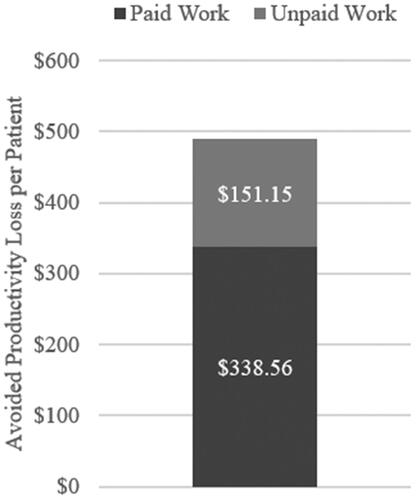?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aims
Japanese cedar pollinosis (JCP) is a form of seasonal allergic rhinitis that affects 38.8% of the Japanese population. Particularly severe and most severe symptoms among JCP patients can lead to impairments of paid work productivity and unpaid work activities. Indeed, the current standard of care (SoC) is not always able to relieve these symptoms. Omalizumab, a novel JCP treatment recently approved in Japan, provides an effective add-on therapy to the SoC. This study estimates the effect of omalizumab on paid and unpaid work activities (i.e. its social impact) in patients with severe and most severe JCP symptoms in Japan.
Methods
The impact of omalizumab was estimated through a one-year static cohort model using the Work Productivity and Activity Impairment Allergy Specific (WPAI-AS) questionnaire derived from a clinical trial on omalizumab enrolling patients with severe and most severe JCP symptoms, which had been conducted in Japan. This effect was quantified using Japanese official statistics on employment and time use. The human capital approach and the proxy good approach were employed to monetize paid and unpaid work activities, respectively. A sensitivity analysis was implemented to account for modeling structural uncertainties.
Results
Our results show that the use of omalizumab might reduce the paid and unpaid work productivity losses due to severe and most severe JCP by nearly one-third. In the severe symptom period of three weeks, 36.6 million hours of lost paid and unpaid work hours could be avoided, which sums up to a monetized productivity loss of 728.3 million USD.
Conclusions
Omalizumab could provide substantial benefits in terms of paid and unpaid work activities in patients with severe and most severe JCP. Our results also highlight the importance of considering unpaid work in estimating productivity costs due to poor health.
Introduction
Allergic rhinitis (AR) is one of the most common chronic allergic respiratory diseases worldwideCitation1 and its burden is considered a challenge for the new centuryCitation2. In Japan, the prevalence of seasonal AR due to Japanese cedar pollen (known as Japanese cedar pollinosis – JCP) has been continuously increasing in the last decadesCitation3. In 2019, JCP affected 38.8% of the entire Japanese populationCitation4. Its symptoms comprise paroxysmal repetitive sneezing, watery rhinorrhoea, and nasal congestion, frequently coexisting with ocular symptoms. These symptoms set in with the pollen spreading in early February and last until March or April, usually with a severe spread in March, depending on the regions across JapanCitation5.
As stated in the Practical Guideline for the Management of Allergic Rhinitis in Japan (PG-MARJ) 2020Citation3, the treatment aims to relieve its symptoms and eliminate difficulties in everyday life. The pharmacotherapy of JCP depends on its severity and typology (i.e. sneezing and rhinorrhea type, nasal blockage type, and combined type) and might vary during the pollen period, as the amount of pollen dispersal influences the severity of the symptoms. According to the PG-MARJ, a combination of second-generation antihistamine and nasal corticosteroid is recommended for severe or most severe JCP patients with sneezing and rhinorrhea typeCitation3. However, a combination consisting of three or more treatments including leukotriene receptor antagonist is recommended for patients with nasal blockage type or the combined type having nasal blockage as a major complaintCitation3.
Despite the available therapies, many patients with JCP remain symptomatic in JapanCitation6 and have to cope with treatment dissatisfaction. These remaining symptoms can affect the well- being and quality of life (QoL) of these patients, by causing fatigue, headache, and cognitive impairmentCitation7. In addition, inadequately controlled symptoms could lead to society’s economic lossesCitation8. A recent analysis of the burden of disease of inadequately controlled symptoms in patients suffering severe forms of JCP has estimated a productivity loss of 26.1 billion US Dollars (USD) in the three weeks of higher pollen spreading in 17.17 million patients assumed to receive the SoC (this patient population does not reflect the patient population of the present study, for more details, see the Supplementary Material S1). These results underline the extent of unmet medical needs among patients with severe and most severe JCP symptoms.
Omalizumab, a humanized monoclonal anti-immunoglobulin E (IgE) antibody, has already shown significant improvements in the QoL of children, adolescents, and adults suffering with severe allergic asthma and other allergic comorbiditiesCitation9,Citation10. Omalizumab has also proven efficacy against placebo in patients suffering moderate to severe JCP symptoms and was approved as new additional indication for the treatment of seasonal allergic rhinitis (for use only in severe or the most severe patients who have not responded sufficiently to conventional therapies in Japan 2019Citation11. In a recent clinical trial enrolling patients with severe or most severe JCP in JapanCitation12, omalizumab (on top of SoC, which consists of antihistamine and nasal corticosteroid) has demonstrated a favourable benefit-risk profile and efficacy. Specifically, compared to placebo (on top of SoC), omalizumab has improved nasal and ocular symptoms, QoL and reduced work productivity loss, as well as activity impairment. By efficiently reducing the symptoms in severe and most severe JCP patients, omalizumab might considerably improve the well-being of patients and the society in Japan.
The impact of AR in many regions of the world is a subject of researchCitation8,Citation13–15, but the studies on the impact of its inadequate managementCitation16,Citation17 or specific pharmaceutical treatmentsCitation17,Citation18 remain fragmented. Especially for Japan, there is a lack of investigations in the therapeutic area of AR despite its high prevalence. Our model-based evaluation contributes to filling this research gap. We assessed the effects of this new treatment by quantifying the effects of omalizumab in terms of avoided paid and unpaid work productivity losses (i.e. the social impact) in patients with inadequately controlled severe and most severe JCP symptoms in Japan. For this purpose, we used the restored impairment of paid and unpaid work productivity due to omalizumab according to the difference between the impairments of the ‘Soc + omalizumab’ and the ‘SoC alone’ arms observed in a clinical trial (NCT03369704).
Methods
Methodological approach
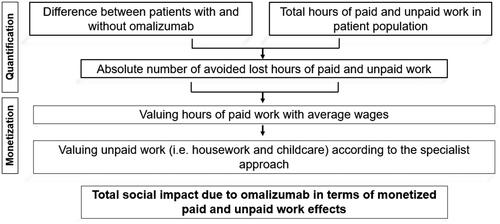
To quantify the social impact of omalizumab in Japan, we built a static cohort model, which considers the prevalent JCP cohort over one year by assuming 21 days of impairment, i.e. the severe symptom period observed in the clinical trial of omalizumabCitation12. Specifically, we retrieved the data from the clinical trial evaluating omalizumab on top of SoC (i.e. antihistamine and nasal corticosteroid) against placebo on top of SoCCitation12, published literatureCitation4,Citation7,Citation19,Citation20, and the Japanese bureau of statisticsCitation21–25. depicts the calculation flow dividing the approach into two main steps. In the first step, the model quantifies the loss of productivity avoided by omalizumab and then monetizes it to determine the social impact of omalizumab.
In what follows, we describe the input criteria needed to determine the social impact.
Patient population
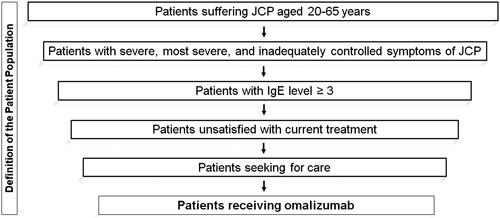
We defined the hypothetical patient population for omalizumab according to the clinical trial’s inclusion criteriaCitation3 and the optimal use guideline for omalizumab in JapanCitation26 (for the steps undertaken to define the patient population, see ). The sample included patients between 20 and 65 years of age with severe and most severe JCP according to PG-MARJFootnotei as well as inadequately controlled symptoms of JCPCitation19. The underlying assumption was that these patients have the need and willingness to search for a new treatment. After determining the number of patients who are unsatisfied with current treatment (i.e. with inadequately controlled symptoms)Citation7, seeking careCitation19, and have a cedar-specific IgE score ≥3Citation20,Footnoteii we derived the cohort of patients potentially eligible for omalizumab in Japan by applying these numbers to the population in Japan in 2020Citation21.
Work productivity and activity impairment
We evaluated the overall work and activity impairment restored due to omalizumab according to the Work Productivity and Activity Impairment Allergy Specific (WPAI-AS) questionnaireCitation18. We used the restored overall work impairment and did not differentiate between absenteeism and presenteeism to determine the productivity effects in terms of paid work and the restored activity impairment as a proxy for impairment in unpaid work activities. Specifically, this restored impairment was given by the difference between the impairment observed in the control group (SoC alone; percent overall work impairment, least squares – LS – mean: 37.10; percent activity impairment, LS mean: 38.51) and the one observed in the treatment group (SoC plus omalizumab; percent overall work impairment, LS mean: 23.49; percent activity impairment, LS mean: 24.35) in the clinical trialCitation27. The resulting impairment reduction we used is listed in .
Table 1. Input Parameters of the Model.
Calculation period
To define the period in our modelling, we focused on the severe symptom period of the clinical trialCitation12, during which the change in the overall work and activity impairment has been analyzed. Concretely, the severe symptom period was defined as three weeks, where the cumulative value of the mean daily nasal symptom score is at its maximumCitation12.
Monetization
The costs associated with the productivity losses, generally defined as ´costs associated with production loss and replacement costs due to illness, disability and death of productive persons, both paid and unpaid’Citation31 are determined. We quantified the paid work loss according to the human capital (HC) approach, where human beings are treated as assets and impaired working time is valued with wages to measure the loss to the economyCitation28. The Japanese employment rate was applied to the patient population and thus its average working hours per day and average wages per hour determinedCitation22–24. The underlying assumption was that these statistics reflect the working pattern of the considered patient population. Based on this assumption, the 15 working days assumed in the symptom period, and the restored work impairment rate, the model quantifies the avoided productivity loss in terms of paid work due to omalizumab as follows:
(1)
(1)
where
represents the costs of age- and gender- specific avoided productivity losses of paid work,
depicts the working days with symptoms,
indicates the age- and gender-specific labor force participation rates,
accounts for the average daily working hours per age and gender, while
indicates the change in the overall work impairment due to omalizumab,
defines the average wage per hour.
To estimate productivity effects beyond paid work, we applied the restored activity impairment to the average hours spend for unpaid work activities to approximate the effects of omalizumab on unpaid work. To value these losses, we used the proxy good (PG) approach. The PG approach consists in applying the Reid’s third-person-criterionCitation29, i.e. it considers what it would cost to hire a worker to perform a specific activity using either its specialist’s or generalist’s wage. For valuing household tasks, we used specialist’s wages, i.e. we valued every household task with the wage of its closest market substitute (e.g. we used a plumber’s wage to evaluate the activity of fixing a leak or a nursing home care worker’s wage to value the activity of informal care)Citation32. According to the Reid’sCitation29 third-person-criterion and by referring to the Japanese time use surveyCitation25, we defined cooking, caring, nursing for children or elderly, and cleaning activities as unpaid work activities. Considering the time spent for each activity per day, the symptom period, and the rate of restored activity impairment, the model calculates the avoided unpaid work loss due to omalizumab as follows:
(2)
(2)
where
represents the costs of age- and gender- specific avoided productivity losses of unpaid work,
depicts the JCP symptom days,
accounts for the age- and gender- specific daily hours spent on several unpaid work activities, and
indicates the change in activity impairment due to omalizumab.
defines the average wage per hour per unpaid work activity.
To calculate the social impact, the model adds up the impact of omalizumab on avoided paid and unpaid work activities as follows:
(3)
(3)
where
is the avoided productivity loss due to paid work in patients using omalizumab and
is the avoided productivity loss due to unpaid work.
Social impact analysis
shows the input values used for obtaining the base case results as well as the range used for the deterministic sensitivity analysis.
Sensitivity analysis
Although we selected the input parameters carefully, the number of patients, the effect of omalizumab, the patient population’s work behavior, and the assessment of paid and unpaid productivity might be subject to some uncertainties in their measurement. To test parameter and model uncertainty, we conducted a deterministic sensitivity analysis (DSA), by changing the parameter values through the upper and lower bounds listed in . The DSA allowed us to assess the impact of changes in parameters’ value on our results and therefore identify the variables that most affect our results. For more detailed information on the data sources used for varying the parameter values, refer to the Supplementary Material S2.
Results
Patient population
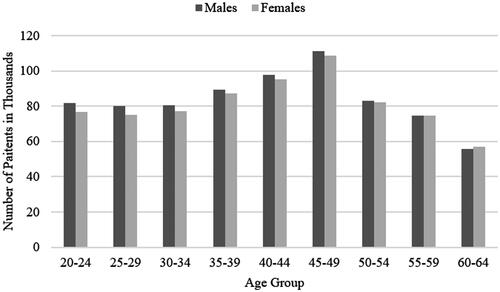
The hypothetical patient population defined in our model amounted to nearly 1.49 million of patients, of which 745,000 (50.7%) were men and 733,000 (49.3%) women. According to the population structure in Japan, the age group 45–49 years was the largest among our patient population ().
Avoided hours of productivity loss per patients and for the entire patient population
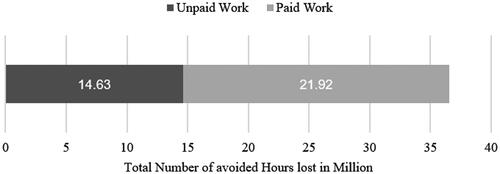
The avoided lost paid and unpaid work hours attributable to the use of omalizumab summed up to 36.6 million. Because a high share of males and females in our patient population was in employment, 60% of this productivity loss was attributable to paid work (). Beyond employment rates, the average working hours and average hours spent for unpaid work, parameters which are age- and gender-specific, influenced the avoided hours of productivity. For more detailed results by age and gender, refer to Table A4 in the Supplementary Appendix.
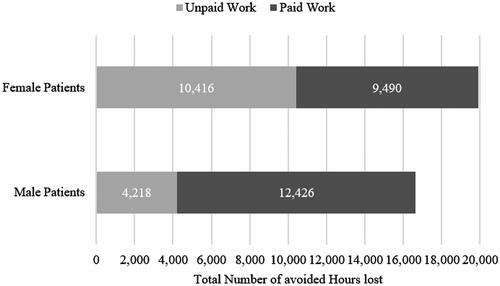
depicts the gender-specific avoided loss of paid and unpaid work. Although the patient population included an almost equal share of men and women, more productivity losses were avoided among women. Indeed, 19.9 out of 36.6 million hours of productivity loss (54%) were avoided among female patients. Of these, about 10.4 million (52%) were achieved by avoiding productivity losses in unpaid work activities. Instead, the largest share of avoided productivity loss for men was attributed to paid work. Among male patients, approximately 12.4 (75%) out of 16.6 million lost hours were avoided through paid work activities. The higher share of avoided productivity losses in unpaid work activities among female patients compared to male patients resulted from women’s higher unpaid labor contributions.
Social impact for the entire patient population
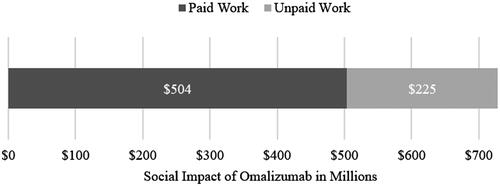
The estimated productivity loss that could be avoided due to the avoidance of 36.6 million hours of paid and unpaid work activities summed up to 728.3 million USD. Most of this productivity loss was attributed to the avoided loss of paid work. The monetized productivity loss from paid work amounted to 503.5 million USD, which represented nearly 70% of the total monetized avoided productivity loss. The high share of unpaid work (60% of the total productivity loss) was only about 30% of monetarized productivity loss, primarily because of the lower valuation of unpaid work compared to paid work in our model. The valuation method that we applied for unpaid work resulted in an hourly wage of about 16 USD. On the other hand, valuing paid work at the average wage per hour resulted in assessing one hour of paid work at 23 USD ().
The patient perspective
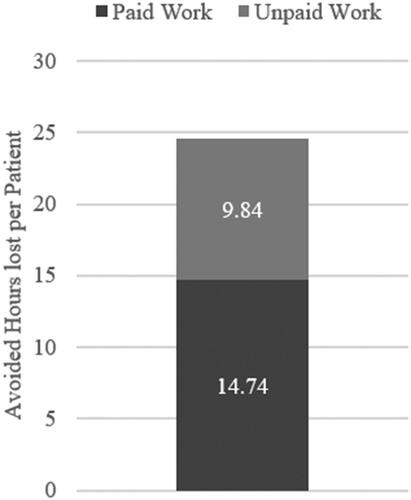
The effects of omalizumab for an average patient underline the importance of omalizumab from a patient perspective. The results showed that omalizumab could potentially avoid a loss of 14.74 h of paid work per patient during the severe JCP symptom period (). Omalizumab could also prevent productivity losses from unpaid work for 9.84 h per patient. In total, the use of omalizumab in this patient population could avoid 24.58 h of productivity loss per patient during the severe JCP symptom period, implicating that patients could experience an entire day without any symptoms during the severe symptoms’ period.
This was equivalent to an overall avoided monetized productivity loss of $489.71 per patient (). This was attributed to the monetarization of paid work since the valuation of hours of paid work was significantly higher than the one of unpaid work hours.
Sensitivity analysis
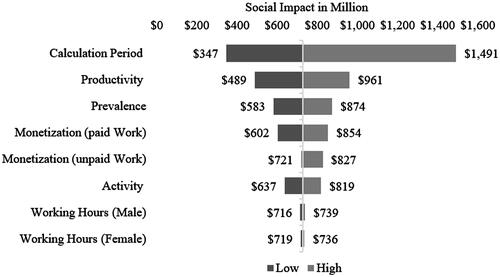
In our base-case scenario (input parameters listed in ), the productivity loss that could be avoided in our patient population through the relief of the severe JCP symptoms by omalizumab amounted to approximately 728.4 million USD. Yet, through the variation of different input parameters, the social impact of omalizumab could be reduced to about 347 million USD or increased to nearly 1.5 billion USD ().
9 indicates parameters that influence results the most during the calculation period. Assuming a severe JCP symptom phase of only 10 days, the avoided productivity loss would amount to ∼346.8 million USD. If, instead, we assumed that the JCP symptom period persists for 43 days, the avoided productivity loss would increase to approximately 1.5 billion USD. Another important influencing factor was the reduction in work impairment reported in the clinical trial of omalizumab. The variation of this parameter within the lower and upper limits of its confidence interval showed that the avoided productivity loss would be reduced to about 489 million USD if the reduction in work impairment corresponded to the lower bound, i.e. to seven percentage points. Instead, if the reduction in work impairment corresponded to the upper bound of the confidence interval, the avoided productivity loss would increase to about 961 million USD. Besides the productivity and calculation period, also the number of patients considered in the model considerably influenced the results. By varying the prevalence by plus and minus 20 percent and maintaining all the other input parameters constant, the avoided loss of productivity would be reduced to about 583 million USD or increased to 874 million USD. In addition, according to the method chosen for valuating paid work, the social impact of omalizumab could increase or decrease by 25 percent. The monetization of unpaid work with the generalist approach hardly affected the result. However, a monetary valuation with the opportunity cost approach would increase the social impact of omalizumab to about 827 million USD. The magnitude of the reduction in the activity impairment also had a significant, but smaller, impact on the results because of the work impairment variation due to the relatively low monetarization. Accordingly, the results would range from 637 million USD to 819 million USD. Lastly, the variation in daily work hours was of relatively little importance for the results ().
Discussion and limitations
To the best of our knowledge, this is the first study estimating work productivity and activity effects of omalizumab in a population with inadequately controlled severe and most severe JCP symptoms in Japan. On average, omalizumab could avoid productivity losses for ∼489.71 USD per patient in the severe JCP pollen period. Considering the large hypothetical patient population of 1.49 million patients, this relief of symptoms is meaningful on both a patient and societal level. Hence, a novel treatment for severe JCP symptoms could substantially benefit the Japanese society. However, the results for the entire patient population should be interpreted cautiously.
Due to the regulatory approval of omalizumab for JCP in December 2019 and its absence of real-world evidence, we considered the optimal use guideline for omalizumabCitation26 as a supporting source to estimate the patient population. Yet, in a real-world situation, physicians’ intention to prescribe (linked, e.g. to effectiveness and administration burden) and patient preference for uptake (related to factors such as out-of-pocket cost, insurance coverage, and waiting time) might influence the eligible patient population for omalizumabCitation30,Citation33 and, therefore, the number of eligible patients could change in a real world setting. The uncertainty regarding the patient population may increase in the future, especially against the background of further enhancement of allergen-specific immunotherapy (AIT). AIT has the potential to cure the disease and over the decades, the severe side effects associated with subcutaneous immunotherapy could be reduced by sublingual immunotherapy. AIT is indicated for patients for whom the diagnosis of the causative allergen has been established and especially recommended for patients who do not respond to general targeted drug therapy and can be expected to improve symptoms according to the Japanese guideline. It has been evaluated that AIT is cost-effective compared with pharmacotherapyCitation34. However, especially in Japan, it may be necessary to evaluate the persistence of the effect of AIT after the end of treatment (duration >3 years), indicating that accurate cost-effectiveness can be determined only when the duration of efficacy and remission rate after the completion of sublingual immunotherapy are clarifiedCitation35. Therefore, we aimed to analyze the potential effects of a novel drug, also given that pharmacotherapy is currently considered an earlier treatment option according to the Japanese guideline for AR.
Patient population
As the DSA shows, the number of patients influences the results the most. To determine the number of patients, we implemented the percentage of patients with severe and most severe JCP, a cedar-specific IgE score ≥3, and patients’ satisfaction with their current treatment and care-seeking behavior, as a starting point for determining the hypothetical patient population.
Prevalence was retrieved from a self-questionnaire asking for symptoms without a test on identified causative allergens, as suggested in the Japanese guidelines for allergic rhinitisCitation4. Thus, self-declaration of JCP prevalence might be inaccurate as it could cover AR due to other pollen or other acute respiratory infections. The findings of questionnaires might, therefore, include false-positive casesCitation36. Additionally, the JCP prevalence varies substantially depending on the regions across Japan and the year of the survey. We accounted for that in the sensitivity analysis, by varying the patient population by plus or minus 20 percent.
To reflect the real-world conditions of AR in Japan, we applied severity rates obtained with the PG-MARJ rather than referring to the Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines established as the international guideline for ARCitation37,Citation38. By doing this, we might have underestimated the percentage of patients with severe and most severe symptoms as Gotoh et al.Citation19 had shown that, compared to the PG-MARJ classification, the ARIA classification resulted in a higher percentage of patients with severe and most severe symptoms. Whereas a classification with the PG-MARJ resulted in 80% of patients having moderate or more severe rhinitis, the classification with the ARIA resulted in 90% of patients having moderate and severe symptoms. In general, severity could be subject to the reporting heterogeneity bias as it is defined based on the patient-reported symptoms and thus perceptions.
We estimated the number of patients with an IgE score ≥3 based on IgE scores of patients between 20 and 29 years old.Citation20 As the analysis of Sakashita et al.Citation39 had indicated that IgE scores might be higher in younger than in older patients, we might have overestimated the share of JCP patients with an IgE score ≥3. However, the allergen-specific IgE score is generally a marker for exposure, but no close relationship between the score and symptoms can be assumedCitation40. By referring to these assumptions, Nakamura et al.Citation20 have shown that although 78.8% of their participants were sensitive to cedar pollen according to their specific IgE score, only 41.2% of the participants reported symptoms during the pollen spreading period.
We assumed that patients who are not satisfied with their treatment demonstrated openness and willingness for a new therapy such as omalizumab. However, to get the recommendation and subcutaneous injection of omalizumab, patients need to visit a medical institution and manifest their dissatisfaction with the current treatments. Therefore, we applied the proportion of patients who visit a clinic for symptomsCitation19 as a proxy for the patient population, acknowledging the fact that we might not capture patient’s preferencesCitation30,Citation33.
Calculation period
The studies measuring the duration of the JCP pollen period have shown a duration of approximately eight weeks, with a peak of about three weeks, starting in the first or second week of MarchCitation5,Citation41,Citation42. However, the pollen period depends on the region and varies between years. We considered this uncertainty in the sensitivity analysis and examine data on pollen dispersal in Tokyo over the last five yearsCitation43 as Tokyo is the capital of Japan accounting for over 10% of its population.
Work productivity and activity impairment
By applying the HC approach to value work productivity and activity impairments, we did not account for multiplier effects or compensation mechanisms between co-workers. It is hard to estimate whether the HC approach leads to an over- or under-estimation of productivity and activity losses. Further, we implicitly assumed that our patient population presents the same work and activity distributions as the general Japanese population. As about 40% of the Japanese population is affected by JCP, this assumption seems reasonable. Nevertheless, we accounted for the uncertainty around the work distribution, by varying the working hours in the sensitivity analysis. In addition, there are different approaches to value unpaid work activities, namely the PG approach with either specialist or generalist wage and the opportunity cost approachCitation31,Citation32. In the sensitivity analysis, we reported the results obtained by applying these different approaches.
We acknowledge that the assumptions made might vary from the real-world situation and that the extrapolation of data collected in the phase III trial might be biased. Especially the self-reported productivity impairment may contain measurement error which might only be overcome by linking survey and register dataCitation44, which was not possible for this study. However, in the base case analysis, we used conservative input parameters, and, in the sensitivity analysis, we varied them to their lower and upper bounds. Our study suggests that add-on therapy with omalizumab improves the symptoms of severely and most severely affected JCP patients.
Since no previous study has investigated the impact of medical innovation on AR’s burden, comparing our results in the existing literature is challenging. A recent observational study assessing the burden of AR in Spain has estimated a paid work productivity loss of 3,120.45 USD per year per patientCitation45. We estimated that the avoided loss of productivity in terms of paid work per year and per patient would be 338.56 USD, equivalent to restored productivity of 14%. If we extrapolate this 14% to 100%, the paid productivity loss based on our calculation would result in a value of 2,418 USD per patient. The difference between our result and the one of Colás et al.Citation45 may be explained by the fact that we considered an exposure period of about three weeks whereas Colás et al.Citation45 have examined patients with perennial AR over a whole year.
Although omalizumab restores work and activity impairment by approximately 37%Footnoteiii, the clinical trial underlines that the impairment due to severe and most severe JCP symptoms is extreme. With a 37.10% overall work impairment and an overall activity impairment of 38.51%, the JCP impairment is similar to the one of psoriasis arthritisCitation46. Furthermore, even though the impairment due to JCP is limited to a few months, its influence on productivity is much more significant than the impairment due to more common diseases such as hypertension or diabetesCitation47.
Conclusions
In the present study, we estimated the effects of omalizumab on productivity in patients with inadequately controlled JCP symptoms. Our findings indicate that omalizumab might provide substantial benefits in avoiding paid and unpaid work productivity losses. In addition, our results suggest that unpaid work is of importance when estimating productivity losses due to JCP. This study shows that widespread diseases, such as the JCP, affect both the individual and the society. A comparison with diseases, such as hypertension, diabetes, and psoriasis arthritis, reveals that the implied burden of JCP is similar to the implied burden of these diseases. As from the epidemiological dataCitation48, a marked increase in incident of JCP being observed in younger age population in recent years, and therefore, the social burden of the JCP may influence the work productivity and activity impairment in the future. Our estimates highlight the importance of an adequate treatment for patients with severe JCP symptoms and underline that a better awareness of social impacts of JCP treatment options might help prescribers and enhance the availability of effective treatments to patients.
Transparency
Declaration of funding
This study was sponsored by Novartis.
Declaration of financial/other relationships
AI reports personal fees from Novartis Pharma Japan Inc. during the conduct of the study; grants and other from Gilead Sciences KK., grants from Intuitive Surgical GK., grants from Boston Scientific Japan Inc., grants and personal fees from Pfizer Japan Inc., grants from Beckton Dickinson and Company, grants from Milliman Inc., personal fees and other from Terumo corporation, personal fees from Chugai Pharmaceuticals Inc., personal fees from Astellas Pharma Inc., other from Fuji film Inc., other from CSL Behring Japan Inc., personal fees from Sanofi Japan Inc., personal fees from Takeda Pharmaceutical Inc., personal fees from Nippon Boeringer Ingelheim Inc., personal fees from Ono pharmaceutical Inc., personal fees from Novartis Pharma Japan Inc., personal fees from Eisai Inc., personal fees from Abbvie GK, personal fees from Sumitomo Dainippon Pharma Inc., personal fees from Ayumi Pharmaceutical Inc., personal fees from Medilead Inc., personal fees from Novo Nordisk Japan Inc., and personal fees from Taiho Pharm Inc. outside the submitted work.
KH reports personal fees from Novartis Pharma during the conduct of the study; personal fees from Tanabe Mitsubishi Pharm outside the submitted work.
MM, MK and FP are employees of WifOR, an independent economic research institute in Darmstadt. The authors indicate no conflicts of interest regarding the content of this article.
MF and HY are employed at Novartis Pharma K.K. (Tokyo, Japan) and declare no conflict of interest concerning this article.
HS works for Novartis Corporation Sdn. Bhd. (Selangor, Malaysia) and declares no conflict of interest with respect to this article.
MO reports personal fees from Novartis Pharma during the conduct of the study; personal fees from Kyorin Pharm Co., personal fees from Sanofi, grants, and personal fees from Tanabe Mitsubishi Pharm, and grants and personal fees from Taiho Pharm Co. outside the submitted work.
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Author contributions
MM and MK contributed equally to the conception, design, data analysis, and drafting of the paper. MM contributed to the final manuscript review. FP provided substantial feedback on the work, contributed to the interpretation of the data, and critically revised the manuscript.
Supplemental Material
Download MS Word (25.8 KB)Supplemental Material
Download MS Word (13.8 KB)Supplemental Material
Download MS Word (13.9 KB)Supplemental Material
Download MS Word (16.8 KB)Supplemental Material
Download MS Word (14 KB)Acknowledgements
None stated.
Notes
i In Japan, the PG-MARJ is used to assess the severity of JCP. The severity is classified based on its three major symptoms, i.e. sneezing, rhinorrhoea, and nasal blockage, which are divided into five grades: severest, severe, moderate, mild, and asymptomaticCitation3.
ii Besides assessing symptoms, serum allergen specific IgE antibody measurements enable a definite diagnosis of AR. Usually, the score ranges from 0 to 4–6Citation49. A score of 0 indicates a negative result and higher scores represent a higher level of IgECitation40.
iii This refers to the reduction of the overall work impairment from 37.10% to 23.49% and the reduction of the activity impairment from 38.51% to 24.35% (change of 13.61% and change of 14.16%, respectively, presented in ).
References
- Titulaer J, Arefian H, Hartmann M, et al. Cost-effectiveness of allergic rhinitis treatment: an exploratory study. SAGE Open Med. 2018;6:205031211879458.
- Baena-Cagnani CE. The global burden of asthma and allergic diseases: the challenge for the new century. Curr Allergy Asthma Rep. 2001;1(4):297–298.
- Okubo K, Kurono Y, Ichimura K, et al. Japanese guidelines for allergic rhinitis 2020. Allergol Int. 2020;69(3):331–345.
- Matsubara A, Sakashita M, Gotoh M, et al. Survey of allergic rhinitis in Japan 2019. Nippon Jibiinkoka Gakkai Kaiho Tokyo. 2020;123(6):485–490.
- Sasaki K, Okamoto Y, Yonekura S, et al. Cedar and cypress pollinosis and allergic rhinitis: quality of life effects of early intervention with leukotriene receptor antagonists. Int Arch Allergy Immunol. 2009;149(4):350–358.
- Yamada T, Saito H, Fujieda S. Present state of japanese cedar pollinosis: the national affliction. J Allergy Clin Immunol. 2014;133(3):632–639.e5.
- Okubo K, Okuda M. An internet survey of allergic rhinitis patients (written in Japanese). 2012;19(1):113–124.
- Vandenplas O, Vinnikov D, Blanc PD, et al. Impact of rhinitis on work productivity: a systematic review. J Allergy Clin Immunol Pract. 2018;6(4):1274–1286.e9.
- Lai T, Wang S, Xu Z, et al. Long-term efficacy and safety of omalizumab in patients with persistent uncontrolled allergic asthma: a systematic review and meta-analysis. Sci Rep. 2015;5(1):8191.
- Nishima S, Kozawa M, Milligan KL, et al. Omalizumab and unmet needs in severe asthma and allergic comorbidities in Japanese children. Asia Pac Allergy. 2019;9(1):e7.
- Okubo K, Ogino S, Nagakura T, et al. Omalizumab is effective and safe in the treatment of japanese cedar pollen-induced seasonal allergic rhinitis. Allergol. Int. 2006;55(4):379–386.
- Okubo K, Okano M, Sato N, et al. Add-On omalizumab for inadequately controlled severe pollinosis despite standard-of-Care: a randomized study. J Allergy Clin Immunol Pract. 2020;8(9):3130–3140.e2.
- Cardell L-O, Olsson P, Andersson M, et al. TOTALL: high cost of allergic rhinitis-a national Swedish population-based questionnaire study. NPJ Prim Care Respir Med. 2016;26(1):15082.
- Meltzer EO, Blaiss MS, Naclerio RM, et al. Burden of allergic rhinitis: allergies in America, Latin America, and Asia-Pacific adult surveys. Allergy Asthma Proc. 2012;33(5):113–141.
- Nishiike S, Ogino S, Irifune M, et al. Measurement of quality of life during different clinical phases of japanese cedar pollinosis. Auris Nasus Larynx. 2004;31(2):135–139.
- Kulthanan K, Chusakul S, Recto MT, et al. Economic burden of the inadequate management of allergic rhinitis and urticaria in asian countries based on the GA²LEN Model. Allergy Asthma Immunol Res. 2018;10(4):370–378.
- Zuberbier T, Lötvall J, Simoens S, et al. Economic burden of inadequate management of allergic diseases in the European Union: a GA(2) LEN review. Allergy. 2014;69(10):1275–1279.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics. 1993;4(5):353–365.
- Gotoh M, Yuta A, Okano M, et al. Severity assessment of Japanese cedar pollinosis using the practical guideline for the management of allergic rhinitis in Japan and the allergic rhinitis and its impact on asthma guideline. Allergol Int. 2013;62(2):181–189.,
- Nakamura S, Tsunoda S, Sakaida H, et al. Analysis of factors associated with cedar pollen sensitization and development of pollinosis in a young Japanese adult population. Allergol Int. 2019;68(1):39–45.
- E-Stat. Population Estimates by Age: February 1, 2020 (Final estimates). 2020; [cited 2020 Sep 29]. http://www.stat.go.jp/data/jinsui/pdf/202007.pdf.
- E-Stat. Basic Survey on Wage Structure in 2019 – Table 1 by job category-Amount of cash earnings, predetermined amount of earnings, annual bonuses, and other special earnings to be regularly paid by job category (industrial total). 2020; [cited 2020 Sep 29]. https://www.e-stat.go.jp/dbview?sid=0003084610.
- E-Stat. Summary tables, Time Series, Table 15 Labour force participation rate by age group for 2019. 2020; [cited 2020 Aug 29]. Available from: https://www.stat.go.jp/english/data/roudou/results/month/index.html.
- E-stat. Labour Force Survey Basic Aggregation All Prefectures Nationwide Monthly -Average monthly working days of employees by industry and occupation (January 2013-)-12th and 13th revised industry classification and December 2009 revised occupation classification, Table 2-11-2. 2020; [cited 2020 Sep 29]. Available from: https://www.e-stat.go.jp/dbview?sid=0003074700.
- Statistic Bureau of Japan. Survey on Time Use and Leisure Activities. 2016; [cited 2020 Sep 29]. Available from: https://www.stat.go.jp/english/data/shakai/index.html.
- Pharmaceuticals and Medical Devices Agency. Omalizumab Optimal Use Guideline (written in Japanese). 2019; [cited 2019 Nov]. Available from:https://www.pmda.go.jp/files/000232879.pdf.
- Pharmaceuticals and Medical Devices Agency. Omalizumab Summary of Application; [cited 2019 Nov 12]. Available from: https://www.pmda.go.jp/drugs/2019/P20191119001/index.html.
- Zhang W, Bansback N, Anis AH. Measuring and valuing productivity loss due to poor health: a critical review. Soc Sci Med. 2011;72(2):185–192.
- Reid MG. Economics of household production. New York: J. Wiley & Sons; 1934.
- Tada Y, Ishii K, Kimura J, et al. Patient preference for biologic treatments of psoriasis in Japan. J Dermatol. 2019;46(6):466–477.
- Brouwer WBF, Koopmanschap MA, Rutten FF. Productivity costs in cost-effectiveness analysis: numerator or denominator: a further discussion. Health Econ. 1997;6(5):511–514.
- Miranda V. Cooking, caring and volunteering: unpaid work around the world. OECD, OECD Social, Employment and Migration Working Papers 116, Sep 2011.
- Gelhorn HL, Balantac Z, Ambrose CS, et al. Patient and physician preferences for attributes of biologic medications for severe asthma. Patient Prefer. Adherence. 2019;13:1253–1268.
- Cox LS, Murphey A, Hankin C. The cost-effectiveness of allergen immunotherapy compared with pharmacotherapy for treatment of allergic rhinitis and asthma. Immunol Allergy Clin North Am. 2020;40(1):69–85. Feb.
- Okamoto Y. Review: prospects for sublingual immunotherapy for allergic rhinitis. Nippon Jibiinkoka Gakkai Kaiho. 2019;122(11):1381–1385.
- Yonekura S, Okamoto Y, Horiguchi S, et al. Effects of aging on the natural history of seasonal allergic rhinitis in middle-aged subjects in South chiba, Japan. Int Arch Allergy Immunol. 2012;157(1):73–80.
- Bousquet J, Schünemann HJ, Samolinski B, et al. Allergic Rhinitis and its Impact on Asthma (ARIA): achievements in 10 years and future needs. J Allergy Clin Immunol. 2012;130(5):1049–1062.
- Bousquet P, van Cauwenberge N. Khaltaev J. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(5 Suppl):S147–S334.
- Sakashita M, Hirota T, Harada M, et al. Prevalence of allergic rhinitis and sensitization to common aeroallergens in a Japanese population. Int Arch Allergy Immunol. 2010;151(3):255–261.
- Spickett G. Oxford handbook of clinical immunology and allergy, Fourth edition. Oxford (UK); New York (NY): Oxford University Press, 2020.
- Sasaki K, Ohshiro T, Sakio R, et al. Efficacy of seasonal allergic rhinitis using an 810 nm diode laser system: 810 nm diode laser for allergic rhinitis. Laser Ther. 2019;28(1):11–18.,
- Minami Y, et al. Comparison of quality of life between patients with Japanese sedar pollinosis ans atopic patients with the SF-8 health status questionnaire. Allergy. 2010;(64):451.
- Tokyo Metropolitan Institute of Public Health. Pollen Disperse Status 2019. 2020; [cited 2020 Sep 29]. Available from: http://www.tokyo-eiken.go.jp/.
- Böckerman P, Ilmakunnas P. The job satisfaction-productivity nexus: a study using matched survey and register data. ILR Rev. 2012;65(2):244–262.
- Colás C, Brosa M, Antón E, et al. Estimate of the total costs of allergic rhinitis in specialized care based on real-world data: the FERIN study. Allergy. 2017;72(6):959–966.
- Himmler S, Mueller M, Sherif B, et al. A case study applying a novel approach to estimate the social impact of a medical innovation – the use of secukinumab for psoriatic arthritis in Germany. Expert Rev. Pharmacoecon. Outcomes Res. 2019;2019:1–9.
- Lamb CE, Ratner PH, Johnson CE, et al. Economic impact of workplace productivity losses due to allergic rhinitis compared with select medical conditions in the United States from an employer perspective. Curr Med Res Opin. 2006;22(6):1203–1210.
- Nakae K, Baba K. Update on epidemiology of pollinosis in Japan: changes over the last 10 years: update on epidemiology of pollinosis in Japan. Clin. Exp. Allergy Rev. 2010;10(1):2–7.
- Lieberman P. and Anderson J. A., Eds., Allergic diseases: diagnosis and treatment, 3rd ed. Totowa (NJ): Humana; 2007.