?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objectives
Treatments for severe hypoglycemia aim to restore blood glucose through successful administration of rescue therapy, and choosing the most effective and cost-effective option will improve outcomes for patients and may reduce costs for healthcare payers. The present analysis aimed to compare costs and use of medical services with nasal glucagon and injectable glucagon in people with type 1 and 2 diabetes in Canada when used to treat severe hypoglycemic events when impaired consciousness precludes treatment with oral carbohydrates using an economic model, based on differences in the frequency of successful administration of the two interventions.
Methods
A decision tree model was prepared in Microsoft Excel to project outcomes with nasal glucagon and injectable glucagon. The model structure reflected real-world decision-making and treatment outcomes, based on Canada-specific sources. The model captured the use of glucagon, emergency medical services (EMS), emergency room, inpatient stay, and follow-up care. Costs were accounted for in 2019 Canadian dollars (CAD).
Results
Nasal glucagon was associated with reduced use of all medical services compared with injectable glucagon. EMS call outs were projected to be reduced by 45%, emergency room treatments by 52%, and inpatient stays by 13%. Use of nasal glucagon was associated with reduced direct, indirect, and combined costs of CAD 1,249, CAD 460, and CAD 1,709 per severe hypoglycemic event, respectively, due to avoided EMS call outs and hospital costs, resulting from a higher proportion of successful administrations.
Conclusions
When a patient with type 1 or type 2 diabetes is being treated for a severe hypoglycemic event when impaired consciousness precludes treatment with oral carbohydrate, use of nasal glucagon was projected to be dominant versus injectable glucagon in Canada reducing costs and use of medical services.
Introduction
Untreated severe hypoglycemia can result in cognitive impairment, loss of consciousness, seizure, coma, or deathCitation1. Moreover, severe hypoglycemia is associated with increased risk of cardiovascular disease and mortality over the longer term, with 5-year mortality found to be 3.4 times higher in patients with diabetes who reported severe hypoglycemia compared with those who reported no/mild hypoglycemiaCitation2,Citation3. Analysis of 15 trials conducted as part of an insulin clinical trial program suggests that severe hypoglycemic events are most common in people with type 1 diabetes receiving basal-bolus therapy, followed by patients with type 2 diabetes using multiple daily insulin injections, and a small number of events occur in people with type 2 diabetes receiving basal-oral therapyCitation4. Real-world studies have shown that severe hypoglycemia remains a challenge for people with diabetes in Canada. In a retrospective analysis of 552 people with diabetes (17% type 1 diabetes, 83% type 2 diabetes) treated with insulin and/or insulin secretagogues, severe hypoglycemia was reported by 41.8% of all respondents in the last year, at an average rate of 2.5 events per person-yearCitation5. Similar outcomes were observed in the Canadian cohort of the global Hypoglycemia Assessment Tool (HAT) studyCitation6. The study enrolled 498 patients with diabetes (37% type 1 diabetes, 63% type 2 diabetes) treated with insulin, and assessed hypoglycemic events retrospectively over 6 months and prospectively over 4 weeks. In the retrospective period, annualized event rates of severe hypoglycemia were 2.5 and 2.0 events per patient per year in those with type 1 and type 2 diabetes, respectively. Similarly, during the prospective period, 1.9 and 1.8 severe events per patient per year were observed in those with type 1 and type 2 diabetes, respectively.
The use of continuous glucose monitoring and flash glucose monitoring may provide opportunities for patients, particularly those receiving intensive insulin therapy, to reduce the frequency of severe hypoglycemia, but technology use does not eliminate the risk entirelyCitation7–9. Severe hypoglycemia represents a barrier to achieving good glycemic control in patients with diabetes, as in an effort to avoid hypoglycemia, patients may target higher blood glucose levels and thereby increase their risk of related complicationsCitation10,Citation11. Severe hypoglycemic events can result in significant medical resource use, such as emergency medical services (EMS) and treatment in the emergency room, and therefore an economic burden to healthcare payersCitation12,Citation13. The majority of severe hypoglycemic events occur outside of a healthcare setting. A survey conducted in 184 people with diabetes and 140 caregivers found that 87.1 and 87.7% of severe hypoglycemic events occurred at home in people with type 1 and type 2 diabetes, respectivelyCitation14.
Treatments for severe hypoglycemia aim to restore blood glucose to physiologic levels through the administration of a rescue therapy, namely oral carbohydrates, intravenous dextrose, or glucagonCitation15. Diabetes Canada guidelines recommend that severe hypoglycemia is treated with 20 g of oral carbohydrate if the patient is able to swallowCitation15. Patients who are unconscious, unable to swallow or tolerate oral carbohydrates, and have intravenous access are recommended to receive 10–25 g of glucose intravenously over 1–3 min, while administration of 1 mg of glucagon intramuscularly or subcutaneously is recommended in patients without intravenous accessCitation15. Glucagon treatments are particularly important for severe hypoglycemic events occurring outside of a healthcare setting, as patients will not have intravenous access. For administration by laypeople, injectable (intramuscular or subcutaneous) and intranasal glucagon are currently available in Canada for treatment of severe hypoglycemic events in insulin-treated people with diabetes when impaired consciousness precludes treatment with oral carbohydratesCitation15.
Administration of injectable glucagon is a multi-step process that requires reconstitution of glucagon powder using a syringe and needle, followed by injection of the glucagon solution into the affected personCitation16. The glucagon kit must be administered by a family member or acquaintance who may or may not have been trained to use the kit. Difficulties may arise during the administration of injectable glucagon, including handling difficulties (such as opening the package, sheath removal, mixing, bent needles), syringe separation and injection being aborted entirely, and injection of air or water onlyCitation17. These difficulties may be enhanced during the stressful emergency situation of a severe hypoglycemic event, particularly if training on the use of injectable glucagon was not completed recentlyCitation17.
Increasing the proportion of severe hypoglycemic events that are successfully treated may improve outcomes for people with diabetes, and may reduce the use of costly medical services, such as EMS. Nasal glucagon was developed to address this unmet needCitation18. Nasal glucagon contains glucagon powder in a ready-to-use, single-use, one-step dispensing device that, upon pressing a plunger, releases a 3 mg dose into the patient’s nostril where it is passively absorbed in the nasal mucosaCitation19,Citation20. The dispensed powder does not need to be inhaled and is therefore well-suited for administration in unconscious patients. The nasal glucagon device can be stored at temperatures up to 30 °C for 2 yearsCitation21. In a clinical trial of adults with type 1 diabetes, nasal glucagon was demonstrated to be non-inferior to injectable glucagon in terms of the percentage of patients successfully treated, where success was defined as an increase in plasma glucose to ≥70 mg/dL or an increase of ≥20 mg/dL from the glucose nadir, within 30 min of receiving study glucagon, without receiving additional actions to increase the plasma glucose levelCitation22. As neither reconstitution nor injection is necessary for the use of nasal glucagon, caregivers, family, friends or passers-by are able to treat a severe hypoglycemic event with greater success than with injectable glucagon. A simulation study demonstrated that 94% of instructed caregivers and 93% of non-instructed acquaintances successfully administered a full dose of nasal glucagon, compared with only 13% of caregivers and 0% of non-instructed acquaintances using injectable glucagonCitation23. Another simulated study found that successful administration was increased with nasal glucagon compared with injectable glucagon in both trained users (90.3% versus 15.6%) and untrained users (90.9% versus 0%), with users and people with diabetes who observed the study reporting feeling safer with nasal glucagon over injectable glucagonCitation24. In a real-world study of nasal glucagon in adults with type 1 diabetes, 96% of moderate and severe hypoglycemic events resolved within 30 min, and all patients with severe hypoglycemic events awakened or returned to normal status within 15 minCitation25. In a real-world study of nasal glucagon in children with type 1 diabetes, all moderate hypoglycemic episodes resolved within 30 min of administrationCitation26. In both real-world studies, no calls for EMS were made for any of the hypoglycemic events and ∼94% of caregivers reported overall satisfaction with the use of nasal glucagonCitation25,Citation26.
The aim of the present analysis was to compare cost and use of medical services with nasal glucagon and injectable glucagon in Canada when used to treat severe hypoglycemic events when impaired consciousness precludes treatment with oral carbohydrates using an economic model, based on differences in the frequency of successful administration of the two interventions.
Methods
Evaluation of cost-effectiveness
A cost-effectiveness analysis was prepared, assessing the incremental cost per a series of medical services resource use avoided with nasal glucagon versus injectable glucagon. These were:
Incremental cost per EMS call out avoided
Incremental cost per emergency room treatment avoided
Incremental cost per inpatient stay avoided
Incremental cost-effectiveness ratios (ICERs) can be used to express whether an intervention represents good value for money by combining cost and effectiveness outcomes. Where an intervention improves outcomes while reducing costs it is said to be dominant, and no ICER is calculated. Where applicable, ICERs in terms of the incremental cost per aspect of care avoided were calculated using the following formula:
Model structure
A decision tree model was developed in Microsoft Excel, reflecting the real-world decision-making and treatment outcomes that follow a severe hypoglycemic event when impaired consciousness precludes treatment with oral carbohydrates. The model structure was developed based on reviews of the published literature, treatment guidelines, and emergency care pathways. The model consists of 15 nodes to reflect the events and decisions that occur, and the model uses the same structure to evaluate outcomes for nasal glucagon and injectable glucagon ().
Figure 1. Model structure. The model uses the same structure to evaluate outcomes for nasal glucagon and injectable glucagon. A decision node representing the choice of glucagon kit is not shown. Treatment success was defined as successful administration of treatment, considered to be delivery of a full dose of nasal glucagon or injectable glucagon.
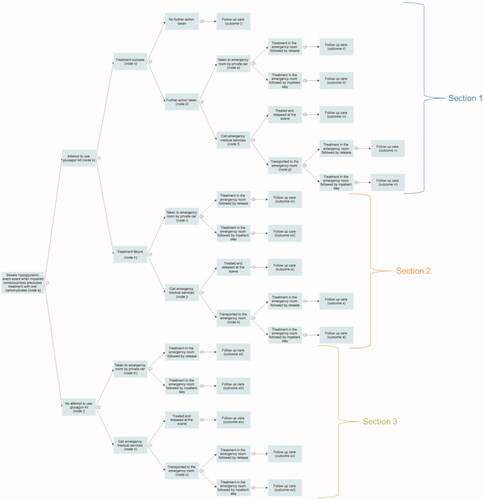
This network results in a total of 16 possible outcomes, each associated with different costs depending on the events and decisions that took place. Severe hypoglycemic events are acute in nature and the modeling analysis, therefore, took a short-term perspective, capturing the acute treatment following an event and follow-up care. No discounting was applied as outcomes were not projected over time horizons >1 year. Outcomes were assessed from a societal perspective, capturing both direct and indirect costs.
Decision probabilities
Decision probabilities were taken from the published literature, with Canadian-specific sources used wherever possible (). The only difference between the treatment arms was at node b, the probability of successful administration of glucagon. Data on successful administration of treatment with nasal glucagon and injectable glucagon were taken from a simulation studyCitation23. In the study, 16 instructed caregivers and 15 non-instructed acquaintances administered nasal glucagon and injectable glucagon to manikins, simulating unconscious people with diabetes during severe hypoglycemic episodes. With nasal glucagon, 15 caregivers (94%) and 14 acquaintances (93%) administered a full dose. One caregiver and one acquaintance did not successfully administer nasal glucagon because they did not fully depress the plunger on the device. With injectable glucagon, only two caregivers (13%) administered a full dose, and no acquaintances gave a full dose. All other decision probabilities were equivalent in the two arms and were taken from published sources (inputs applied in the base case are described below alongside alternative values applied in sensitivity analyses)Citation6,Citation23–29. For each model input, the most recent value from a large-scale Canadian study was used where ever possible.
Table 1. Treatment and decision probabilities.
Costs
The modeling analysis captured the direct costs of nasal glucagon, injectable glucagon, EMS call out, emergency room treatment, inpatient stay, follow-up with a healthcare professional, and self-monitoring of blood glucose testing ()Citation28,Citation30–32,Citation35–37.
Table 2. Resource use and costs associated with severe hypoglycemic events.
The modeling analysis also captured indirect costs. Despite the extensive review of the literature, the only robust sources that could be identified were those informing indirect costs relating to emergency room treatment and emergency room treatment followed by inpatient stay (). Therefore, a conservative approach was taken; indirect costs were applied for these two aspects of care only, and indirect costs for other aspects of care were not included (acquisition of glucagon, EMS treatment, an appointment with a healthcare professional, and acquisition of SMBG testing supplies). Total indirect costs for emergency room treatment and emergency room treatment followed by inpatient stay were comprised of the indirect cost identified by the OCCI and lost productivityCitation32. Time off work for an emergency room visit was assumed to be 5.19 h, based on a retrospective cohort study conducted in Alberta for a 5-year periodCitation33. Time off work for an emergency room treatment followed by inpatient stay was based on the mean length of stay identified in the OCCI for admissions related to hypoglycemia (5.3 days)Citation32. Lost productivity was calculated by applying the mean hourly wage in Canada to these time off work estimatesCitation34.
Sensitivity analyses
A series of sensitivity analyses were conducted, with alternative inputs applied at each node of the model (). Alternative inputs around treatment attempts, the success of treatment attempts, further action taken, and action following transport to the emergency room were considered in the sensitivity analyses and were sourced from published literatureCitation14,Citation24,Citation27,Citation28,Citation33,Citation38,Citation39.
Table 3. Summary of sensitivity analyses.
Results
Cost outcomes
Compared with the use of injectable glucagon, the use of nasal glucagon was associated with reduced direct, indirect, and combined costs of a severe hypoglycemic event when impaired consciousness precludes treatment with oral carbohydrate (). Nasal glucagon was associated with a higher drug cost by CAD 36.73, due to the higher acquisition cost compared with injectable glucagon. However, this was completely offset by direct costs savings due to avoided EMS call outs and hospital costs (emergency room treatment and inpatient stays). Cost savings were driven by a reduced frequency of hospitalization, with equal costs accrued in both the nasal glucagon and injectable glucagon arms when hospitalization occurred. Follow-up costs were equal with nasal glucagon and injectable glucagon. Overall, nasal glucagon was associated with direct cost savings of CAD 1,249 per severe hypoglycemic event compared with injectable glucagon. Further indirect cost savings versus injectable glucagon of CAD 460 were identified due to avoided emergency room treatments and inpatient stays. When direct and indirect costs were combined, nasal glucagon was associated with overall cost savings of CAD 1,709 per event compared with injectable glucagon.
Table 4. Mean cost of a severe hypoglycemic event when impaired consciousness precludes treatment with oral carbohydrate.
Effectiveness outcomes
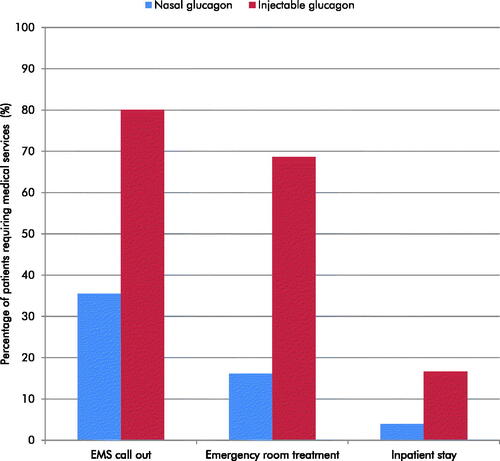
The use of nasal glucagon was associated with reduced use of all medical services compared with the use of injectable glucagon (). EMS call outs were reduced by 45% (36% versus 80%), emergency room treatments by 52% (16% versus 69%), and inpatient stays by 13% (4% versus 17%). Reduced use of medical services was driven by the higher rates of successful delivery of a full dose of nasal glucagon compared with injectable glucagon, supported by two usability studies, and therefore a reduced requirement for professional medical care.
Cost-effectiveness outcomes
Nasal glucagon was associated with reduced direct and combined costs, reduced frequency of EMS call out, emergency room treatment, and inpatient stay versus injectable glucagon. Therefore nasal glucagon was considered dominant versus injectable glucagon, reducing the frequency of EMS call out, emergency room treatment and inpatient stay with cost savings, and ICERs were not calculated.
Sensitivity analyses
Variation in the decision probabilities applied showed that nasal glucagon was dominant over injectable glucagon for treatment of a severe hypoglycemic event when impaired consciousness precludes treatment with oral carbohydrate was robust (). Nasal glucagon was associated with reduced costs versus injectable glucagon in all sensitivity analyses investigated. Furthermore, nasal glucagon was associated with reduced requirements for emergency room treatment and inpatient stays in all scenarios, and therefore was considered dominant in all sensitivity analyses.
Table 5. One-way and multi-way sensitivity analysis results.
Nasal glucagon was associated with equal EMS call out use in one scenario (analysis 9), when probabilities downstream from successful administration of a full dose of glucagon were varied (node c). When it was assumed that further action was taken in 100% of severe hypoglycemic events after successful administration of a full dose of glucagon, EMS use was equal, as the successful treatment of the severe hypoglycemic event did not result in a change in action from the caregiver. However, emergency room and inpatient stay use remained lower with nasal glucagon as it was assumed that the successful treatment of the severe hypoglycemic event would lead to reduced resource use. This assumption was based on real-world data showing that the majority of successfully treated severe hypoglycemic events resolve within 15 min, with median target EMS response times in urban, rural, and remote areas of 8, 20, and 40 min, respectivelyCitation25,Citation26,Citation40. Therefore severe hypoglycemic events are likely to have resolved either before the EMS arrive or before transportation to the emergency room has been initiated.
Discussion
Use of nasal glucagon for treatment of severe hypoglycemic events when impaired consciousness precludes treatment with oral carbohydrate was projected to result in reduced costs for healthcare payers and reduced use of medical services compared with the use of injectable glucagon. Cost savings and reduced use of medical services with nasal glucagon were driven by the higher proportion of successful, full-dose administrations and a corresponding reduction in professional medical care. Nasal glucagon was associated with overall cost savings of CAD 1,709 per severe hypoglycemic event when impaired consciousness precludes treatment with oral carbohydrates. The modeling analysis found that the use of nasal glucagon to treat severe hypoglycemic events was associated with reduced use of all medical services compared with the use of injectable glucagon. Nasal glucagon was dominant versus injectable glucagon, reducing the use of medical services and resulting in cost savings.
A key strength of the analysis is that it follows the treatment pathways after a severe hypoglycemic event when impaired consciousness precludes treatment with oral carbohydrates. The model was designed to reflect real-world decision-making and treatment outcomes in the context of a severe hypoglycemic event, capturing the subsequent events, and using Canadian-specific data wherever possible. This results in a highly relevant analysis, reflecting clinical practice in the Canadian setting. The U.S. Food and Drug Administration has recently approved two new formulations of injectable glucagon that do not require reconstitution before useCitation41,Citation42. These interventions have not yet been approved for use and are not currently under review by Health Canada. Therefore they were not included in the present analysis, as they are not a treatment option available to clinicians and patients. As new treatments for severe hypoglycemia become available in Canada, it will be important for updated cost-effectiveness analyses to capture all available treatment options. For such analyses to be accurate, additional head-to-head clinical data comparing ready-to-use injectable glucagon with nasal glucagon will be required.
The only difference between nasal glucagon and injectable glucagon in the base case analysis was the probability of successful administration of a full dose (node b), with all other parameters equal in both arms, including the probability of the resolution of a severe hypoglycemic event if the glucagon kit were administered successfully. This results in a fair analysis, with data sources to inform resource use following successful or unsuccessful treatment equal in both arms.
In the present analysis, cost-effectiveness was assessed only in patients experiencing a severe hypoglycemic event when impaired consciousness precludes treatment with oral carbohydrate who used a glucagon kit. However, it is likely that only a small proportion of the dispensed glucagon kits are used, and the cost of these unused kits will have an impact on the overall cost-effectiveness of glucagon therapies. A previously published budget impact analysis for the US setting aimed to capture the impact of unused kits on the overall cost at a population levelCitation43. This analysis found that nasal glucagon was associated with cost savings versus injectable glucagon even when a glucagon kit was not used in 75% of events meeting the criteria for treatment, despite a glucagon kit having been dispensed to the patient. The present analysis was focused on a Canadian healthcare payer perspective, rather than a budget impact analysis as used in the analysis for the US, but similar results could be expected in the Canadian setting. Appropriate prescription to patients at risk of severe hypoglycemic events, followed by appropriate education and training is key to maximizing the cost-effectiveness of nasal glucagon. Kits will also be unused if a device is considered difficult to use or intimidating by caregivers and acquaintances. In the Yale et al. study, 81% of caregivers and 100% of acquaintances were reported as recommending nasal glucagon over injectable glucagonCitation23. A real-world study has shown that in 100% of events, caregivers of adults with diabetes agreed that nasal glucagon would be less intimidating for caregivers than injectable glucagon, while an equivalent study found that in 96.9% of events, caregivers of children with diabetes agreed with this statementCitation25,Citation26. Kits will also be unused if unavailable at the time of the severe hypoglycemic episode, such as severe hypoglycemia occurring outside the home or workplace. The easy portability of a nasal glucagon kit due to the small size and room temperature storage may result in greater availability of nasal glucagon versus injectable glucagon. Currently, it is not possible to accurately quantify the proportion of glucagon kits that are unused when a severe hypoglycemic event occurs, provide reasons why they were not used, and how these could change due to the introduction of nasal glucagon. As such, this represents an area for future research in order to conduct more accurate cost-effectiveness analyses.
Numerous studies have shown that hypoglycemic events have a substantial impact on quality of lifeCitation44–47. However, to date, no study has investigated the impact of the resolution of severe hypoglycemia on quality of life. It is likely that earlier resolution and avoidance of treatment by the EMS, in the emergency room, and hospitalization are associated with improved quality of life, but additional studies are required to quantify this. The modeling analysis did not capture mortality, as the costs accrued would depend on when this occurred in the model flow diagram (), which is difficult to quantify. However, improved treatment of severe hypoglycemia with nasal glucagon may likely result in reduced mortality.
Conclusions
When a patient with type 1 or type 2 diabetes is being treated for a severe hypoglycemic event when impaired consciousness precludes treatment with oral carbohydrate, the use of nasal glucagon was projected to reduce costs for healthcare payers. The higher likelihood of successful administration with nasal glucagon was projected to reduce EMS call outs, emergency room treatments, and inpatient stays in the modeling analysis. Nasal glucagon was projected to be dominant versus injectable glucagon, reducing the use of medical services and resulting in cost savings.
Transparency
Declaration of funding
The study was funded by Eli Lilly and Company.
Declaration of financial/other relationships
JFY has received consulting fees for participation in advisory boards and giving lectures from Eli Lilly and Company Limited and Novo Nordisk Canada Inc. BO is an employee of Eli Lilly and Company Limited. BM is an employee of Eli Lilly and Company. DM is an employee of Eli Lilly Canada Inc. BH and WV are employees of Ossian Health Economics and Communications. Ossian received consulting fees from Eli Lilly and Company Limited to support the preparation of the analysis. GS was an employee of Eli Lilly Canada Inc. when the study was conducted. MJ was an employee of Eli Lilly Canada Inc. when the study was conducted. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors designed the study. Data from previously published studies were identified by GS. The analysis was performed by BH. All authors interpreted the results. The manuscript was drafted by BH and revised critically for important intellectual content by all other authors. All authors have approved the final version of the manuscript, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgements
No assistance in the preparation of this article is to be declared.
References
- Cryer PE. Hypoglycemia, functional brain failure, and brain death. J Clin Invest. 2007;117(4):868–870.
- Goto A, Arah OA, Goto M, et al. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ. 2013;347(jul29 3):f4533–f4533.
- McCoy RG, Van Houten HK, Ziegenfuss JY, et al. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care. 2012;35(9):1897–1901.
- Heller SR, Frier BM, Hersløv ML, et al. Severe hypoglycaemia in adults with insulin-treated diabetes: impact on healthcare resources. Diabet Med. 2016;33(4):471–477.
- Ratzki-Leewing A, Harris SB, Mequanint S, et al. Real-world crude incidence of hypoglycemia in adults with diabetes: results of the InHypo-DM study, Canada. BMJ Open Diabetes Res Care. 2018;6(1):e000503.
- Aronson R, Goldenberg R, Boras D, et al. The canadian hypoglycemia assessment tool program: insights into rates and implications of hypoglycemia from an observational study. Can J Diabetes. 2018;42(1):11–17.
- Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–1640.
- Hermanns N, Heinemann L, Freckmann G, et al. Impact of CGM on the management of hypoglycemia problems: overview and secondary analysis of the HypoDE study. J Diabetes Sci Technol. 2019;13(4):636–644.
- Messaaoui A, Tenoutasse S, Crenier L. Flash glucose monitoring accepted in daily life of children and adolescents with type 1 diabetes and reduction of severe hypoglycemia in real-Life use. Diabetes Technol Ther. 2019;21(6):329–335.
- International Hypoglycaemia Study Group. Minimizing hypoglycemia in diabetes. Diabetes Care. 2015;38(8):1583–1591.
- Wild D, von Maltzahn R, Brohan E, et al. A critical review of the literature on fear of hypoglycemia in diabetes: implications for diabetes management and patient education. Patient Educ Couns. 2007;68(1):10–15.
- Foos V, Varol N, Curtis BH, et al. Economic impact of severe and non-severe hypoglycemia in patients with type 1 and type 2 diabetes in the United States. J Med Econ. 2015;18(6):420–432.
- Meneghini LF, Lee LK, Gupta S, et al. Association of hypoglycaemia severity with clinical, patient-reported and economic outcomes in US patients with type 2 diabetes using basal insulin. Diabetes Obes Metab. 2018;20(5):1156–1165.
- Mojdami D, Peyrot M, Spaepen E, et al. 115 – Conversations and reactions around severe hypoglycemia (CRASH study): Canadian results. Can J Diabetes. 2019;43(7):S40.
- Diabetes Canada Clinical Practice Guidelines Expert Committee; Yale JF, Paty B, Senior PA. 2018 Clinical practice guidelines hypoglycemia. Can J Diabetes. 2018;42(Supplement 1):S104–S108.
- Eli Lilly Canada Inc. GLUCACON (rDNA Origin) Product Monograph [cited 2020 Apr 3]. Available from: https://www.lilly.ca/en/pdf/consumer-information/04_rglucagon-ci_9july2012.pdf
- Harris G, Diment A, Sulway M, et al. Glucagon administration – underevaluated and undertaught. Pract Diab Int. 2001;18(1):22–25.
- Pontiroli AE. Intranasal glucagon: a promising approach for treatment of severe hypoglycemia. J Diabetes Sci Technol. 2015;9(1):38–43.
- Eli Lilly Canada Inc. BAQSIMI™ glucagon nasal powder Product Monograph [cited 2020 Apr 3]. http://pi.lilly.com/ca/baqsimi-ca-pm.pdf
- Sherr JL, Ruedy KJ, Foster NC, et al. Glucagon nasal powder: a promising alternative to intramuscular glucagon in youth with type 1 diabetes. Diabetes Care. 2016;39(4):555–562. ;.
- Pack BW, Melnick JP, Breen CC, et al. (IP11) Portability of nasal glucagon for the rescue of severe hypoglycemia: Stability and performance when exposed to temperature extremes. Association of Diabetes Care & Education Specialists. 2021 Annual Conference; Virtual; August 12–15; 2021.
- Rickels MR, Ruedy KJ, Foster NC, et al. Intranasal glucagon for treatment of insulin-induced hypoglycemia in adults with type 1 diabetes: a randomized crossover noninferiority study. Diabetes Care. 2016;39(2):264–270.
- Yale JF, Dulude H, Egeth M, et al. Faster use and fewer failures with needle-free nasal glucagon versus injectable glucagon in severe hypoglycemia rescue: a simulation study. Diabetes Technol Ther. 2017;19(7):423–432.
- Settles J, Child CJ, Bajpai SK, et al. 13-LB: nasal vs. injected glucagon: user experience results of a simulated severe hypoglycemia study. Diabetes. 2019;68(Supplement 1):13-LB.
- Seaquist ER, Dulude H, Zhang XM, et al. Prospective study evaluating the use of nasal glucagon for the treatment of moderate to severe hypoglycaemia in adults with type 1 diabetes in a real-world setting. Diabetes Obes Metab. 2018;20(5):1316–1320.
- Deeb LC, Dulude H, Guzman CB, et al. A phase 3 multicenter, open-label, prospective study designed to evaluate the effectiveness and ease of use of nasal glucagon in the treatment of moderate and severe hypoglycemia in children and adolescents with type 1 diabetes in the home or school setting. Pediatr Diabetes. 2018;19(5):1007–1013.
- Sinclair JE, Austin M, Froats M, et al. Characteristics, prehospital management, and outcomes in patients assessed for hypoglycemia: repeat access to prehospital or emergency care. Prehosp Emerg Care. 2019;23(3):364–376.
- Statistics Canada. Families, households and marital status: key results from the 2016 Census [cited 2019 Jun 4]. Available from: https://www150.statcan.gc.ca/n1/daily-quotidien/170802/dq170802a-eng.htm
- Rowe BH, Singh M, Villa-Roel C, et al. Acute management and outcomes of patients with diabetes mellitus presenting to Canadian emergency departments with hypoglycemia. Can J Diabetes. 2015;39:9–18.
- Ontario Drug Benefit Formulary/Comparative Drug Index [cited 2019 Jun 4]. Available from: https://www.formulary.health.gov.on.ca/formulary/results.xhtml?q=glucagon&type=1
- Toronto Paramedic Service. Annual report 2017 [cited 2019 Jun 4]. Available from: https://www.toronto.ca/wp-content/uploads/2018/03/9730-Toronto-Paramedic-Services-Annual-Report-2017-sm.pdf
- Government of Ontario. Ontario Case Costing Initiative (OCCI) [cited 2019 Jun 4]. Available from: https://www.ontario.ca/data/ontario-case-costing-initiative-occi
- Alexiu CJ, Chuck A, Jelinski SE, et al. Presentations for hypoglycemia associated with diabetes mellitus to emergency departments in a Canadian province: a database and epidemiological analysis. Diabetes Res Clin Pract. 2017;130:229–236.
- Statistics Canada. Labour force survey estimates (LFS), wages of employees by type of work, National Occupational Classification for Statistics (NOC-S), sex and age group, unadjusted for seasonality [cited 2019 Jun 4]. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1410006101&pickMembers%5B0%5D=1.1&pickMembers%5B1%5D=2.2&pickMembers%5B2%5D=3.1&pickMembers%5B3%5D=5.1&pickMembers%5B4%5D=6.1
- Ministry of Health and Long Term Care. Schedule of benefits [cited 2021 Jun 28]. Available from: http://www.health.gov.on.ca/en/pro/programs/ohip/sob/physserv/sob_master20181115.pdf
- Ontario Drug Benefit Formulary/Comparative Drug Index [cited 2019 Jun 4]. Available from: https://www.formulary.health.gov.on.ca/formulary/results.xhtml?q=glucose%2Bstrip&type=2
- Canadian Agency for Drugs and Technologies in Health. Guidance document for the costing of health care resources in the Canadian setting. 2nd ed. [cited 2019 Jun 4]. Available from: https://www.cadth.ca/guidance-document-costing-process-2e
- Daneman D, Frank M, Perlman K, et al. Severe hypoglycemia in children with insulin-dependent diabetes mellitus: frequency and predisposing factors. J Pediatr. 1989;115(5 Pt 1):681–685.
- Spaic T, Peddle M, Mahon J, et al. Emergency medical services (EMS) assist-requiring hypoglycemia in diabetes patients. Can J Diabetes. 2016;40(5):S12.
- Alberta Health Services. Quarterly Emergency Medical Services Dashboard [cited 2019 Jun 4]. Available from: https://www.albertahealthservices.ca/assets/about/ems/ahs-ems-dashboard.pdf
- Food and Drug Administration. ZEGALOGUE (dasiglucagon) injection, for subcutaneous use: Prescribing information [cited 2022 Jan 16]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/214231Orig1s000lbl.pdf
- Food and Drug Administration. GVOKE (glucagon) injection, for subcutaneous use: prescribing information [cited 2022 Jan 16]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/212097Orig1s000lbl.pdf
- Pöhlmann J, Mitchell BD, Bajpai S, et al. Nasal glucagon versus injectable glucagon for severe hypoglycemia: a cost-offset and budget impact analysis. J Diabetes Sci Technol. 2019;13(5):910–918.
- Harris S, Mamdani M, Galbo-Jørgensen CB, et al. The effect of hypoglycemia on health-related quality of life: Canadian results from a multinational time trade-off survey. Can J Diabetes. 2014;38(1):45–52.
- Currie CJ, Morgan CL, Poole CD, et al. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin. 2006;22(8):1523–1534.
- Davis RE, Morrissey M, Peters JR, et al. Impact of hypoglycaemia on quality of life and productivity in type 1 and type 2 diabetes. Curr Med Res Opin. 2005;21(9):1477–1483.
- Evans M, Khunti K, Mamdani M, et al. Health-related quality of life associated with daytime and nocturnal hypoglycaemic events: a time trade-off survey in five countries. Health Qual Life Outcomes. 2013;11:90.