Abstract
Aims
To support reimbursement requests in Canada, we evaluated the cost-effectiveness of brentuximab vedotin (Adcetris) in combination with cyclophosphamide, doxorubicin, and prednisone (A + CHP) compared with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) as frontline treatment for CD30-expressing peripheral T-cell lymphomas (PTCLs) using results from the ECHELON-2 clinical trial. The PTCL subtypes included were systemic anaplastic large cell lymphoma (sALCL), PTCL–not otherwise specified (PTCL-NOS), and angioimmunoblastic T-cell lymphoma (AITL).
Materials and methods
A partitioned survival model consisting of three health states (progression-free survival [PFS], post-progression survival [PPS], and death) was constructed from the perspective of the Canadian publicly funded healthcare system over a lifetime horizon. Efficacy, safety, and health-related quality-of-life (HRQoL) data were obtained from ECHELON-2. Medical resource use and costs were derived from Canadian literature and standard sources. Incremental cost-effectiveness ratios (ICERs) per life-years (LYs) and quality-adjusted life-years (QALYs) gained were calculated. Sensitivity analyses were performed to account for uncertainty in key parameters. All costs are reported in Canadian dollars.
Results
A + CHP, when compared with CHOP, was associated with an estimated mean gain of 2.90 LYs and 2.38 QALYs and a mean incremental cost of $76,491. The ICER for A + CHP compared with CHOP was estimated at $26,340 per LY gained and $32,177 per QALY gained. In sensitivity analyses, the ICERs remained below $60,000 per QALY gained. Time horizon, patient starting age, and discount rate affected the results, as the ICER was driven by long-term survival gains observed with A + CHP compared with CHOP.
Limitations
Real-world downstream treatments (such as stem cell transplantation) may differ from the treatment protocol followed in the ECHELON-2 trial.
Conclusions
A + CHP compared with CHOP provides a cost-effective treatment option with improved clinical outcomes that are clinically relevant and a comparable safety profile for adults with previously untreated CD30-expressing sALCL, PTCL-NOS, or AITL in Canada.
Introduction
Peripheral T-cell lymphomas (PTCLs), categorized as a group of non-Hodgkin lymphomas (NHLs), are uncommon, aggressive hematologic tumors that originate from mature T-cellsCitation1,Citation2. In North America, the most common subtypes are PTCL–not otherwise specified (PTCL-NOS), angioimmunoblastic T-cell lymphoma (AITL), systemic anaplastic large cell lymphoma (sALCL), anaplastic lymphoma kinase (ALK)-negative, and sALCL ALK-positive; these subtypes compose over one-half of PTCL casesCitation3,Citation4. In 2020, an estimated 10,400 Canadians were diagnosed with NHLCitation5. Subtypes of PTCL represent approximately 7–15% of all NHL cases, and PTCL incidence is estimated at 0.72 cases per 100,000 per year in CanadaCitation6.
Patients with PTCL commonly present with advanced disease and have a poor prognosis even with aggressive chemotherapy treatmentCitation1,Citation3. Although frontline treatment with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or a CHOP-like regimen was previously recommended for the treatment of nodal PTCL subtypes (i.e. sALCL, PTCL-NOS, or AITL)Citation7, progression-free survival (PFS) and overall survival (OS) rates are disappointing, with fewer than 50% of patients surviving for 5 yearsCitation3. The addition of active therapies to a CHOP regimen have generally shown only small improvements in response rates, with limited PFS benefit potentially due to increased toxicityCitation8.
Brentuximab vedotin (Adcetris; Seagen Inc.; Bothell, WA) is a CD30-directed antibody-drug conjugate recommended in Canada for certain patients with sALCL and Hodgkin lymphoma after failure of at least one multidrug regimen or after stem cell transplant (SCT)Citation9–14. Recently, Health Canada approved brentuximab vedotin in combination with cyclophosphamide, doxorubicin, and prednisone (A + CHP) for the treatment of previously untreated adult patients with sALCL, PTCL-NOS, or AITL whose tumors express CD30Citation15. Although the expression of CD30 varies by PTCL subtype, among patients with sALCL, PTCL-NOS, or AITL, CD30 expression is estimated at 59–100%Citation16. As of mid-2021, CD30 expression in PTCL had no prognostic significance, and no predictive value was established due to the very limited use of anti-CD30 targeted therapyCitation16.
The clinical efficacy of A + CHP compared with CHOP for previously untreated CD30-expressing PTCL was investigated in ECHELON-2 (ClinicalTrials.gov NCT01777152), a randomized, placebo-controlled, active-comparator phase 3 trialCitation17. In the ECHELON-2 trial, 97.8% (442 out of 452) of the trial population was diagnosed with CD30-expressing sALCL, PTCL-NOS, or AITL. As previously reported, 3-year PFS in patients receiving A + CHP was 57.1% (49.9–63.7%) versus 44.4% (37.6–50.9%) in patients receiving CHOP. Although median OS was not reached for either treatment group at a median follow-up of 42.1 (95% CI = 40.4–43.8) months, 23% of patients randomized to A + CHP had died compared with 32% of patients randomized to CHOP. Clinically meaningful improvement in PFS and OS continued with A + CHP versus CHOP at 5 years. In the ECHELON-2 5-year update, 5-year PFS rates were 51.4% (95% CI = 42.8–59.4%) with A + CHP compared with 43.0% (95% CI = 35.8–50.0%) with CHOP (hazard ratio [HR] = 0.70, 95% CI = 0.53–0.91, p = 0.0077), at a median follow-up of 47.6 (range = 0.03–83.98) monthsCitation18. ECHELON-2 remains the only frontline PTCL trial to demonstrate an OS benefit, where the overall risk of death was reduced by 34% in patients receiving A + CHP compared with those receiving CHOP (HR = 0.66, 95% CI = 0.46–0.95, p = 0.0244). In the subset of patients with sALCL, approximately 60% remained in remissionCitation19. Adverse events (AEs) were similar and manageable in both groups, with resolution or improvement of peripheral neuropathy reported at 5 yearsCitation17,Citation19.
Results from cost-effectiveness analyses suggest brentuximab vedotin is a cost-effective treatment option compared with conventional chemotherapy in patients with relapsedCitation17,Citation19 or refractory sALCL from the United Kingdom (UK) National Health Service perspectiveCitation20 and compared with CHOP as frontline therapy for CD30-expressing PTCLs from a United States (US) healthcare payer perspectiveCitation21. To support reimbursement requests in Canada, we estimated from the perspective of the Canadian publicly funded healthcare system the cost-effectiveness of A + CHP relative to CHOP for the treatment of previously untreated adult patients with sALCL, PTCL-NOS, or AITL whose tumors express CD30.
Methods
Patient population
The model patient population included previously untreated adult patients with sALCL, PTCL-NOS, or AITL whose tumors express CD30, consistent with the PTCL patient population approved for frontline A + CHP use in Canada. This population is aligned with the majority of the ECHELON-2 trial population, which included patients (N = 452) with sALCL (n = 316, 69.9%), PTCL-NOS (n = 72, 15.9%), AITL (n = 54, 11.9%), and other PTCL (n = 10, 2.2%)Citation17.
Model overview
A three-health state model was used to follow patients from active treatment through progression until death (). A partitioned survival approach was used to calculate the proportion of patients in each of three health states – PFS, post-progression survival (PPS), and death – over time. Costs in Canadian dollars and utilities were computed based on the proportion of patients in each health state at each model cycle. As the model progressed cycle by cycle to the time horizon, cost and utility data were summed for each treatment arm, allowing differences to be calculated in accumulated costs and effectiveness between comparators at model completion.
Figure 1. Model diagram of survival partition approach. Dotted lines represent the transitions between health states that are not directly tracked; instead, proportions of patients in each health state are calculated through the partition approach at each time point. Abbreviations. OS, overall survival; PFS, progression-free survival; PPS, post-progression survival.
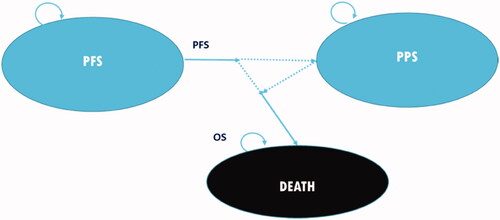
The model used a 3-week cycle length and a lifetime horizon (45 years, by which >99% of patients were in the “death” state) to fully demonstrate the long-term clinical and economic impacts of treatment. This time horizon aligns with the Canadian Agency for Drugs and Technologies in Health (CADTH) guidelinesCitation22, where a lifetime horizon is preferred for an oncology economic evaluation. The effect of applying shorter time horizons (15 and 30 years) on the modeled results was explored in sensitivity analyses.
Costs and health-related outcomes in the base case analysis were discounted at 1.5% per annum, as recommended by CADTH guidelines for economic evaluationsCitation22. In addition, sensitivity analyses using discounting rates of 0% and 3% were conducted. The base case analysis takes the perspective of the Canadian publicly funded healthcare system (). The data obtained from the ECHELON-2 trial are all based on the subgroup of patients with sALCL, PTCL-NOS, or AITLCitation23. The analyses considered direct medical costs and did not include indirect costs.
Table 1. Model settings/specifications for the base case analyses.
Model inputs
Extrapolation of PFS and OS
Survival extrapolation for PFS and OS, derived from observed data from ECHELON-2, was used in the base case following recommendations by the National Institute of Clinical Excellence (NICE) Decision Support UnitCitation24. The analysis was based on the ECHELON-2 trial results from a median follow-up of 36.2 months for PFS and 42.1 months for OS. Six parametric distributions (i.e. exponential, Weibull, Gompertz, log-logistic, log-normal, and generalized gamma) were fitted to model OS and PFS. The best-fitting distributions were selected based on statistical goodness of fit (assessed by Akaike and Bayesian information criteria both), clinical plausibility of the long-term projections, and input from Canadian clinician experts.
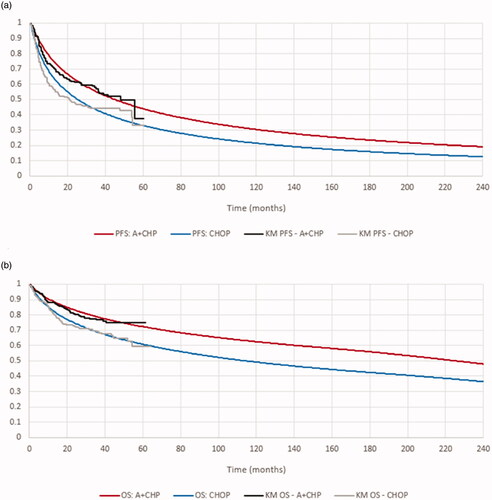
Based on these factors, the log-normal distribution was applied in the base case to project long-term PFS and OS for the A + CHP and CHOP arms (). Long-term OS projections in the model were bound to ensure that the mortality rate in modeled patients was never lower than the Canadian general mortality rate of a similarly aged population. The Kaplan–Meier (KM) curves and fitted distributions in the modeled subgroup for PFS are presented in and OS (adjusted to account for general mortality) in .
Figure 2. The Kaplan–Meier curves and fitted distributions for PFS and OS in the modeled subgroup. (a) Progression-free survival for A + CHP and CHOP–base case. (b) Overall survival for A + CHP and CHOP–base case.* Source. ECHELON-2 trial (sALCL, PTCL-NOS, and AITL subgroup).Citation17 *Adjusted to account for general mortality. Abbreviations. A + CHP, brentuximab vedotin in combination with cyclophosphamide, doxorubicin, and prednisone; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; KM, Kaplan–Meier; OS, overall survival; PFS, progression-free survival.
Subsequent treatment and consolidative stem cell transplant
Twenty-six percent of patients in the A + CHP arm and 41% in the CHOP arm were estimated to receive subsequent systemic therapy in the modelCitation23. Among patients in ECHELON-2 with sALCL, PTCL-NOS, or AITL, consolidative SCT was received by 49 (22.2%) patients in the A + CHP group and 39 (17.6%) patients in the CHOP group following frontline treatmentCitation23. The SCT rates, which may reflect the patients recruited in the ECHELON-2 trial (i.e. with 22% ALK + sALCL patients, who are less likely to receive SCT compared with other PTCL subtypes), were similar to those of registry reportsCitation25,Citation26 and verified with Canadian clinicians; sensitivity analyses were also performed on the SCT rates for both treatment groups. The clinical efficacy of mixed subsequent treatments and SCT was implicitly captured by the PFS and OS outcomes in the ECHELON-2 trial; the costs associated with subsequent treatments and SCT procedures were separately considered in the analysis.
Adverse events
The model considered the effect of treatment-related AEs on costs and quality of life. Grade 3 and 4 AEs reported in at least 5% of patients in either the A + CHP or the CHOP arm in ECHELON-2 were included (). This inclusion rule was considered appropriate by clinicians and sufficient to capture AEs that would affect patients who receive brentuximab vedotin in a real-world setting.
Table 2. Incidence of treatment-related adverse events in ECHELON-2.
Utilities
Utility values associated with health states and safety events in the model were derived from quality-of-life data collected in ECHELON-2 using the US value set for the EQ-5D-3L instrumentCitation27. Average pre- and post-progression utilities were estimated using a random intercept repeated-measures mixed-effects model for repeated measures of the same patient. Utilities for A + CHP and CHOP were not significantly different for patients in the same health state (PFS or PPS) following adjustments for baseline utilities between the two arms; as a result, utilities were not further stratified by treatment. Patients in the PFS state were assigned a utility value of 0.83, and patients in the PPS state were assigned a utility value of 0.79. In the model, quality-adjusted life-years (QALYs) were estimated as the proportion of patients in each health state in a particular cycle multiplied by the utility weights for each of these states, then multiplied again by the proportion of a year represented by the cycle.
Medical resource use and costs
Disease- and treatment-related costs were applied for each health state and event in the model. The model included costs for drug acquisition and administration (applied for the duration of active treatment and determined by dosing regimen from clinical trials), medical resource use during specific disease stages, SCTs, management of AEs, and terminal care. Routine medical resource use costs and one-off costs for terminal care were based on published studies. Unit costs regarding drug acquisition, drug administration, and AE management were based on standard costing sources in Ontario, Canada. All cost inputs were in Canadian dollars.
Drug acquisition costs
Unit drug acquisition costs are presented in . The unit cost of brentuximab vedotin was based on the final recommendation from the pan-Canadian Oncology Drug Review (pCODR) Expert Review Committee (pERC)Citation28. Treatment costs used in the model were based on dosing regimens derived from the ECHELON-2 trialCitation17,Citation28. The treatment cost was computed based on the mean number of treatment cycles received in ECHELON-2 (6.0 for A + CHP and 5.8 for CHOP) and applied as a one-time cost in the first cycle. For therapies that use weight-based or body surface area (BSA)-based dosing, ECHELON-2-specific values were used in the base case analysis: an average weight of 74.40 kg and a BSA of 1.80/m2. Drug wastage was accounted for by rounding up to the next full single-use vial size for each dose administered.
Table 3. Drug acquisition costs.
To reduce the risk of febrile neutropenia, prophylactic medications such as filgrastim and pegfilgrastim may be administered to patients. Per Canadian clinicians’ input, prophylaxis is used mostly in older patients. Therefore, it was assumed that 31% of patients – the percentage of patients 65 years of age or older in ECHELON-2 – received prophylaxis both in the A + CHP and CHOP arms. Also, given the reimbursement status for febrile neutropenia prophylaxis, it was assumed that filgrastim (5 mcg/kg/day intravenous [IV] for up to 2 weeks, beginning with Cycle 1) was used by 90% of patients receiving prophylaxis, whereas 10% of patients received pegfilgrastim (6 mg subcutaneous [SC] once per chemotherapy cycle, beginning with Cycle 1)Citation29,Citation30.
Subsequent treatment costs
A proportion of patients who progressed following frontline treatments were assumed to receive a mixture of chemotherapies as subsequent treatments in the model; based on results from the ECHELON-2 trial, 26% of patients receiving A + CHP and 41% of patients receiving CHOP were assumed to receive subsequent treatmentsCitation23.
Drug administration costs
Administration of IV and SC treatments required an outpatient visit that may have included additional nursing and pharmacist preparation time. Based on the Ontario Health Insurance PlanCitation31, a cost of $54 was applied to each IV and $4 to each SC administration.
SCT and terminal care costs
The aggregate costs of SCTs were sourced from the Ontario Ministry of Health and Long-Term CareCitation32; the cost for autologous SCT was $67,693. One-off terminal care costs were derived from Walker et al.Citation33, which reported an average end-of-life care cost per patient for lymphoma/leukemia of $28,580 in 2002–2003 Canadian dollars, which equated to $35,783 following inflation to 2019 Canadian dollars. SCT costs and terminal care costs were assumed to be the same regardless of prior treatment received.
Routine follow-up care costs
Routine follow-up care costs were calculated using the aggregate cost per model cycle and the time patients stayed in each health state. The same cost per cycle was assumed for patients in the A + CHP and CHOP arms. Due to the paucity of data in the PTCL patient population, information for another type of aggressive NHL – diffuse large B-cell lymphoma (DLBCL) – was used as a proxy based on inputs from Canadian clinicians. Per-cycle costs were derived from 6-month aggregate costs in the patient record from a retrospective cohort of patients with DLBCL diagnosed in British Columbia during 2004–2013Citation34. The per-cycle costs beyond the record period (60 months) were assumed to be the same as the costs incurred during the last 6-month period. Per clinicians’ input, patients were assumed to incur resource use during the first 5 years in PFS and throughout PPS.
Adverse event costs
To account for differences in exposure time, the overall cost of grade 3/4 AEs was calculated using treatment-specific cumulative probabilities of AEs for the safety population over the ECHELON-2 trial duration. A per-patient overall AE cost was applied as a lump sum at the start of treatment. AE management costs for inpatient and outpatient treatments were obtained from the Ontario Case Costing tool ()Citation35. Half of all AEs were assumed to be treated in an inpatient setting in the base case. The costs of treating grade 3/4 AEs were applied to the probability of each event to derive the total cost of AE management.
Table 4. Adverse event unit costs (CAD).
Analyses
Base case analyses were conducted using the model specifications noted in .
Sensitivity analyses
Deterministic sensitivity analyses were conducted to assess how individual parameters impact the base case ICER and associated uncertainties. These analyses included: alternative time horizons, discount rates, and treatment initiation ages; other extrapolated PFS and OS parameter distributions; drug wastage assumptions; different neutropenia prophylaxis utilization; variable disease management and SCT costs; and utility values for health states.
Probabilistic sensitivity analyses were also run using 1,000 Monte Carlo simulations for each model input to assess uncertainties of the ICER by randomly sampling from distributions of probable values for each parameter. Distributions built in the probabilistic sensitivity analyses were beta, gamma, normal, and uniformCitation36.
Results
Base case analysis
Over a lifetime horizon, A + CHP compared with CHOP had an estimated discounted PFS advantage (2.24 years; mean 8.49 vs 6.25 years) and OS advantage (2.90 years; mean 15.13 vs 12.23 years). A + CHP relative to CHOP yielded higher average QALYs gained (12.29 vs 9.91) and higher healthcare costs ($235,467 vs $158,976; ). The cost differential was driven mainly by the drug acquisition cost of A + CHP compared with CHOP ($97,392 vs $10,285). The estimated incremental costs and QALYs accrued by A + CHP relative to CHOP led to an ICER of $26,340 per LY gained and $32,177 per QALY gained.
Table 5. Results: base case cost-effectiveness analysis.
Sensitivity analyses
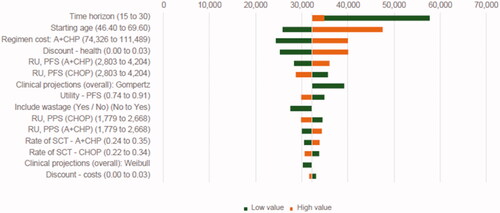
The most influential model parameters in the deterministic sensitivity analyses included time horizon, patient starting age, cost of A + CHP, health discount rate, and application of survival data based on alternative parametric distribution for both arms (). The results showed that, compared with the base case result, the implementation of Gompertz had a greater impact on the ICER than the other distributions: use of Gompertz increased the ICER by 22% to $39,185. As the ICER for A + CHP versus CHOP was driven mainly by the survival gain of A + CHP, longer time horizon, younger patient starting age, lower health discount rate, lower resource use cost for A + CHP, and higher utility value during PFS decreased the ICER. Rates of SCT had a minimal impact on the base case ICER.
Figure 3. Sensitivity analyses, A + CHP vs CHOP. Abbreviations. A + CHP, brentuximab vedotin in combination with cyclophosphamide, doxorubicin, and prednisone; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; PFS, progression-free survival; PPS, post-progression survival; RU, resource use; SCT, stem cell transplant.
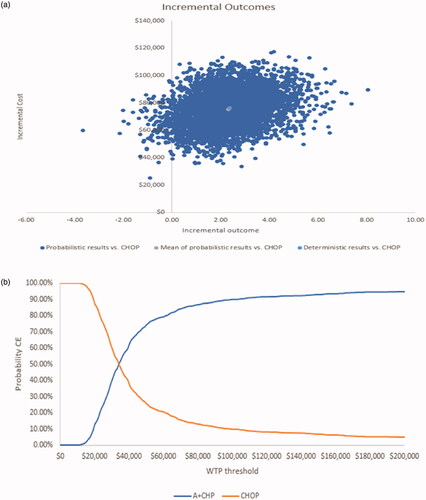
Overall, results from the probabilistic sensitivity analyses show A + CHP was more effective in the majority of iterations (). When results across all probabilistic sensitivity analyses were averaged, the incremental results showed that A + CHP was associated with an additional mean cost of $75,444 and an additional mean 2.32 QALYs gained, for a mean ICER of $32,470 per additional QALY gained. In the cost-effectiveness acceptability curve, the probability of A + CHP being more cost-effective than CHOP was achieved at a threshold of approximately $34,000 per QALY gained (). At a willingness-to-pay threshold of $50,000 and $100,000, the probability that A + CHP is cost-effective compared with CHOP was 75% and 90%, respectively.
Figure 4. Probabilistic sensitivity analysis cost-effectiveness results. (a) Cost-effectiveness plane (A + CHP vs CHOP). (b) Cost-effectiveness acceptability curve. Abbreviations. A + CHP, brentuximab vedotin in combination with cyclophosphamide, doxorubicin, and prednisone; CE, cost-effective; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; WTP, willingness-to-pay.
Discussion
It is commonly accepted that reimbursement decisions should be based on obtaining sufficient clinical value for money – that is, enough gain in health benefits to justify any increased costs. Results from this cost-effectiveness evaluation demonstrate that the economic value of adopting A + CHP in previously untreated adult patients with sALCL, PTCL-NOS, or AITL whose tumors express CD30 is within accepted ranges for oncology medicines from the perspective of the Canadian publicly funded healthcare system. The analysis was submitted, along with clinical evidence from the first prospective trial in PTCL to show clinically meaningful PFS and OS benefits over the standard therapy, CHOPCitation18,Citation19, without an observed increase in toxicity, to support the manufacturer’s reimbursement request. In the base case analysis, the estimated incremental costs and QALYs accrued by A + CHP relative to CHOP led to an ICER of $26,340 per LY gained and $32,177 per QALY gained. In a wide range of sensitivity analyses, ICERs were also below $60,000 per QALY gained. The model parameters that affected long-term benefits – time horizon, discount rates, and starting age – were those that most affected the ICER, reflecting that the central driver of value of A + CHP treatment in this population is extended survival.
Although previously published cost-effectiveness analyses for PTCL are limited, their results support our findings. In a cost-effectiveness analysis of brentuximab vedotin compared with conventional chemotherapy in patients with relapsed or refractory sALCL from the UK National Health Service perspectiveCitation20, the base case ICER for brentuximab vedotin was £35,390 per QALY gained versus conventional chemotherapy. In sensitivity analyses, ICERs were between £28,112 and £61,921, suggesting that brentuximab vedotin may be a cost-effective treatment option relative to conventional chemotherapy. An economic analysis from a US healthcare payer perspective evaluated the cost-effectiveness of A + CHP compared with CHOP in the frontline setting for CD30-expressing PTCLsCitation21. In this evaluation, data from the ECHELON-2 trial for the full intent-to-treat population indicate that the base case ICER was $89,217 (ICERs in sensitivity analyses ranged from $57,000 to $138,000), which is below the widely accepted threshold of $150,000 for oncology medicines in the USCitation21.
From a clinical perspective, the ECHELON-2 trial established that frontline treatment with A + CHP is superior to CHOP for patients with CD30-expressing PTCL based on a statistically significant and clinically meaningful improvement in PFS and OS with no observed increase in toxicityCitation17. With its 5-year results, ECHELON-2 is the only trial demonstrating significant improvements in OS for patients with PTCLCitation18. The current economic analysis was based on data from ECHELON-2. The trial enrolled patients with a broad range of PTCL subtypes, which is reflective of the heterogeneous nature of PTCL in clinical practice. However, as with other newly approved therapies, there is uncertainty around the extrapolation of survival data beyond the follow-up period of the clinical trial. In the model base case, the same distribution (i.e. log-normal) for A + CHP and CHOP was used to estimate long-term PFS and OS and assumed that similar trends in progression and death would be observed in both groups of patients. Use of the same distribution for both treatments follows best modeling practices, based on the lack of available statistical or clinical evidence to suggest long-term survival may be differentCitation24.
Landmark results from the ECHELON-2 trial showed frontline treatment with A + CHP continued to provide clinically meaningful improvements in PFS and OS through 5 years compared to CHOPCitation18. To test the long-term OS projection beyond the ECHELON-2 trial duration, a scenario was conducted where the hazard of death for the A + CHP arm was assumed to be the same as for CHOP post the maximum duration of ECHELON-2 trial follow-up (i.e. 62 months). This scenario is considered conservative toward A + CHP given its significant relative OS benefit compared with CHOP that was observed during the ECHELON-2 trial and the continued clinically meaningful improvement in PFS and OS observed at 5 yearsCitation19. Within this scenario, a shorter time horizon of 10 years (maximum ECHELON-2 follow-up duration plus 5 years past trial period) was also explored. When assuming the same mortality after the ECHELON-2 observation period, the ICER in the new scenario increased to $43,344 from $32,177 (base case result) due to a decreased incremental OS benefit (2.08 [new scenario] vs 2.90 LYs [base case]) for A + CHP versus CHOP, within a lifetime horizon. When the time horizon was decreased to 10 years, the ICER for the new scenario was $100,661 compared with $90,271 (corresponding to the base case settings where the time horizon is reduced to 10 years). However, it is likely that an analysis based on a 10-year time horizon cannot fully capture the survival benefits associated with A + CHP. The base case analysis shows at year 10, in the A + CHP arm 30% of patients remained free of progression and 62% of patients were alive; in the CHOP arm, 10-year PFS was 21% and OS 49%.
Strengths and limitations
A partitioned survival analysis was performed, thus enabling the most direct use of PFS and OS data from the ECHELON-2 trial. The use of partitioned survival models is preferred for many oncology cost-effectiveness evaluations, as clinical trial data can be used directly or extrapolated beyond the trial follow-up using the observed event history and clinical judgment to confirm face validityCitation37.
However, the impact of downstream treatments, including the use of SCT, on clinical outcomes in a real-world clinical practice that might differ from the protocol followed in the ECHELON-2 trial cannot be fully captured by the current model, where the observed survival data from ECHELON-2 representing the subsequent treatments received in the trial were applied. Despite this, the use of robust clinical and quality-of-life data that directly compare A + CHP with a standard frontline treatment (i.e. CHOP) available to the patient population in Canada ensures that the results generated by the model are meaningful for real-world clinical practice. Overall, this cost-effectiveness analysis demonstrates that the net clinical benefit of brentuximab vedotin observed in the ECHELON-2 trial is also associated with good value for money, which is supported by a positive reimbursement decision by pCODR for this new indication.
Conclusions
Based on the clinical data from the ECHELON-2 trial, this model shows that A + CHP is associated with longer PFS and OS and higher quality-adjusted survival outcomes compared with CHOP. Although there is no formal cost-effectiveness threshold for oncology therapies in Canada, ICERs in the base case analysis and sensitivity analyses fell below $60,000 per QALY, the ICER of other reimbursed therapies for oncology indicationsCitation38. For previously untreated adult patients in Canada with sALCL, PTCL-NOS, or AITL whose tumors express CD30, A + CHP provides a cost-effective treatment option with superior efficacy and comparable safety relative to CHOP.
Transparency
Declaration of funding
This work was sponsored by Seagen Inc.
Declaration of other financial/other relationships
DZ and MH are employees of Evidera, which received funding from Seagen Inc. for the conduct of this study. JL, AK, and WM are former employees of Evidera, which received funding from Seagen Inc. for the conduct of this study. KSY, JL, and KF are employees and shareholders of Seagen Inc.
No potential conflicts of interest were reported by the authors.
Previous presentations
Zou D, Lee J, Kansal A, et al. Cost-effectiveness Of Brentuximab Vedotin For The Frontline Treatment Of Peripheral T-Cell Lymphomas In Canada [abstract]. Value in Health, Volume 23, S444.
Acknowledgements
The authors thank Drs. Isabelle Fleury, Minakshi Taparia, Gizelle Popradi, Eugenia Piliotis, Bence Bruckler, and Mary Margaret Keating for their contributions to the clinical treatment pathways, assumptions, resource use, and data sources in this economic model as they relate to the Canadian healthcare setting. Medical writing support was provided by Ann Cameron, PhD, of Curo (part of the Envision Pharma Group) and Beth Lesher, PharmD, BCPS, of OPEN Health, and was funded by Seagen Inc.
References
- Rodriguez-Abreu D, Filho VB, Zucca E. Peripheral T-cell lymphomas, unspecified (or not otherwise specified): a review. Hematol Oncol. 2008;26(1):8–20.
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390.
- Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–4130.
- Foss FM, Zinzani PL, Vose JM, et al. Peripheral T-cell lymphoma. Blood. 2011;117(25):6756–6767.
- Brenner DR, Weir HK, Demers AA, et al. Projected estimates of cancer in Canada in 2020. CMAJ. 2020;192(9):E199–e205.
- Mahe ER, Pugh T, Stockley T, et al. Filling the void of Canadian T-cell lymphoma epidemiology: data from the Canadian Institute for Health Information discharge abstract database. Am Assoc Cancer Res. 2015;75:15.
- d’Amore F, Gaulard P, Trümper L, et al. Peripheral T-cell lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v108–15. Sep
- Moskowitz AJ, Lunning MA, Horwitz SM. How I treat the peripheral T-cell lymphomas. Blood. 2014;123(17):2636–2644.
- Pan-Canadian Oncology Drug Review. pCODR Expert Review Committee (pERC) final recommendation (Adcetris). 2018.
- Pan-Canadian Oncology Drug Review. pCODR Expert Review Committee (pERC) final recommendation (Adcetris) for HL 2013. Available from: https://www.cadth.ca/sites/default/files/pcodr/pcodr-adcetrishl-fn-rec.pdf
- Pan-Canadian Oncology Drug Review. pCODR Expert Review Committee (pERC) final recommendation (Adcetris) for sALCL 2013. Available from: https://www.cadth.ca/sites/default/files/pcodr/pcodr-adcetris-salcl-fn-rec.pdf
- L’Institut National d'Excellence En Santé Et En Services Sociaux. ADCETRISMC – lymphome anaplasique à grandes cellules systémique 2014. Available from: https://www.inesss.qc.ca/fileadmin/doc/INESSS/Inscription_medicaments/Avis_au_ministre/Fevrier_2014/adcetris_lagcs_2014_02_CAV.pdf
- L’Institut National d'Excellence En Santé Et En Services Sociaux. ADCETRISMC – lymphome de Hodgkin 2014. Available from: https://www.inesss.qc.ca/fileadmin/doc/INESSS/Inscription_medicaments/Avis_au_ministre/Juin_2014/Adcetris__LH_2014_06_CAV.pdf
- L’Institut National d'Excellence En Santé Et En Services Sociaux. ADCETRISMC – lymphome de Hodgkin 2018. Available from: https://www.inesss.qc.ca/fileadmin/doc/INESSS/Inscription_medicaments/Avis_au_ministre/Fevrier_2018/Adcetris_2018_02.pdf
- Adcetris [Product Monograph]. Bothell, WA: Seagen Inc.; 2019.
- Federico M, Bellei M, Luminari S, et al. CD30+ expression in peripheral T-cell lymphomas (PTCLs): a subset analysis from the international, prospective T-Cell Project. J Clin Oncol. 2015;33(15_suppl):8552–8552.
- Horwitz S, O’Connor OA, Pro B, et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019;393(10168):229–240.
- Horwitz S, O’Connor OA, Pro B, et al. The ECHELON-2 trial: 5-year results of a randomized, phase 3 study of brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma. Ann Oncol. 2021;2021:S0923-7534(21)04875-4.
- Horowitz SM, O’Connor OA, Pro B, et al., editors. The ECHELON-2 trial: 5-year results of a randomized, double-blind, phase 3 study of brentuximab vedotin and CHP (A + CHP) versus CHOP in frontline treatment of patients with CD30-positive peripheral T-cell lymphoma. 62nd ASH Annual Meeting and Exposition; 2020 December 5–8; Virtual.
- Hux M, Zou D, Ma E, et al. Cost-effectiveness of brentuximab vedotin in relapsed or refractory systemic anaplastic large cell lymphoma. J Clin Oncol. 2016;34(7_suppl):18–18.
- Feldman T, Zou D, Rebeira M, et al. Cost-effectiveness of brentuximab vedotin with chemotherapy in treatment of CD30-expressing PTCL. Am J Manag Care. 2020;26(2):e41–e49.
- Canadian Agency for Drugs and Technologies in Health (CADTH). Guidelines for the economic evaluation of health technologies: Canada. 4th edition. 2017.
- Seagen Inc. Document on file – Cost-effectiveness of brentuximab vedotin for the treatment of previously untreated adult patients with systemic anaplastic large cell lymphoma, peripheral T-cell lymphomas not otherwise specified, or angioimmunoblastic T-cell lymphoma whose tumors express CD30: Canadian pCODR technical report. 2019.
- Latimer NR. Survival analysis for economic evaluations alongside clinical trials – extrapolation with patient-level data: inconsistencies, limitations, and a practical guide. Med Decis Making. 2013;33(6):743–754.
- Bellei M, Foss FM, Shustov AR, et al. The outcome of peripheral T-cell lymphoma patients failing first-line therapy: a report from the prospective, international T-Cell Project. Haematologica. 2018;103(7):1191–1197.
- Park SI, Horwitz SM, Foss FM, et al. The role of autologous stem cell transplantation in patients with nodal peripheral T-cell lymphomas in first complete remission: report from COMPLETE, a prospective, multicenter cohort study. Cancer. 2019;125(9):1507–1517.
- EuroQol Research Foundation. EQ-5D-3L User Guide. 2018. [June 8, 2021].
- Pan-Canadian Oncology Drug Review. pCODR Expert Review Committee (pERC) final recommendation for brentuximab vedotin (Adcetris) for peripheral T-cell lymphoma 2020. Available from: https://cadth.ca/sites/default/files/pcodr/Reviews2020/10199BrentuximabVedotinPTCL_FnRec_approved_Post04Jun2020_final.pdf
- NEUPOGEN (filgrastim) [package insert]. U.S. Food and Drug Administration website 2015 [cited 2021 Mar 16]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/103353s5184lbl.pdf
- ADCETRIS (brentuximab vedotin) [package insert] U.S. Food and Drug Administration website 2014. [cited 2021 Mar 16]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125388_s056s078lbl.pdf
- Ontario Ministry of Health and Long-Term Care (MOHLTC). Schedule of benefits. Physician services. [cited 2019 Mar 20]. Available from: https://www.health.gov.on.ca/en/pro/programs/ohip/sob/
- Ontario Ministry of Health and Long-Term Care (MOHLTC). Interprovincial billing rates for designated high cost transplants effective for discharges on or after April 1, 2018. 2018 [cited 2019 Mar 20]. Available from: https://www.health.gov.on.ca/en/pro/programs/ohip/bulletins/na_84/high_cost_procedures.pdf
- Walker H, Anderson M, Farahati F, et al. Resource use and costs of end-of-life/palliative care: Ontario adult cancer patients dying during 2002 and 2003. J Palliat Care. 2011;27(2):79–88.
- Costa S, Scott DW, Steidl C, et al. Real-world costing analysis for diffuse large B-cell lymphoma in British Columbia. Curr Oncol. 2019;26(2):108–113.
- Ontario Case Costing Initiative. Cost analysis tool – Acute inpatient and ambulatory 2016/2017 statistics by most responsible diagnosis [March] 2019.
- Briggs AH, Schulpher CK. Decision modelling for health economic evaluation. Oxford, UK: Oxford University Press; 2006.
- Woods B, Sideris E, Palmer N, et al. NICE DSU technical support document 19: partitioned survival analysis for decision modelling in health care: a critical review. 2017. [January 3, 2019].
- Chabot I, Rocchi A. How do cost-effectiveness analyses inform reimbursement decisions for oncology medicines in Canada? The example of sunitinib for first-line treatment of metastatic renal cell carcinoma. Value Health. 2010;13(6):837–845.
- Ontario Case Costing Initiative. Cost analysis tool – Acute inpatient and ambulatory 2016/2017 statistics by most responsible diagnosis [March 2019].
