Abstract
Aim
To conduct a cost-effectiveness analysis (CEA) on the use of andexanet alfa for the treatment of factor Xa inhibitor-related intracranial hemorrhage (ICH) from the US third-party payer and societal perspectives.
Methods
CEA compared andexanet alfa to prothrombin complex concentrate for the treatment of patients receiving factor Xa inhibitors admitted to hospital inpatient care with an ICH. The model comprised two linked phases. Phase 1 utilized a decision tree to model the acute treatment phase (admission of a patient with ICH into intensive care for the first 30 days). Phase 2 modeled long-term costs and outcomes using three linked Markov models comprising the six health states defined by the modified Rankin score.
Results
The analysis showed that the strategy of using andexanet alfa for the treatment of factor Xa inhibitor-related ICH is cost-effective, with incremental cost-effectiveness per quality-adjusted life-year gained of $35,872 from a third-party payer perspective and $40,997 from a societal perspective over 20 years.
Limitations
(1) Absence of head-to-head trials comparing therapies included in the economic model, (2) lack of comparative long-term data on treatment efficacy, and (3) bias resulting from the study designs of published literature.
Conclusion
Given these results, the use of andexanet alfa for the reversal of anticoagulation in patients with factor Xa inhibitor-related ICH may improve quality of life and is likely to be cost-effective in a US context.
Introduction
Direct oral factor Xa inhibitors, such as apixaban and rivaroxaban, are used for the prevention of ischemic stroke, as well as for the prevention and treatment of venous thromboembolismCitation1,Citation2. Like vitamin K antagonists, these agents, as a class, carry a small risk for major bleeding events, which are associated with considerable morbidity and mortality and result in substantial economic burdenCitation3–7. More than 84,000 patients who were treated with factor Xa inhibitors in 2015 were hospitalized in the United States for major bleedingCitation4, with annualized rates of major bleeding ranging from 1% to 4%Citation6,Citation8. Intracranial hemorrhage (ICH) contributes to considerable healthcare resource use and economic burden. Real-world studies have shown that in patients with ICH, morbidity, resource utilization, and mortality rates are particularly high. In the 3-year, prospective ORANGE (ORal ANticoagulant aGEnt-associated bleeding adverse events reporting system) study conducted in the United Kingdom, ICH was a strong predictor of deathCitation9. In a US MarketScan analysis, ICH was associated with substantially higher mortality than any other type of major bleeding and was associated with a high healthcare resource burden, including hospitalization costs of >$45,000Citation4. Other studies have shown high rates of mortality in patients with factor Xa inhibitor-related ICH, ranging from 27% in-hospital mortality among patients with intracerebral hemorrhage (also known as an intraparenchymal hemorrhage, a subset of ICH)Citation10 to 37% 30-day mortality among patients with ICHCitation3.
Andexanet alfa (coagulation factor Xa [recombinant] inactivated-zhzo) is a specific reversal agent that binds to and sequesters factor Xa inhibitor molecules, thereby rapidly reducing anti-factor Xa activity and restoring endogenous factor Xa activityCitation11,Citation12. In phase 3, randomized, placebo-controlled trials, andexanet alfa demonstrated significant and effective reversal of the anticoagulant activity of apixaban and rivaroxaban in older healthy participants within minutes following bolus and sustained throughout the 2-h intravenous (IV) infusionCitation12. In the multicenter, prospective, single-arm, phase 3b/4 ANNEXA-4 (Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of Factor Xa Inhibitors) trial, an analysis of 352 adults who had experienced acute major bleeding within 18 h of treatment with a direct factor Xa inhibitor demonstrated that treatment with andexanet alfa resulted in excellent or good hemostatic efficacy within 12 h in 82% of patients, a 30-day mortality rate of 14%, and an incidence of adjudicated thrombotic events of 9.7%Citation11. A subgroup analysis of 98 patients treated for spontaneous ICH (sICH) showed that 79% of patients achieved excellent or good hemostasis over the 12-h period following treatment with andexanet alfaCitation13. Andexanet alfa received US Food and Drug Administration (FDA) approval in May 2018 under the accelerated approval pathway for the treatment of life-threatening or uncontrolled bleeding resulting from factor Xa inhibitors apixaban and rivaroxabanCitation14.
Prior to the approval of andexanet alfa, there were no FDA-approved agents available for the reversal of factor Xa inhibitors in the setting of life-threatening or uncontrolled bleedsCitation15. Non-specific factor replacement products, including prothrombin complex concentrates (PCCs), have been used off-label for this purposeCitation15. Current 2020 American College of CardiologyCitation16 and American College of Emergency PhysiciansCitation17 guidelines recommend the use of andexanet alfa for the reversal of factor Xa inhibitors and the use of PCCs if andexanet alfa is not available. Further, the European Medicines Agency granted conditional marketing authorization for andexanet alfa in April 2019Citation18.
To support evidence-based decision-making on the part of hospital administrators and payers, we conducted a study that included a wide range of both short- and long-term healthcare costs. The objective of this study was to conduct a cost-effectiveness analysis (CEA) of andexanet alfa to estimate the cost-utility of andexanet alfa for the treatment of factor Xa inhibitor-related ICH in clinical practice in the United States.
Methods
A decision-analytic model was built in Microsoft Excel for Microsoft 365 (Redmond, WA, USA). The analysis adhered to the International Society for Pharmacoeconomics and Outcomes Research Good Practice Guidelines for CEACitation19. Costs and outcomes were discounted at 3.5%.
Cost-effectiveness analysis
Model structure
The CEA model compared the long-term healthcare costs and quality-adjusted survival of patients treated with andexanet alfa versus PCCs for the treatment of oral factor Xa inhibitor-related ICH. The quality-adjusted life-year (QALY) was calculated based on the utility values associated with each treatment. The clinical scenario in which a patient received a combination of PCC and andexanet alfa was not included. The model was developed from the US third-party payer and societal perspectives. The time horizon was 20 years with a cycle length of 1 month. Considering the age and morbidity of the patient population included in the model, 20 years is likely to represent a lifetime horizon.
The model comprised two linked phases (). Phase 1 was the acute treatment phase comprising admission of a patient with ICH into the intensive care unit (ICU) for the first 30 days. Phase 2 included the long-term healthcare costs and quality of life modeled after patients were discharged from the acute treatment phase.
Figure 1. Model structure: (a) phase 1 – acute care from hospital admission to 30 days; (b) phase 2 – long-term care from 30 days to model time horizon. Abbreviations. ICH, intracranial hemorrhage; mech. vent., mechanical ventilation; mRS, modified Rankin score; PCC, prothrombin complex concentrate.
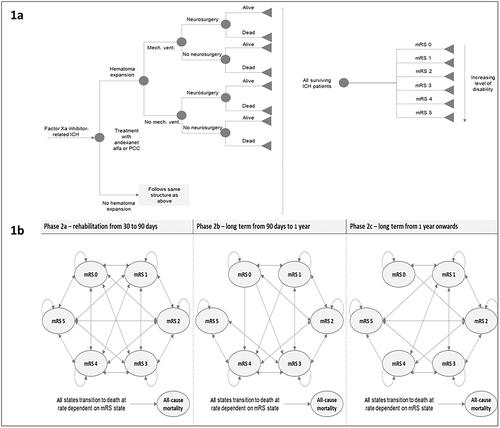
For phase 1 (), the modeling approach utilized a decision tree, which allowed the explicit consideration of critical elements in the care of patients with ICH. First, hematoma expansion, defined as an expansion in hematoma volume >35%, increases the risk of adverse outcomes, such as mechanical ventilation and a longer length of ICU stayCitation20. Based on prior researchCitation21, the model assumed that the administration of andexanet alfa in this patient cohort would result in a lower risk of hematoma expansion () relative to the administration of PCCs. Second, the risk of mechanical ventilation was considered; ventilated patients incur more costs during their stay in the ICU than non-ventilated patients. Third, the neurosurgical intervention was considered; patients who require neurosurgical intervention incur the additional cost associated with the surgery. The model also incorporated the risk of readmission, as readmission incurs additional costs. Due to the absence of available data on functional outcomes following readmission, it was assumed that readmission does not affect functional outcomes.
Table 1. Treatment-dependent and treatment-independent clinical inputs.
For phase 2 (), the modeling approach utilized three linked Markov models comprising the six health states defined by the modified Rankin score (mRS). The movement of patients between mRS health states was modeled during each of the three subphases: (1) patient rehabilitation, (2) discharge from rehabilitation to 1 year post-ICH, and (3) long term (1 year post-ICH onwards). The following assumptions were made: based on the functional status, patients could undergo rehabilitation in intensive inpatient settings, in skilled nursing facilities, or at home. This was assumed to occur between 30 and 90 days post-ICH. After discharge from a rehabilitation setting, patients could continue to experience excess healthcare costs associated with their recovery for up to 1 year. Patients could also experience functional improvement for up to 1 year. After the first year post-ICH, patients could continue to accrue increased healthcare costs compared to a healthy population; the model assumed increased risk of death and reduced chances of functional improvement after 1 year.
Model assumptions
The following additional assumptions were made for this model: (a) the acute-phase hospitalization and healthcare resource utilization is a function of hematoma expansion only; (b) all additional costs not accounted for explicitly in the model are equal between therapies (e.g. the total length of hospital stay); and (c) the risk of mechanical ventilation and length of ICU stay is only associated with the risk of hematoma expansion.
Healthcare resource use and survival benefit
The number of lives saved, incremental ICU days avoided, and the number needed to treat with increased uptake of andexanet alfa were also assessed. The number needed to treat represents the number of patients that one would need to treat to save one additional life over a given time period and was calculated as 1/(the risk of death with PCC – the risk of death with andexanet alfa).
Clinical inputs
Clinical inputs were sourced from the peer-reviewed literature, where available ()Citation20–29. All the clinical inputs for PCC came from real-world observational data as there are no randomized controlled trials available and PCCs are used off-label in this indication. In addition, as ICH is a stroke subtype, some clinical inputs were derived from stroke data.
Acute-phase clinical inputs dependent on treatment
Hematoma expansion is used within the model to estimate the in-hospital resource utilization. It is not linked in the model to mortality (i.e. independent parameters accounting for hematoma expansion and 30-day mortality are employed). The risk of hematoma expansion was based on the proportion of patients with poor hemostasis at follow-up imaging, which was defined based on the International Society on Thrombosis and Haemostasis criteriaCitation30 of an increase in hematoma volume of >35% from baseline scan. The estimates for risk of hematoma expansion were derived from Huttner et al.Citation21. In brief, Huttner et al. was an indirect propensity score-matched analysis that compared data extracted from the prospective ANNEXA-4 study (85 andexanet alfa–treated patients) and from a multicenter observational cohort study, RETRACE II (97 PCC-treated patients). Both studies included patients who had treatment with apixaban or rivaroxaban within 18 h of admission, intracerebral bleeds not caused by trauma or cancer, a Glasgow Coma Scale score of ≥7, a baseline intracerebral volume of ≤60 ml, and no record of abnormal liver function or alcohol abuse. The start of follow-up was defined in ANNEXA-4 as the date of administration of andexanet alfa and in RETRACE II as the date of hospital admission for intracerebral hemorrhage. Patients received follow-up computed tomography or magnetic resonance imaging scans at about 12 h in ANNEXA-4 and up to 36 h after the initial scan in RETRACE II. Duration of follow-up was defined in ANNEXA-4 as 30 days after treatment with andexanet alfa and in RETRACE II as the time spent in the hospital. Importantly, regression models estimating the risk of hematoma expansion in Huttner et al.Citation21 were adjusted for baseline bleed severity, including hematoma volume and other clinical factors that are critical for comparing outcomes in patients with intracerebral hemorrhageCitation21. Although other sources of evidence are available for assessing the effectiveness of PCCCitation31, Huttner et al.Citation21 was selected as it is a propensity score-matched analysis of sICH patients from ANNEXA-4Citation11 and RETRACE II)Citation32. The use of estimates from Panos et al.Citation31 for the PCC arm of the model would have involved making a naïve comparison that did not account for the difference in the proportion of more severe ICH in ANNEXA-4 versus the Panos et al. study or the fact that the Panos et al. study excluded patients with involvement of intraventricular extension, both of which would have likely biased in favor of the PCC arm of the model.
The estimates for the risk of neurosurgical intervention for patients on andexanet alfa and PCC were derived from ANNEXA-4 and published observational studies. For andexanet alfa, the data were pooled from two retrospective observational studies and unpublished data on file from ANNEXA-4Citation22,Citation23,Citation25. For PCCs, the data were pooled from four observational studies, including three prospective studies and one retrospective studyCitation26–29.
The estimates for the risk of 30-day mortality for patients treated with andexanet alfa and PCCs were derived from an indirect, propensity score–weighted comparison of data from ANNEXA-4 and the prospective, observational ORANGE study, respectivelyCitation24. Matching covariates included age, bleed site, history of atrial fibrillation, venous thromboembolism, stroke, renal dysfunction, and cancerCitation24. Cohen et al.Citation24 was used as the source for these data because it is a propensity score-matched analysis of 30-day mortality rates from ANNEXA-4 and ORANGECitation9, a retrospective UK study of direct oral anticoagulant (DOAC)–related bleeding. Huttner et al.Citation21 also reported the results of a propensity score matching between ANNEXA-4 and RETRACE, and Panos et al.Citation31 reported mortality results of a US PCC cohort; however, both analyses only examined in-hospital mortality and thus were less suitable for the current analysis. Additionally, the ORANGE studyCitation24 was considered the most suitable source as it reported results from all ICH whereas RETRACE was specific to intracerebral bleeds and included both trauma-related and spontaneous bleeds.
The distribution of mRS scores of surviving patients was obtained from an indirect, propensity score–weighted analysis of data derived from PCC-treated patients from RETRACE II and andexanet alfa–treated patients from ANNEXA-4Citation25. The mRS distributions were derived from a subgroup analysis of a larger indirect comparison of andexanet alfa versus usual care, and results for the PCC subgroup were similar to the overall results for the usual care armCitation33. In ANNEXA-4, mRS was measured 30 days after hospital admission; in RETRACE II, mRS was measured at hospital discharge and 90 daysCitation33. As it is not possible to adjust for differences between the discharge and 30-day results since patients were discharged at different time points up to 30 days with RETRACE, mRS at discharge was deemed the best proxy for 30-day results. Results at 90 days were not included due to the potential for the introduction of bias from rehabilitation treatments.
Treatment-independent clinical inputs
The estimates for the probability of mechanical ventilation and mean length of ICU stay were derived from a retrospective analysis, which quantified the impact of hematoma expansion on clinical and economic outcomes in 200 ICH patients between 2009 and 2012Citation20. Studies that directly quantify the impact of hematoma expansion on resource utilization are lacking. This study was chosen because (a) it defined hematoma expansion as a ≥33% increase in hematoma volume over 24 h, which is a definition similar to that utilized in the RETRACE II studyCitation32, and (b) it utilized multivariate regression analysis to account for confounding due to age, warfarin use, or other variables that may impact the associations observed between hematoma expansion and key outcomes, such as length of stay and necessity for mechanical ventilation.
The estimates for the transition probability between different mRS states were derived from published observational studies (Supplemental Table 1)Citation34,Citation35. The estimates for the transition probability from hospital discharge to the end of rehabilitation (90 days) were sourced from a retrospective observational study that analyzed data from a nationwide multicenter Korean registryCitation34. Post-discharge recovery was defined as an improvement in mRSCitation34. The transition probability estimates from 90 to 365 days and from 1 year post-ICH as well as all-cause mortality hazard ratio estimates for the general population were sourced from a population-based cohort study of ischemic stroke conducted from 2002 to 2014Citation35. The study looked at the relationship of 3-month mRS scores with disability (defined as mRS >2) at 1 and 5 years and with age/sex-adjusted death ratesCitation35.
Cost inputs
Cost inputs were sourced from the peer-reviewed literature, where available, and inflated to 2020 USD using the medical care component of the consumer price index ()Citation13,Citation14,Citation25,Citation36–46.
Table 2. Treatment-dependent and treatment-independent cost data.
Treatment costs
The mean cost of therapy was calculated based on the drug acquisition costs, drug dosage, proportion of patients receiving additional therapy, and cost of additional therapy (Supplemental Table 2)Citation13,Citation14,Citation36–39,Citation47,Citation48.
Wholesale acquisition costs for andexanet alfa (Andexxa, Alexion Pharmaceuticals, Inc., Boston, MA, USA) and 4-factor PCC (Kcentra, CSL Behring LLC., Kankakee, IL, USA), assumed to represent PCC, were obtained from Micromedex 2020. The costs of drugs used as add-on therapy comprised fresh frozen plasmaCitation39, vitamin KCitation48, activated 4-factor PCC (Feiba NF, Baxter Healthcare Corp, IL, USA)Citation36, and rFactor VIIa (NovoSeven RT, Novo Nordisk, Denmark)Citation36.
The cost of therapy for andexanet alfa was based on the proportion of patients receiving the high dose (18%) and low dose (82%). The proportion of patients receiving low-dose andexanet alfa was derived from the ICH subanalysis of ANNEXA-4Citation13. The definition of low and high dosing was based on the andexanet alfa package insert. The low dose comprised a 400-mg IV bolus, followed by an IV infusion of 4 mg/min for 120 min (880 mg total). The high dose comprised an 800-mg IV bolus, followed by an IV infusion of 8 mg/min for 120 min (1,760 mg total)Citation14.
The cost of therapy for PCC was based on a mean patient weight of 85 kg and mean dosing of 50 IU/kgCitation49,Citation50. The mean patient weight of 85 kg was obtained from a retrospective observational study conducted from 2014 to 2018 on adult patients who received 4-factor PCC (Kcentra) for factor Xa inhibitor-related major bleedingCitation37. The mean dose of PCCs of 50 IU/kg was based on Neurocritical Care Society guidelines in the care of patients with antithrombotic-associated ICHCitation38.
The mean cost of additional therapy was calculated based on the proportion of patients who received additional therapy after initial treatment with andexanet or PCC. The proportion of andexanet alfa and PCC patients using additional treatment was sourced from a hospital chart auditCitation51, in which electronic records from 45 US hospitals were queried; 3,030 patients were hospitalized for anticoagulation-related bleeding (International Classification of Diseases, Tenth Revision billing code of D68.32, T45.515, or T45.525) after having received an oral factor Xa inhibitor just prior to admission and managed with either andexanet alfa or PCC. Based on these inputs, the mean cost of therapy was estimated at $29,167 for andexanet alfa–based strategies and $12,226 for PCC-based strategies (Supplemental Table 2)Citation13,Citation14,Citation36–39,Citation47,Citation48.
Resource use costs
The daily cost of mechanical ventilation was sourced from a retrospective cohort study on patients ≥18 years of age admitted to an ICU between 1 October 2002 and 31 December 2002, using data from NDC Health's Hospital Patient Level Database (National Data Corporation, Atlanta, GA, USA). The daily cost of ICU stay was sourced from a retrospective study on Medicaid and Medicare beneficiaries (2000–2010) that utilized data from the federal Healthcare Cost Report Information System, the American Hospital Association, and the US Census Bureau that estimated comprehensive critical care medicine costs per day by using the modified Russell equation, a “top-down” approach that examines broad costing without patient-level detailsCitation41. The cost of craniotomy was sourced from a retrospective chart review study of all adult patients requiring surgical drainage of a chronic subdural hematoma from 1 January 2007 to 31 December 2009Citation42. All patients included underwent either burr hole drainage or a mini-craniotomy. Readmission cost was sourced from a retrospective study on patients with a primary diagnosis of non-traumatic ICH utilizing the Nationwide Readmission Database between 2010 and 2014. Rehabilitation costs were sourced from a retrospective study on elderly Medicare beneficiaries with stroke or hip fracture enrolled in the fee-for-service Medicare program and discharged from an acute care hospital from January 2002 to June 2003. Post-ICH care costs were sourced from a prospective economic substudy of the acute ischemic stroke SWIFT-PRIME trial, in which costs were measured for patients using detailed medical resource utilization and hospital billing data for patients in the clinical trialCitation45. Post-trial costs were estimated for each surviving patient using a model based on trial data and inputs derived from a contemporary cohort of ischemic stroke survivorsCitation45.
Indirect costs
Indirect costs were used in addition to the therapy and medical costs for assessing the cost-effectiveness of andexanet alfa versus PCC from a societal perspective (Supplemental Table 3). Because the population modeled has a baseline age of 80 years, it is assumed that there are no productivity differences between treatment arms due to very low employment in this age group. The estimates for the informal care costs were sourced from Kunz et al.Citation52.
Utilities
The European Quality of Life Score with five dimensions (EQ-5D) was used to calculate utilities in this CEA. The utilities were sourced from a retrospective observational study that utilized European Quality of Life Score (EQ-5D-3L) data from the Virtual International Stroke Trials Archive (VISTA) to calculate health utility, stratified by mRS at 3 monthsCitation53. Health utilities for mRS 0, mRS 1, mRS 2, mRS 3, mRS 4, and mRS 5 were 0.92, 0.85, 0.77, 0.64, 0.41, and 0.14, respectivelyCitation53.
Scenario analysis
Scenario analysis was conducted by including the New Technology Add-on Payment (NTAP) reimbursement. NTAP is provided to hospitals by the Centers for Medicare & Medicaid Services in addition to the standard Medicare Severity-Diagnosis Related Group (MS-DRG) payment. NTAP is granted to new, high-cost technologies in the inpatient setting based on criteria including the newness of the technology, inadequately paid cost under the existing MS-DRG system, and significant clinical improvement for a specific patient population as compared to currently available treatments. The reimbursement amount for the NTAP was granted to andexanet alfa in October 2018Citation54; the current maximum NTAP reimbursement amount for andexanet alfa is $18,281.25Citation47.
Sensitivity analysis
To account for the uncertainty around model parameters, and to test the robustness of the model, both deterministic sensitivity analysis (DSA) and probabilistic sensitivity analysis (PSA) were conducted. One-way DSA was performed on all model parameters. The DSA was conducted by varying the estimates by 25%. The PSA randomly sampled parameters from within chosen distributions over 1,000 iterations.
Results
Cost-effectiveness analysis
Base-case analysis
Considering a payer perspective and a time horizon of 20 years, the base-case settings resulted in an incremental increase of $47,398 in the cost with andexanet alfa compared to PCCs (). The incremental QALY improvement between the two treatments was 1.32, resulting in an incremental cost-effectiveness ratio (ICER) of $35,872. Detailed costs are provided in Supplemental Table 4.
Table 3. Base-case ICERs: payer perspective (in 2020 USD).
Considering a societal perspective and a time horizon of 20 years, the base-case settings resulted in an incremental increase in costs of $54,169 with andexanet alfa compared to PCCs. The indirect costs were $18,061 and $11,289 for andexanet alfa and PCCs, respectively. The incremental QALY improvement between the two treatments was 1.32, resulting in an ICER of $40,997.
Scenario analysis
Considering a payer perspective and a time horizon of 20 years, the inclusion of NTAP reduction resulted in an incremental increase of $29,117 in the cost with andexanet alfa compared to PCCs. The incremental QALY improvement between the two treatments was 1.32, resulting in an ICER of $22,036. Further, patients on andexanet alfa had reduced acute care costs ($42,473 vs $52,174 for PCC).
Considering a societal perspective and a time horizon of 20 years, the base-case settings resulted in an incremental increase in costs of $35,888 with andexanet alfa compared to PCCs. The incremental QALY improvement between the two treatments was 1.32, resulting in an ICER of $27,161.
Sensitivity analysis
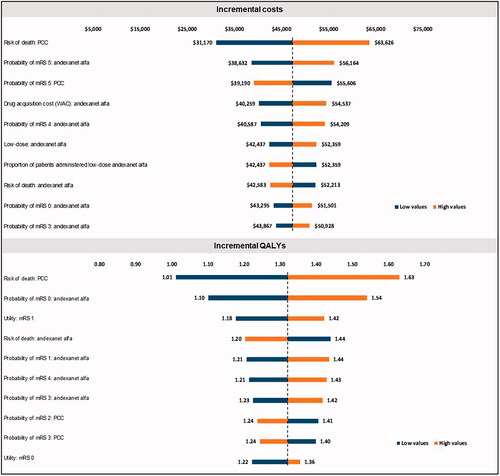
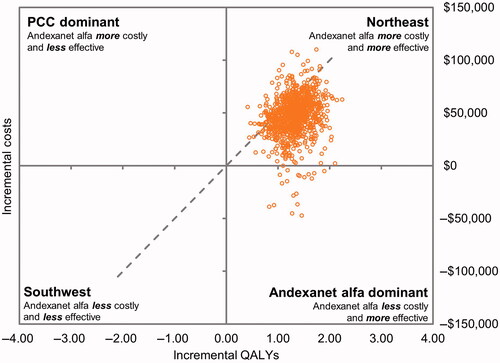
The DSA results () showed that the most influential parameters for the incremental cost component of ICER were the risk of death among patients on PCCs, probability of mRS 5 states for patients on andexanet alfa or PCC, probability of the mRS 4 state for patients on andexanet alfa, and drug acquisition costs of andexanet alfa. The most influential parameters for the incremental QALY component of ICER were risk of death among patients on PCCs, probability of mRS of 0 or 1 for patients on andexanet alfa, the health utility value for patients with an mRS of 1, and the risk of death among patients on andexanet alfa. The PSA findings () were consistent with the base-case analysis. In the current model scenario using discounted costs and QALYs, andexanet alfa was cost-effective at a willingness-to-pay (WTP) threshold of $50,000.
Healthcare resource use and survival benefit
The health outcomes achieved from increased andexanet alfa uptake were 16 lives saved, 38.6 incremental ICU days avoided, and 2.97 number (of patients) needed to treat to save a life.
Discussion
Andexanet alfa, which is currently the only FDA-approved treatment indicated for the reversal of factor Xa inhibitor activity in cases of life-threatening or uncontrolled bleeding, specifically binds and sequesters factor Xa inhibitor molecules, thereby restoring endogenous factor Xa activityCitation11,Citation12. This mechanism of action differs from that of currently available non-specific factor replacement agents such as PCCsCitation14. As replacement agents such as PCCs continue to be used even though they are not FDA approved and their efficacy in ICH has not been provenCitation27–29,Citation32, it is critical to understand the comparative safety, efficacy, and cost-effectiveness of using these agents compared to andexanet alfa in patients with factor Xa inhibitor-related ICH.
To date, limited data have compared the higher wholesale acquisition costs of andexanet alfa to PCCsCitation23, and there is a lack of dataCitation55,Citation56 regarding the health economic impact of utilization of andexanet alfa compared to PCCs in the context of factor Xa inhibitor-related ICH. Micieli et al.Citation57 evaluated CEA of andexanet alfa versus PCCs in the Canadian populationCitation57. The current study includes a CEA from a US payer and societal perspective.
This CEA suggests that from the payer and societal perspectives, treatment with andexanet alfa for the reversal of factor Xa inhibitor-related ICH is a cost-effective option over the long term when compared to PCCs. When considered over a time horizon of 20 years, andexanet alfa had an ICER of $35,872 per gain in QALY without the inclusion of the NTAP reduction, which is below the commonly applied WTP threshold of $50,000. The ICER after the inclusion of the NTAP reduction was lower ($22,036 per gain in QALY). Although cost-effectiveness of andexanet alfa will vary with rebates, drug price discounts, and variable market-specific assumptions, andexanet alfa remained cost-effective when parameters were varied in the sensitivity analyses.
The incremental QALY improvement between the two treatments was 1.32. These findings suggest that treatment with andexanet alfa may be of greater value to patients than treatment with PCCs. Treatment with andexanet alfa for the reversal of factor Xa inhibitor-related ICH is projected to reduce time in the ICU, reduce the need for mechanical ventilation and surgery, and therefore improve the quality of life of surviving patients. Thus, when evaluating a treatment, a patient perspective should be considered in addition to acute hospitalization costs. Although pharmacy costs with PCCs are less than with andexanet alfa, the andexanet alfa pharmacy costs can be partially offset by lower costs of ICU days, mechanical ventilation, and surgery. These results are likely to be largely driven by the higher rate of good/excellent hemostasis with andexanet alfa than with PCCsCitation56.
These conclusions, however, differ from the conclusions of the main analysis of the only other peer-reviewed published CEA (Micieli et al.)Citation57, which showed that, compared to PCC, andexanet alfa use was not cost-effective. This published CEA from a Canadian payer perspective had substantial uncertainty, reflecting the limitations of the currently available dataCitation57. Further, the model design, inputs, and use of naïve comparisons of two independent datasets to inform mortality and thromboembolic events from which the costs, utilities, and long-term survival are obtained drive the differences in results from the current study. The differences are noted as follows. First, whereas the current study utilized a decision tree in combination with three linked Markov models to estimate acute and long-term health states, Micieli et al.Citation57 used a simple Markov structure to model disease, in which a patient can either survive or die after an ICH, after which the surviving patient may experience a thromboembolic event. Second, Micieli et al.Citation57 did not model any functional outcome difference and did not account for differences in functional outcome after administration of andexanet alfa, though they partially address this in a one-way sensitivity analysis. Third, Micieli et al. did not adjust for potential differences in efficacy between study designs, using data from ANNEXA-4 to model the risk of 30-day mortality from ICH, and data on efficacy and safety of 4-factor PCCs from UPRATECitation28, a single-arm observational study. Although the mortality data used was different in Micieli et al.Citation57 (18.5% for andexanet alfa vs 33.9% for PCC) and the current work (15.3% for andexanet alfa vs 48.9% for PCC), when the mortality gap is decreased in the current model, the CEA results improve slightly as a result of fewer highly morbid patients in the andexanet arm surviving to incur long-term healthcare costs. Fourth, the differences in results between the two modeling approaches are driven largely by the segregation of our model into different functional outcomes after treatment. We used unpublished propensity score matching to show how the administration of andexanet alfa improves functional outcomes compared to PCC. This improvement in functional outcomes drives the cost-effectiveness of andexanet alfa because a lower mRS improves utility values, lowers the rehabilitation and long-term costs, and improves overall survival of the andexanet cohort compared to the PCC cohort because long-term mortality in the model is mRS-dependent. Time-dependent, but not functional outcome-dependent, hazard ratios of death were applied by Micieli et al. after the index ICH, and additional hazard ratios were applied for the patients who experience thromboembolic events. The authors concluded that PCCs are more likely to be cost-effective than andexanet alfa since the thromboembolic event rate in ANNXEA-4 is higher than that in UPRATE. Fifth, multiplicative hazard ratios sourced from different studies were used to estimate the risk of death in patients who had more than one condition (i.e. ischemic stroke after ICH), which could have biased the risk of long-term mortality. Lastly, any differences in the acute hospitalization costs associated with a lower probability of hematoma expansion were not considered.
Limitations
The analysis presented in this manuscript has several limitations. First, there are no data from prospective randomized trials comparing 4-factor PCC with andexanet alfa, and thus conclusions about the products’ comparative efficacy cannot be made reliably. Second, some of the key data inputs were obtained from unpublished literature, and references for mechanical ventilation and rehabilitation costs were relatively oldCitation40,Citation44,Citation46. The data were also pooled from multiple sources for some clinical inputs. These data might present some bias resulting from the variable study designs. Thirty-day mortality was obtained from Cohen et al.Citation24, which used propensity score matching analysis from ANNEXA-4 and ORANGE. This approach has limitations as there are differences in the exclusion criteria between ANNXEA-4 and ORANGE, with the former excluding patients who were expected to live <30 days, patients with a baseline Glasgow Coma Scale score of <7, and those with bleed volumes >60 mlCitation11. There was also a lack of bleed volumes and severity data from ORANGE, and thus the propensity score matching was unable to account for potentially key prognostic indicatorsCitation9. A consideration of these limitations should be balanced against the fact that the propensity score matching did match against patients who had been administered a PCC for DOAC reversal. Therefore, it is possible that this would have taken place only with patients having a reasonable expectation of survival. Huttner et al. also conducted a propensity score matching analysis in sICH patients from ANNEXA-4 and RETRACE IICitation33. Importantly, they were able to match factors such as bleed volume and intraventricular extension. Although the study was lacking in statistical power, a numerical advantage to andexanet alfa was observed in the in-hospital mortality (hazard ratio = 0.49; confidence interval = 0.24–1.04). The rate ratio of 30-day mortality from the Cohen et al. analysis of 0.31Citation24 is not inconsistent with this analysis. Regarding mRS distribution, discharge results from RETRACE II were used as a proxy for 30-day resultsCitation33; this should be noted as a limitation. To account for these uncertainties in clinical and cost inputs, sensitivity analyses were conducted and showed results similar to those of the main analysis. Third, several knowledge gaps exist and could have influenced results. The evidence informing the length of ICU stay as a function of hematoma expansion is limitedCitation20. Additionally, the data source for outcomes associated with hematoma expansion was limited by the paucity of information regarding direct anticoagulant use, as some patients in the study were taking warfarinCitation20. However, although the patient cohort is not identical to the cohort captured in ANNEXA-4, it was assumed that hematoma expansion, regardless of cause, would result in similar resource utilization. Lastly, only a few studies with limited sample sizes included data on the proportion of patients treated with andexanet alfa who subsequently required surgery or the proportion of patients given andexanet alfa/PCC combination therapy. There is a knowledge gap in the understanding of why, in whom, under which circumstances, and in which order combination therapy is used.
Conclusions
The results of this study using a comprehensive model to assess both acute and long-term outcomes suggest that the use of andexanet alfa for the reversal of factor Xa inhibitor-related ICH may improve patient quality of life and is likely to be cost-effective in a US context.
Transparency
Declaration of funding
This study was supported by Portola Pharmaceuticals, Inc., now Alexion, AstraZeneca Rare Disease.
Declaration of financial/other interests
JF reports no conflicts of interest. JNG has received research funding from Portola Pharmaceuticals, Octapharma Biopharmaceuticals, and Pfizer Pharmaceuticals; and has received consulting fees from Portola Pharmaceuticals, CSL Behring, and Philips Healthcare. BL is employed by Alexion Pharmaceuticals. ACB was formerly employed by Alexion Pharmaceuticals. RSB and FA are employed by Maple Health Group, which received funding from Portola Pharmaceuticals, now Alexion Pharmaceuticals, to perform this study. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Study concept and design: JF, JNG, BL, ACB, RSB, FA. Analysis: RSB, FA. Interpretation: JF, JNG, BL, ACB, RSB, FA. Manuscript drafting: RSB, FA. All authors were responsible for revising and critically appraising the manuscript for intellectual content and approved the final submission. All authors agree to be responsible for all aspects of the work.
Previous presentations
These data were submitted for presentation at the American Society of Health-System Pharmacists Mid-year 2020 virtual conference.
Supplemental Material
Download MS Word (79.9 KB)Acknowledgements
The authors would like to thank Ishveen Chopra, Ph.D., for medical writing and editorial assistance during the development of the manuscript. Alexion, AstraZeneca Rare Disease (the sponsor) funded the medical writing and editorial assistance and provided a formal review of the publication. Authors retain control and final authority of publication content and decisions, including the choice of journal.
Data availability statement
Alexion will consider requests for disclosure of clinical study participant-level data provided that participant privacy is assured through methods like data de-identification, pseudonymization, or anonymization (as required by applicable law), and if such disclosure was included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant-level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion-sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at https://alexion.com/our-research/research-and-development. Link to Data Request Form (https://alexion.com/contact-alexion/medical-information).
References
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125–e151.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–352.
- Milling TJ Jr, Clark CL, Feronti C, et al. Management of factor Xa inhibitor-associated life-threatening major hemorrhage: a retrospective multi-center analysis. Am J Emerg Med. 2018;36(3):396–402.
- Deitelzweig S, Neuman WR, Lingohr-Smith M, et al. Incremental economic burden associated with major bleeding among atrial fibrillation patients treated with factor Xa inhibitors. J Med Econ. 2017;20(12):1217–1223.
- Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799–808.
- Obonska K, Navarese EP, Lansky A, et al. Low-dose of oral factor Xa inhibitors in patients with a recent acute coronary syndrome: a systematic review and meta-analysis of randomized trials. Atherosclerosis. 2013;229(2):482–488.
- Investigators E, Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499–2510.
- Milling TJ Jr, Frontera J. Exploring indications for the use of direct oral anticoagulants and the associated risks of major bleeding. Am J Manag Care. 2017;23(4 suppl):S67–S80.
- Green L, Tan J, Morris JK, et al. A three-year prospective study of the presentation and clinical outcomes of major bleeding episodes associated with oral anticoagulant use in the UK (ORANGE study). Haematologica. 2018;103(4):738–745.
- Xian Y, Zhang S, Inohara T, et al. Clinical characteristics and outcomes of intracerebral hemorrhage related to factor Xa inhibitors. JAMA Netw Open. 2021;4(2):e2037438.
- Connolly SJ, Crowther M, Eikelboom JW, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380(14):1326–1335.
- Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373(25):2413–2424.
- Demchuk AM, Yue P, Zotova E, et al. Hemostatic efficacy and anti-FXa (factor Xa) reversal with andexanet alfa in intracranial hemorrhage: ANNEXA-4 substudy. Stroke. 2021;52(6):2096–2105.
- ANDEXXA® (coagulation factor Xa [recombinant], inactivated-zhzo). Lyophilized powder for solution for intravenous injection. [package insert]. Boston, MA: Alexion Pharmaceuticals, Inc.; 2021.
- Harrison SK, Garrett JS, Kohman KN, et al. Comparison of outcomes in patients with intracranial hemorrhage on factor Xa inhibitors versus vitamin K antagonists treated with 4-factor prothrombin complex concentrate. Proc (Bayl Univ Med Cent). 2018;31(2):153–156.
- Tomaselli GF, Mahaffey KW, Cuker A, et al. 2020 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(5):594–622.
- Baugh CW, Levine M, Cornutt D, et al. Anticoagulant reversal strategies in the emergency department setting: recommendations of a multidisciplinary expert panel. Ann Emerg Med. 2020;76(4):470–485.
- European Medicines Agency. Ondexxya (andexanet alfa). Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/ondexxya.
- Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016;316(10):1093–1103.
- Yaghi S, Dibu J, Achi E, et al. Hematoma expansion in spontaneous intracerebral hemorrhage: predictors and outcome. Int J Neurosci. 2014;124(12):890–893.
- Huttner HB, Gerner ST, Connolly S, et al. Andexanet alfa versus usual care in patients with DOAC associated intracerebral hemorrhage: a propensity-score based historical comparison of patients from ANNEXA4 and RETRACE II. Int J Stroke. 2020;15(1 suppl):62.
- Brown CS, Scott RA, Sridharan M, et al. Real-world utilization of andexanet alfa. Am J Emerg Med. 2020;38(4):810–814.
- Barra ME, Das AS, Hayes BD, et al. Evaluation of andexanet alfa and four-factor prothrombin complex concentrate (4F-PCC) for reversal of rivaroxaban- and apixaban-associated intracranial hemorrhages. J Thromb Haemost. 2020;18(7):1637–1647.
- Cohen AT, Lewis M, Connor A, et al. 30 day mortality following andexanet alfa in ANNEXA-4 compared with prothrombin complex concentrate (PCC) therapy in the ORANGE study for life-threatening non-vitamin K oral anticoagulant (NOAC) related bleeding. J Am Coll Cardiol. 2020;75(11):2242.
- Alexion Pharmaceuticals. Data on file. [unpublished]. 2020.
- Gerner ST, Kuramatsu JB, Sembill JA, et al. Characteristics in non–vitamin K antagonist oral anticoagulant-related intracerebral hemorrhage. Stroke. 2019;50(6):1392–1402.
- Schulman S, Gross PL, Ritchie B, et al. Prothrombin complex concentrate for major bleeding on factor Xa inhibitors: a prospective cohort study. Thromb Haemost. 2018;118(5):842–851.
- Majeed A, Agren A, Holmstrom M, et al. Management of rivaroxaban- or apixaban-associated major bleeding with prothrombin complex concentrates: a cohort study. Blood. 2017;130(15):1706–1712.
- Arachchillage DRJ, Alavian S, Griffin J, et al. Efficacy and safety of prothrombin complex concentrate in patients treated with rivaroxaban or apixaban compared to warfarin presenting with major bleeding. Br J Haematol. 2019;184(5):808–816.
- Khorsand N, Majeed A, Sarode R, et al. Assessment of effectiveness of major bleeding management: proposed definitions for effective hemostasis: communication from the SSC of the ISTH. J Thromb Haemost. 2016;14(1):211–214.
- Panos NG, Cook AM, John S, et al. Neurocritical Care Society (NCS) Pharmacy Study Group. Factor Xa inhibitor-related intracranial hemorrhage: results from a multicenter, observational cohort receiving prothrombin complex concentrates. Circulation. 2020;141(21):1681–1689.
- Gerner ST, Kuramatsu JB, Sembill JA, et al. Association of prothrombin complex concentrate administration and hematoma enlargement in non-vitamin K antagonist oral anticoagulant-related intracerebral hemorrhage. Ann Neurol. 2018;83(1):186–196.
- Huttner HB, Gerner ST, Kuramatsu JB, et al. Haematoma expansion and clinical outcomes in patients with factor-Xa inhibitor–related atraumatic intracerebral haemorrhage treated within the ANNEXA-4 trial versus real-world usual care: a comparative effectiveness study. Stroke. 2022;53(2):532–543.
- Jang MU, Kang J, Kim BJ, et al. In-hospital and post-discharge recovery after acute ischemic stroke: a nationwide multicenter stroke registry-base study. J Korean Med Sci. 2019;34(36):e240.
- Ganesh A, Luengo-Fernandez R, Wharton RM, et al. Time course of evolution of disability and cause-specific mortality after ischemic stroke: implications for trial design. J Am Heart Assoc. 2017;6(6):e005788.
- IBM Micromedex. Micromedex Red Book. Available from: https://www.micromedexsolutions.com/micromedex2/librarian/PFDefaultActionId/redbook.ModifyRedBookSearch/ssl/true.
- Smith MN, Deloney L, Carter C, et al. Safety, efficacy, and cost of four-factor prothrombin complex concentrate (4F-PCC) in patients with factor Xa inhibitor-related bleeding: a retrospective study. J Thromb Thrombolysis. 2019;48(2):250–255.
- Frontera JA, Lewin JJ 3rd, Rabinstein AA, et al. Guideline for reversal of antithrombotics in intracranial hemorrhage: executive summary. A statement for healthcare professionals from the Neurocritical Care Society and the Society of Critical Care Medicine. Crit Care Med. 2016;44(12):2251–2257.
- Shander A, Ozawa S, Hofmann A. Activity-based costs of plasma transfusions in medical and surgical inpatients at a US hospital. Vox Sang. 2016;111(1):55–61.
- Dasta JF, McLaughlin TP, Mody SH, et al. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266–1271.
- Halpern NA, Goldman DA, Tan KS, et al. Trends in critical care beds and use among population groups and Medicare and Medicaid beneficiaries in the United States: 2000–2010. Crit Care Med. 2016;44(8):1490–1499.
- Regan JM, Worley E, Shelburne C, et al. Burr hole washout versus craniotomy for chronic subdural hematoma: patient outcome and cost analysis. PLoS One. 2015;10(1):e0115085.
- Hoffman H, Furst T, Jalal MS, et al. Annual incidences and predictors of 30-day readmissions following spontaneous intracerebral hemorrhage from 2010 to 2014 in the United States: a retrospective nationwide analysis. Heliyon. 2020;6(1):e03109.
- Buntin MB, Colla CH, Deb P, et al. Medicare spending and outcomes after postacute care for stroke and hip fracture. Med Care. 2010;48(9):776–784.
- Shireman TI, Wang K, Saver JL, et al. Cost-effectiveness of solitaire stent retriever thrombectomy for acute ischemic stroke: results from the SWIFT-PRIME trial (Solitaire With the Intention for Thrombectomy as Primary Endovascular Treatment for Acute Ischemic Stroke). Stroke. 2017;48(2):379–387.
- Qureshi AI, Chaudhry SA, Sapkota BL, et al. Discharge destination as a surrogate for Modified Rankin Scale defined outcomes at 3- and 12-months poststroke among stroke survivors. Arch Phys Med Rehabil. 2012;93(8):1408–1413.e1.
- Centers for Medicare & Medicaid Services, HHS Medicare Program. Hospital inpatient prospective payment systems for acute care hospitals and the Long-Term Care Hospital Prospective Payment System and policy changes and fiscal year 2020 rates; quality reporting requirements for specific providers; Medicare and Medicaid promoting interoperability programs requirements for eligible hospitals and critical access hospitals; 2019. Available from: www.federalregister.gov/documents/2019/08/16/2019-16762/medicare-program-hospital-inpatientprospective-payment-systems-for-acute-care-hospitals-and-the%0D.
- John Hopkins Medicine. Appendix B: phytonadione (vitamin K) dosing guidelines. Interdisciplinary clinical practice manual, medication drug use, warfarin, management of the adult patient receiving, MDU023; 2009. [cited 2020 March 25]. Available from: https://www.hopkinsmedicine.org/hematology/anticoagulation/provider_information/guideline_pdfs/warfarin%20reversal.pdf.
- Tao J, Bukanova EN, Akhtar S. Safety of 4-factor prothrombin complex concentrate (4F-PCC) for emergent reversal of factor Xa inhibitors. J Intensive Care. 2018;6:34.
- Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association practical guide on the use of non-vitamin-K antagonist anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J. 2017;38(27):2137–2149.
- Coleman CI, Dobesh PP, Danese S, et al. Real-world management of oral factor Xa inhibitor-related bleeds with reversal or replacement agents including andexanet alfa and four-factor prothrombin complex concentrate: a multicenter study. Future Cardiol. 2021;17(1):127–135.
- Kunz WG, Hunink MG, Dimitriadis K, et al. Cost-effectiveness of endovascular therapy for acute ischemic stroke: a systematic review of the impact of patient age. Radiology. 2018;288(2):518–526.
- Ali M, MacIsaac R, Quinn TJ, et al. Dependency and health utilities in stroke: data to inform cost-effectiveness analyses. Eur Stroke J. 2017;2(1):70–76.
- Portola Pharmaceuticals. U.S. Centers for Medicare & Medicaid Services (CMS) grants new technology add-on payment for Portola Pharmaceuticals’ Andexxa; 2018.
- Haque M, Gratson M, Woerle J, et al. Beginning to understand the cost-effectiveness of Andexxa. Georgetown Medical Review. 2019;3(1). DOI:10.52504/001c.7777
- Frontera JA, Bhatt P, Lalchan R, et al. Cost comparison of andexanet versus prothrombin complex concentrates for direct factor Xa inhibitor reversal after hemorrhage. J Thromb Thrombolysis. 2020;49(1):121–131.
- Micieli A, Demchuk AM, Wijeysundera HC. Economic evaluation of andexanet versus prothrombin complex concentrate for reversal of factor Xa-associated intracranial hemorrhage. Stroke. 2021;52(4):1390–1397.