality, time in ICU, and time on IMV, in addition to an economic benefit from the US hospital perspective associated with adding lenzilumab to SOC for patients with COVID-19 pneumonia. Lenzilumab provides clinical benefits to a broad population of patients with characteristics similar to the full study cohort from the LIVE-AIR trial. Notably, the drug appears to be particularly effective in patients aged <85 years with CRP <150 mg/L and it is estimated to result in cost savings to US hospitals when used in this patient population. These clinical benefits and cost savings extend to the Black and African American subgroup as well. This is a critical finding as Black and African American persons are hyper-vulnerable to COVID-19Citation26. Concomitant use of remdesivir with lenzilumab further improves clinical benefits and cost savings. These findings support the use of lenzilumab as a standard option in the treatment of COVID-19 pneumonia and may help to assist hospital formulary decision-makers with their consideration regarding its adoption in the hospital setting should a EUA or full approval through a Biologics License Application be granted.
Transparency
Declaration of funding
This work was supported by Humanigen Inc.
Declaration of financial/other interests
AK, EJ, DC, MA are employees of Humanigen Inc. AZ, KT, ANP, AH, MT are employees of EVERSANA which received funding from Humanigen Inc. to conduct this study.
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Author contributions
AK, EJ, AZ, KT, ANP, MT made substantial contributions to the conception and design of the calculator. Clinical data acquisition and analysis was conducted by DC. AZ, KT, ANP, AH conducted the literature review, developed the calculator structure, and prepared the manuscript. All authors reviewed the calculator and manuscript for interpretation of data and important intellectual content and have given their approval for the final version of the manuscript.
Supplemental Material
Download MS Word (54.1 KB)Acknowledgements
The authors acknowledge Cameron Durant (Humanigen Inc.), Ari Mendell (EVERSANA), and Karson Theriault (EVERSANA) for their specific contributions to this project.
Data availability statement
The data that support the findings of this study are available through the link below: https://doi.org/10.1101/2021.05.01.21256470
Notes
i Humanigen, Inc., Burlingame, CA, USA; manufactured by Calalent in the USA.
ii Microsoft Corporation, Redmond, WA, USA.
iii The mITT population was the analysis set used for the primary analysis of efficacy, defined as all randomized patients who received at least one dose of study drug under the documented supervision of the principal investigator or sub-investigator and excluding sites that experienced documented limitations to access of basic supportive care for COVID-19.
iv Time to recovery was a pre-specified secondary outcome of the clinical trial and was defined as the first day on which a patient was discharged or ready for discharge by satisfying one of the following 3 categories from the 8-point ordinal scale: hospitalized, not requiring supplemental oxygen, no longer requiring ongoing medical care; not hospitalized, limitation on activities and/or requiring home oxygen; not hospitalized, no limitations on activities.
References
- Sarkodie SA, Owusu PA. Global assessment of environment, health and economic impact of the novel coronavirus (COVID-19). Environ Dev Sustain. 2021;23(4):5005–5011.
- Kaye AD, Okeagu CN, Pham AD, et al. Economic impact of COVID-19 pandemic on healthcare facilities and systems: International perspectives. Best Practice & Research Clinical Anaesthesiology. 2020;35(3):293–306.
- Nicola M, Alsafi Z, Sohrabi C, et al. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg. 2020;78:185–193.
- Centers for Disease Control and Prevention: COVID Data Tracker United States COVID-19 Cases, Deaths, and Laboratory Testing (NAATs) by State, Territory, and Jurisdiction [Internet]. 2021 [updated 2021 July 3; cited 2021 July 5]. Available from: https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days.
- Centers for Disease Control and Prevention: COVID Data Tracker United States – New Admissions of Patients with Confirmed COVID-19 [Internet]. [updated 2021. July 19; cited 2021 July 22]. Available from: https://covid.cdc.gov/covid-data-tracker/#new-hospital-admissions.
- Centers for Disease Control and Prevention: COVID Data Tracker United States – COVID-19 Vaccinations in the United States [Internet]. 2021 [updated 2021 November 7; cited 2021 November 8]. Available from: https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-people-fully-percent-pop12.
- Centers for Disease Control and Prevention: COVID Forecasts: Hospitalizations – Reported and forecasted new COVID-19 hospital admissions as of November 1, 2021. [Internet]. [updated 2021 November 3; cited 2021 November 3]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/science/forecasting/hospitalizations-forecasts.html.
- Centers for Disease Control and Prevention: COVID Data Tracker United States – COVID-19 Hospitalizations and Disease Severity [Internet]. [updated 2021. July 19; cited 2021 July 22]. Available from: https://covid.cdc.gov/covid-data-tracker/#hospitalizations-severity.
- Rae M, Claxton G, Kurani N, et al. Potential costs of COVID-19 treatment for people with employer coverage [updated March 13, 2021; July 6, 2021]. Available from: https://www.healthsystemtracker.org/brief/potential-costs-of-coronavirus-treatment-for-people-with-employer-coverage/.
- Di Fusco M, Shea KM, Lin J, et al. Health outcomes and economic burden of hospitalized COVID-19 patients in the United States. J Med Econ. 2021;24(1):308–317.
- Centers for Medicare & Medicaid Services. COVID-19 Frequently Asked Questions (FAQs) on Medicare Fee-for-Service (FFS) Billing. July 2, 2021. p. 1–180.
- Sheinson D, Dang J, Shah A, et al. A Cost-Effectiveness framework for COVID-19 treatments for hospitalized patients in the United States. Adv Ther. 2021;38(4):1811–1831.
- Goletti D, Cantini F. Baricitinib therapy in covid-19 Pneumonia – an unmet need fulfilled. N Engl J Med. 2021;384(9):867–869.
- Liu C-H, Lu C-H, Wong SH, et al. Update on antiviral strategies against COVID-19: unmet needs and prospects [review]. Front Immunol. 2020;11(3845):616595.
- Temesgen Z, Burger CD, Baker J, et al. Lenzilumab in hospitalised patients with COVID-19 pneumonia (LIVE-AIR): a phase 3, randomised, placebo-controlled trial. Lancet Respir Med. 2021. DOI:10.1016/S2213-2600(21)00494-X
- Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19(3):141–154.
- Xiong Y, Liu Y, Cao L, et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect. 2020;9(1):761–770.
- Zhou Y, Fu B, Zheng X, et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. 2020;7(6):998–1002.
- Kox M, Waalders NJB, Kooistra EJ, et al. Cytokine levels in critically ill patients with COVID-19 and other conditions. Jama. 2020;324(15):1565–1567.
- Thwaites RS, Sanchez Sevilla Uruchurtu A, Siggins MK, et al. Inflammatory profiles across the spectrum of disease reveal a distinct role for GM-CSF in severe COVID-19. Sci Immunol. 2021;6(57):eabg9873.
- Novack V, Beitler JR, Yitshak-Sade M, et al. Alive and ventilator free: a hierarchical, composite outcome for clinical trials in the acute respiratory distress syndrome. Crit Care Med. 2020;48(2):158–166.
- Temesgen Z, Kelley CF, Cerasoli F, et al. Early lenzilumab treatment of COVID-19 patients using C-Reactive protein as a biomarker improves efficacy: results from the phase 3 'AIR’. Trial. medRxiv. 2022. DOI:10.1101/2021.12.30.21267140
- Lavillegrand JR, Garnier M, Spaeth A, et al. Elevated plasma IL-6 and CRP levels are associated with adverse clinical outcomes and death in critically ill SARS-CoV-2 patients: inflammatory response of SARS-CoV-2 patients. Ann Intensive Care. 2021;11(1):9.
- Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York city: prospective cohort study. Bmj. 2020;369:m1966.
- Humanigen: Lenzilumab Treatment May Provide Enhanced Likelihood of Survival Without Ventilation in Hospitalized Black and African-American COVID-19 Patients [Internet]. [updated 2021 August 4; cited 2021 September 24]. Available from: https://ir.humanigen.com/English/news/news-details/2021/Lenzilumab-TreatmentMay-Provide-Enhanced-Likelihood-of-Survival-Without-Ventilation-in-Hospitalized-Black-and-African-American-COVID-19-Patients/default.aspx.
- Centers for Disease Control and Prevention: Risk for COVID-19 Infection, Hospitalization, and Death by Race/Ethnicity [Internet]. [updated 2021 September 9; cited 2021 September 24]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html#print.
- Ndugga N, Hill L, Artiga S. Latest Data on COVID-19 Vaccinations by Race/Ethnicity [Internet]. [updated 2021 September 22; cited September 24]. Available from: https://www.kff.org/coronavirus-covid-19/issue-brief/latest-data-on-covid-19-vaccinations-race-ethnicity/.
- Centers for Disease Control and Prevention: People with Certain Medical Conditions [Internet]. [updated 2021 August 20; cited 2021 September 24]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html.
- Rodriguez F, Solomon N, de Lemos JA, et al. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19: findings from the American Heart Association's COVID-19 cardiovascular disease registry. Circulation. 2021;143(24):2332–2342.
- Chastain DB, Osae SP, Henao-Martinez AF, et al. Racial disproportionality in covid clinical trials. N Engl J Med. 2020;383(9):e59.
- United States Census Bureau: QuickFacts Table [Internet]. [updated 2020. cited 2021 September 24]. Available from: https://www.census.gov/quickfacts/fact/table/US/RHI225219#qf-headnote-a.
- National Institutes of Health: COVID-19 Treatment Guidelines – Therapeutic Management of Hospitalized Adults with COVID-19 [Internet]. [updated 2021 July 8; cited 2021 July 22]. Available from: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults–therapeutic-management/.
- ClinicalTrials.gov: ACTIV-5/Big Effect Trial (BET-B) for the Treatment of COVID-19 [Internet]. [updated 2021 September 22; cited 2021 September 24]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04583969.
- Humanigen. Additional data analyses for LIVE-AIR Phase 3 randomized double-blind placebo-controlled trial. Data on file. 2021.
- Bureau of Labor Statistics: CPI-All Urban Consumers (Current Series); Medical care in U.S. city average, all urban consumers, not seasonally adjusted [Internet]. [updated 2021 April; cited 2021 June 3]. Available from: https://data.bls.gov/cgi-bin/surveymost?cu.
- Humanigen. Lenzilumab drug cost. Data on file. 2021.
- Food and Drug Administration: Fact Sheet for Healthcare Providers: Emergency Use Authorization for ACTEMRA® (tocilizumab) [Internet]. [updated 2021 June; cited 2021 November 10]. Available from: https://www.fda.gov/media/150321/download.
- Pricentric One by EVERSANA: MNF(WAC) price for ACTEMRA Infusion 1 vial 20 mL 400 mg [Internet]. [updated 2021 November 09. cited 2021 November 10]. Available from: https://pricentric.alscg.com/.
- Schmier J, Ogden K, Nickman N, et al. Costs of providing infusion therapy for rheumatoid arthritis in a hospital-based infusion center setting. Clin Ther. 2017;39(8):1600–1617.
- Wu N, Hanrahan J, Bornstein J, et al. Healthcare costs utilization and costs of patients hospitalized with acute respiratory distress syndrome (ARDS) in US commercially-insured individuals and medicare beneficiaries. Eur Respiratory Soc. 2015;46(59):PA2139.
- Ruhl AP, Lord RK, Panek JA, et al. Health care resource use and costs of two-year survivors of acute lung injury. An observational cohort study. Ann Am Thorac Soc. 2015;12(3):392–401.
- Hazard D, Kaier K, von Cube M, et al. Joint analysis of duration of ventilation, length of intensive care, and mortality of COVID-19 patients: a multistate approach. BMC Med Res Methodol. 2020;20(1):206.
- Luo X, Zhou W, Yan X, et al. Prognostic value of C-Reactive protein in patients with coronavirus 2019. Clin Infect Dis. 2020;71(16):2174–2179.
- Ali N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J Med Virol. 2020;92(11):2409–2411.
- Manson JJ, Crooks C, Naja M, et al. COVID-19-associated hyperinflammation and escalation of patient care: a retrospective longitudinal cohort study. Lancet Rheumatol. 2020;2(10):e594–e602.
- Sharifpour M, Rangaraju S, Liu M, et al. C-Reactive protein as a prognostic indicator in hospitalized patients with COVID-19. PLOS One. 2020;15(11):e0242400.
- Centers for Disease Control and Prevention: Post-COVID Conditions [Internet]. [updated 2021 July 12; cited 2021 July 19]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects.html.
- Leung TYM, Chan AYL, Chan EW, et al. Short- and potential long-term adverse health outcomes of COVID-19: a rapid review. Emerg Microbes Infect. 2020;9(1):2190–2199.
- Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611–627.
- Zhang P, Li J, Liu H, et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res. 2020;8(1):8.
- Lam MH, Wing YK, Yu MW, et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med. 2009;169(22):2142–2147.
- Wu Q, Zhou L, Sun X, et al. Altered lipid metabolism in recovered SARS patients twelve years after Infection. Sci Rep. 2017;7(1):9110.
- King CS, Sahjwani D, Brown AW, et al. Outcomes of mechanically ventilated patients with COVID-19 associated respiratory failure. PLOS One. 2020;15(11):e0242651.
- Auld SC, Caridi-Scheible M, Blum JM, et al. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med. 2020;48(9):e799–e804.
- Humanigen: FDA has declined Humanigen’s Emergency Use Authorization (EUA) Request for Lenzilumab in Hospitalized COVID-19 Patients [Internet]. [updated 2021 September 9; cited 2021 September 24]. Available from: https://ir.humanigen.com/English/news/news-details/2021/FDA-has-declined-Humanigens-Emergency-Use-Authorization-EUA-Request-for-Lenzilumab-in-Hospitalized-COVID-19-Patients/default.aspx.
- Humanigen: Humanigen Announces Target Enrollment in Phase 2/3 ACTIV-5/BET-B Trial of Lenzilumab for the Treatment of COVID-19 has Been Achieved [Internet]. 2022 [updated 2022 January 5; cited 2022 January 6]. Available from: https://ir.humanigen.com/English/news/news-details/2022/Humanigen-Announces-Target-Enrollment-in-Phase-23-ACTIV-5BET-B-Trial-of-Lenzilumab-for-the-Treatment-of-COVID-19-has-Been-Achieved/default.aspx.
- Shelburne P: Global Vaccine Tracking - Across the Globe, Rates of Vaccine Skepticism Have Stalled [Internet]. [updated 2021 November 4; cited November 8]. Available from: https://morningconsult.com/global-vaccine-tracking/.
- Centers for Disease Control and Prevention: Estimates of Vaccine Hesitancy for COVID-19 [Internet]. [cited 2021 November 2]. Available from: https://data.cdc.gov/stories/s/Vaccine-Hesitancy-for-COVID-19/cnd2-a6zw/.
- Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407–1416.
- Cohn BA, Cirillo PM, Murphy CC, et al. Breakthrough SARS-CoV-2 infections in 620,000 U.S. Veterans. medRxiv. 2021. DOI:10.1101/2021.10.13.21264966
- Dolgin E. COVID vaccine immunity is waning – how much does that matter? Nature. 2021;597(7878):606–607.
- Centers for Disease Control and Prevention: What You Need to Know about Variants [Internet]. [updated 2021 November 1; cited 2021 November 2]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant.html.
- European Commission: European Health Union – Commission Establishes Portfolio of 10 Most Promising Treatments for COVID-19 [Internet]. [updated 2021 October 22. cited 2021 November 2]. Available from: https://ec.europa.eu/commission/presscorner/detail/en/IP_21_5366.
