Abstract
Aims
Gene therapy trials aim to provide a functional cure for patients with haemophilia B (HB), and treatment impact is analyzed by factor IX expression levels (FELs). We investigated the relationship of FELs with health-related quality of life (HRQoL) and costs.
Materials and methods
This was a retrospective cross-sectional analysis of the European (CHESS I-II) and US (CHESS-US) CHESS population studies. Physicians recruited consecutive patients and extracted information from the medical records; patients completed questionnaires between 2014 and 2015 (CHESS-I), 2018–2019 (CHESS-II) and 2019 (CHESS US). Patients with inhibitors were excluded. HRQoL was assessed using the EQ-5D-5L. Twelve-month haemophilia-related direct medical costs included office visits and hospitalizations based on country-level unit costs. A Tobit model was used to analyze FELs and HRQoL and generalized linear models for direct medical costs.
Results
A total of 191 men with HB completed the EQ-5D questionnaire; the mean age was 36.8 years, with a mean FEL of 10.1 IU/dL (median, 4.0). Mean EQ-5D was 0.77 (SD, 0.23). The Tobit model adjusting for age, body mass index and blood-borne viruses showed every 1% increase in FEL was associated with +0.006 points in the mean EQ-5D score (p = .003). Mean haemophilia-related direct medical costs excluding factor replacement therapy were €2,028/year (median, €919) in CHESS I-II (EU, n = 226), and $7,171/year (median, $586) in CHESS US (n = 181). Adjusted EU and US models showed every 1% increase in FEL was associated with a decrease in haemophilia-related direct medical costs of €108/year and $529/year, respectively.
Limitations
Direct medical costs were based on physician extraction of encounters from medical records, potentially underestimating costs of care. The voluntary nature of participation may have introduced selection biases.
Conclusions
We observed a significant association of increases in FEL with increased HRQoL and decreased costs in Europe and the United States among men with HB and no inhibitors.
Introduction
Haemophilia B (HB) is a rare genetic disorder characterized by excessive bleeding due to insufficient or absent clotting factor IX. People with HB may have some endogenous clotting factor, such that having 5% to <40% of normal factor IX levels are classified as a mild disease, 1–5% is moderate, and having <1% is severe.Citation1 A bleeding prevention strategy with regular prophylactic administration of factor IX therapy is the standard of care for people with HB, particularly those with moderate or severe HB, though the on-demand treatment of bleeding events is used when necessary or preferred by the patientCitation2. The vast majority (92%) of costs associated with HB are from clotting factor treatment costs.Citation3 Lifelong management requirements bear a treatment burden for patients, and breakthrough bleeding events still happen.Citation4–6 Breakthrough bleeding events cause progressive joint damage with profound effects on health-related quality of life (HRQoL), work productivity for employed patients, and subsequent direct and indirect costs to patients and societyCitation7–9,Citation10.
Despite the advances of modern prophylactic treatment regimens to reduce the morbidity and mortality associated with HB, clinical research efforts continue to pursue a functional cure for this debilitating, lifelong conditionCitation11. Newer generation gene therapy candidates in particular offer potentially curative levels of effectivenessCitation12. Gene therapy trials evaluate the impact of treatment on factor IX expression levels (FELs), which have been associated with joint bleeds, with the aim of providing consistent coverage by reducing the frequency of troughs in factor IX levelsCitation13,Citation14. The relationship between FELs and clinical, humanistic, and economic outcomes is not well understood, especially among patients with mild or moderate HB.
We previously evaluated associations between FELs and annual bleeding rate (ABR) among people with mild, moderate or severe HB in order to facilitate broader interpretation of emerging results from ongoing gene therapy trials.Citation15 This analysis of the CHESS population studies explored the association of FEL with HRQoL and costs in patients with HB in Europe and the United States.
Methods
Study design and data
This was a retrospective cross-sectional analysis of data from the ‘Cost of Haemophilia in Europe: a Socioeconomic Survey’ (CHESS EU) and the ‘Cost of Severe Haemophilia across the US: a Socioeconomic Survey’ (CHESS US) population studies, the methods and primary findings from which have been reported.Citation16,Citation17 In order to fortify our sample of patients with mild or moderate HB, we supplemented the existing CHESS data with additional recruitment efforts.
The CHESS EU studies (CHESS I and CHESS II) included European adult males with haemophilia A or B from France, Germany, Italy, Spain, the United Kingdom (EU5), Denmark, Romania, and The Netherlands. CHESS I was only included people with severe haemophilia A or B; CHESS II included those with mild or moderate disease. Physicians recruited patients from consecutive office visits and completed electronic case report forms (eCRF) with information extracted from the patients’ charts. Patient-reported outcomes were collected from participating patients recruited by their haemophilia treating physicians from 2014 to 2015 for CHESS I and 2018–2019 for CHESS II.
CHESS US was a retrospective cross-sectional study of adult males with severe haemophilia in the United States. The CHESS US + study was conducted with the same methodological framework to supplement the original CHESS US data set with patient-reported outcomes. CHESS US participants completed online forms on costs and productivity outcomes over the past 12 months (conducted in 2019). Both CHESS US/US + sample populations recruited approximately the same proportions of patients with haemophilia A or B as observed in the US haemophilia population.
This study excluded patients with a current diagnosis of inhibitors. All data were anonymized to protect patient confidentiality. The CHESS studies, as well as this study, were reviewed and approved in accordance with the ethical requirements of the University of Chester’s Faculty of Health & Social Care. All patients provided informed consent.
Study participants
We recruited additional adult males (≥ 18 years) using the same approaches like those in the CHESS studies, where haemophilia treating physicians offered consecutive patients to participate in the study (adult males diagnosed with HB at least 12 months prior to the date of the office visit, which was defined as the index date).
A fieldwork agency recruited haematology specialists in the EU or US (Haemophilia Care Providers could participate in France) using a convenience sampling method. Eligible physicians had to have been in practice for ≥3 years and have ≥4 male patients with haemophilia per month. Physicians could enroll an average of 4 patients and retrospectively extract information from their medical records covering the prior 12 months.
Male adults with any severity of HB who provided informed consent were eligible to participate and completed Patient Public Involvement Engagement (PPIE) questionnaires regarding demographic and clinical characteristics and self-reported HRQoL. Since FELs tend to fluctuate for patients receiving prophylaxis regimens, where FELs are high after infusions and lower just before infusions, we excluded patients receiving prophylaxis regimens in order to avoid potential confounding of results. Only patients receiving on-demand regimens were included. Patients diagnosed with an inhibitor on enrollment, those with haemophilia A or another bleeding disorder, and those unable to complete the study materials were also excluded. For consistency, only eligible patients with HB from the original CHESS EU and CHESS US datasets receiving on-demand treatment were included in this analysis.
Variables and outcomes
Physicians reported demographic and clinical characteristics from the medical chart, including age, ethnicity, height, weight, body mass index (BMI), smoking status, HB severity, problem joints, and comorbidities. FELs were extracted from the medical chart by the physician. FELs among patients receiving on-demand treatment was the endogenous FIX expression used to characterize disease severity. Because no treatment was administered on a regular basis, this allowed an assessment of the relationship between endogenous expression and the outcomes of interest. Patients also self-reported key socio-demographic and clinical variables including employment status, the highest level of education, household income, perception of HB severity (mild, moderate, severe), frequency of bleeding events, pain, frequency of factor IX treatment infusions, and compliance/adherence with treatment.
Patient-reported HRQoL was assessed using the 5-level EQ-5D (EQ-5D-5L; www.euroqol.org). The EQ-5D-5L has five domains (mobility, self-care, performance of usual activities, pain/discomfort and anxiety/depression), each evaluated within 5 levels of severity (“no problems,” “slight problems,” “moderate problems,” “severe problems,” or “extreme problems”). Health state index utility scores across all domains ranged from zero, indicating health status rating equivalent to “death”, to 1 indicating “perfect health”; scores less than zero were possible (“worse than dead”)Citation18.
Haemophilia-related direct medical costs were based on healthcare resource utilization reported by the physicians, including office visits and hospital admissions. Office visit information included the frequency of visits, type of provider (haematologist or other specialties), and manner of visit (in-person or virtual consultation). Hospital admission information included the reason for the admission (haemophilia-related or other), planned or emergency admission, length of stay (calculated as time spent in hospital, with or without time in intensive care), and factor replacement therapy requirements during the hospitalization. On-demand treatment regimen information included the frequency of administrations (IU/kg per week) and estimated compliance. Twelve-month costs were calculated at the patient level based on the respective country-level unit costs. Unit costs for each medical resource were based on general public data sources for each country. The same costing year was used with local indexation applied where necessary.
Statistical analysis
Demographic and clinical characteristics were summarized using frequencies, proportions and measures of central tendency. For the primary objective of exploring associations with FEL, correlation analyses and scatterplots assessed the relationships between baseline FEL and HRQoL and costs. Kruskal-Wallis tests were performed to test for differences across groups followed by Mann-Whitney U tests for pairwise comparisons (including Bonferroni correction as applicable). No missing values were imputed; records with missing data were excluded.
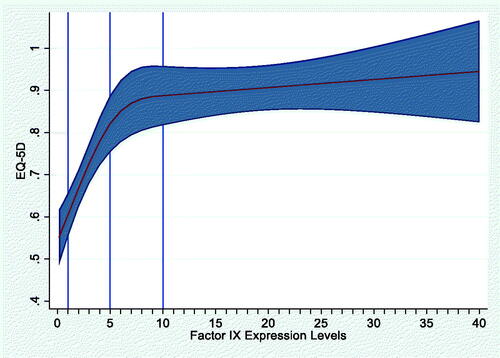
We evaluated two regression models for the analysis of FEL and HRQoL—an ordinary least squares (OLS) model and a Tobit model. A primary Tobit model was run as well as a second Tobit model that considered the disability paradox reported among patients with haemophilia.Citation19 The disability paradox shows higher than expected HRQoL scores among people with severe disease relative to how the general population ranks HRQoL associated with severe disease. This suggests that these patients have adapted to a markedly different basis for expectations related to HRQoL, the second Tobit model applied a decrement of −0.175 points to EQ-5D scores from patients with severe HB.
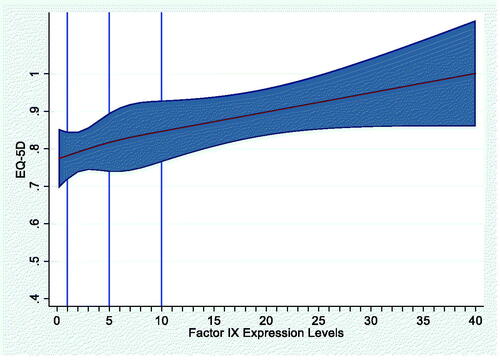
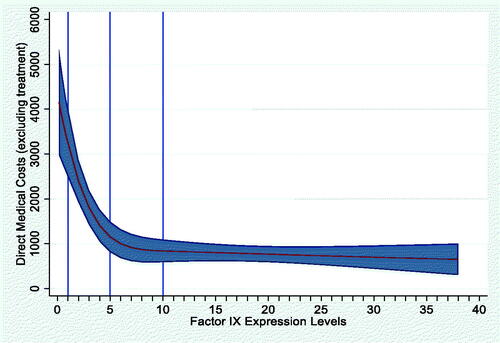
We evaluated three models for the analysis of FEL and haemophilia-related direct medical costs, including an OLS model, a generalized linear model (GLM) with gamma distribution and log link, and a GLM with inverse Gaussian distribution and identity link. EU and US cost models were evaluated separately. Multivariable restricted cubic spline (RCS) models were also developed to evaluate the potential non-linear relationship between FEL and direct medical costs.Citation20 The RCS regression models utilized 3 knots at baseline FEL values of 1, 5, and 10 IU/dL. All models excluded factor replacement therapy costs and controlled for covariates for age, BMI, and presence of blood-borne viruses (human immunodeficiency virus [HIV], hepatitis B virus [HBV], and hepatitis C virus [HCV]). All analyses were performed using STATA 16 (StataCorp LLC, College Station, TX, USA; www.stata.com).
Results
Study population
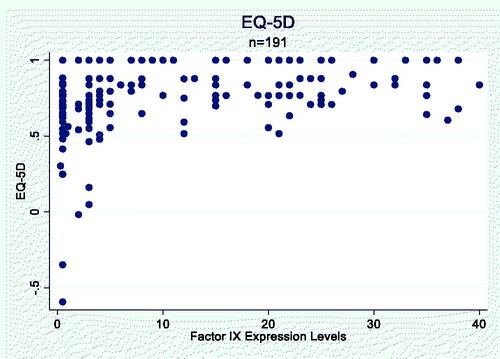
Across the CHESS EU and CHESS US cohorts, a total of 191 men with HB and no inhibitors completed the EQ-5D questionnaire. The mean age of the HRQoL cohort was 36.8 years, with a mean BMI of 25.42 kg/m2, and a mean ABR of 2.8 bleeds/year (). The HRQoL cohort had a mean baseline FEL of 10.1 IU/dL (SD, 11.4) with a median of 4.0 (range, 0.3–40.0).
Table 1. Demographic and clinical characteristics of the study cohort.
The EU and US cost analysis cohorts included 226 and 181 men with HB, respectively, with mean ages of 38.6 and 34.3 years (). The EU cohort had a mean BMI of 24.6 kg/m2, mean ABR of 2.9 bleeds/year, and mean baseline FEL of 9.6 IU/dL (SD, 11.0). The US cohort had a mean BMI of 31.4 kg/m2, a mean ABR of 1.7 bleeds/year, and a mean baseline FEL of 10.4 IU/dL (SD, 9.7).
Association of FEL with HRQoL
The mean EQ-5D score for the HRQoL cohort was 0.77 (SD, 0.23). The primary Tobit model provided the best fit for modeling the association of FEL with HRQoL. The scatterplot showed a generally positive relationship between FEL and HRQoL (EQ-5D score; Appendix ). The primary Tobit model adjusting for age, BMI and presence of blood-borne viruses (HIV, HBV, HCV) showed that every 1% increase in FEL was associated with an increase of 0.006 points in average EQ-5D score (p = .003; ). The RCS model did not show a non-linear relationship between FEL and HRQoL (p = .7991; Appendix A). Results from the second Tobit model accounting for the disability paradox in HRQoL responses from patients with severe HB are provided in Appendix B.
Table 2. Association between FEL and HRQoL (Tobit model; AIC, 148.6126).
Association of FEL with haemophilia-related direct medical costs
The mean haemophilia-related direct medical costs excluding factor replacement therapy were €2,028/year (median, €919) in the European analysis, and $7,171/year (median, $586) in the US analysis. For both models, the GLM with gamma distribution and log link provided the best model fit. After adjusting for age, BMI, and presence of blood-borne viruses, the EU model showed that for every 1% increase in FEL, haemophilia-related direct medical costs decreased by €108 per year (AIC, 16.93; ). In the US model, every 1% increase in FEL was associated with a $529 decrease in mean direct medical costs per year (AIC, 19.23; ). The RCS model showed a significant non-linear relationship between FEL and direct medical costs for the EU model only (p < .001; Appendix A).
Table 3. Association between FEL and direct medical costs.
Discussion
We observed a significant association of increases in FEL with increased HRQoL and decreased costs in Europe and the United States among men with HB and no inhibitors. Every 1% increase in FEL was associated with a 0.006-point increase in EQ-5D score for the entire multinational cohort, suggesting that substantial improvements in FELs for men with HB may be expected to yield marked improvements in HRQoL. Every 1% increase in FEL was also associated with reductions in mean annual haemophilia-related direct medical costs of €108 and $529 in the EU and US, respectively. These costs were modeled without the costs of factor replacement therapy to consider other treatment strategies that may have entirely different cost structures, and because of the overwhelming proportion of total costs accounted for by current factor replacement treatments.
This is the first study illustrating the relationship between FELs and HRQoL and direct medical costs in patients with HB. We included an international cohort of working-age men reporting all severities of HB, most often with mild or moderate disease (64–88% across cohorts) where data are particularly absent. Any level of chronic pain (52–64%) and having at least one problem joint (41–49%) were commonly reported in this sample, both of which are associated with reduced HRQoL and increased healthcare and societal costsCitation7–9,Citation10. However, the nature of these relationships with FELs among people with HB had not previously been quantified. This study offers the first tangible estimates of how improving FEL may increase HRQoL and decrease direct medical costs, both of which are essential to share patient-provider clinical decisions and health technology assessments. This study also supports prior work that ABR alone is not sufficient to differentiate current standards of care from gene therapy,Citation21 emphasizing the need for more relevant measures and the multi-factor core outcome set.Citation22
We expanded the HB sample of patients from the CHESS population health studies to include a larger proportion of people with mild to moderate HB (44–59%), as many burdens of illness studies focus on severe disease. Application of the validated and widely used EQ-5D instrument in our population provided consistent, reliable patient-reported measures of HRQoL across countries. It should be noted that direct medical costs were based on physician extraction of medical encounters that were captured in the patients’ medical records, potentially underestimating the costs of care. European society-level health system data and US insurance claim data may provide additional cost components with more granular cost detail. The voluntary nature of both physician and patient participation in the CHESS studies may have introduced potential selection biases, and the extraction of information by physicians may have been subject to reporting bias. It should be noted that the application of these findings is relevant to patients with HB receiving a prophylaxis treatment regimen, but these patients were necessarily excluded in order to analyze the relationship between FELs and outcomes without the inherent variability of FELs associated with prophylactic treatment administrations.
Conclusions
Quantifying the relationship between FELs and HRQoL and direct medical costs may lend important contextual information to emerging data on changes in FELs from gene therapy clinical trials. Clinical and health policy evaluations of treatment strategies that have the potential to provide curative relief of common haemophilia challenges, including those associated with treatment burden, should consider the humanistic and economic impact on patients and society. Our study may help patients, providers, and population health managers to contextualize FEL data from this perspective, as therapeutic goals may more closely approximate the reduced risk of bleeding and morbidity for people with HB as that observed in the general population.
Transparency
Declaration of funding
This study was supported by uniQure Inc. TMA and NL are employees of uniQure Inc., and participated in the study design, interpretation of findings, and drafting or critical review of the manuscript, including accountability for all aspects of the work.
Declaration of financial interests
TB, AS, and JOH are employees of HCD Economics which received research funding for this study from uniQure Inc. TMA and NL are employees of uniQure Inc. RC, DAGD, MR, TS and MS have no relevant relationships or financial interests related to this work. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors were involved in the conception and design of the study, as well as interpretation of data, drafting or critical revision of the paper for intellectual content, and final approval of the version to be published. TB and AS performed the data analyses. All authors are accountable for all aspects of the work.
Acknowledgements
Medical writing/editorial support was provided by Jeff Frimpter, MPH, of Integrative Life Sciences, sponsored by HCD Economics.
Data availability statement
The data that support the findings of this study may be available from HCD Economics, Ltd but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data may be available from the authors upon reasonable request and with permission of HCD Economics Ltd.
References
- Blanchette VS, Key NS, Ljung LR, et al. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12(11):1935–1939.
- Franchini M. The modern treatment of haemophilia: a narrative review. Blood Transfus. 2013;11(2):178–182.
- Chen CX, Baker JR, Nichol MB. Economic burden of illness among person with hemophilia B from HUGS Vb: examining the association of severity and treatment regimens with costs and annual bleed rates. Value Health. 2017;20(8):1074–1082.
- Martin LR, Williams SL, Haskard KB, et al. The challenge of patient adherence. Ther Clin Risk Manag. 2005;1(3):189–199.
- Thornburg CD, Duncan NA. Treatment adherence in hemophilia. Patient Prefer Adherence. 2017;11:1677–1686.
- Schrijvers LH, Beijlevelt‐van der Zande M, Peters M, et al. Adherence to prophylaxis and bleeding outcome in haemophilia: a multicentre study. Br J Haematol. 2016;174(3):454–460.
- O’Hara J, Walsh S, Camp C, et al. The impact of severe haemophilia and the presence of target joints on health-related quality-of-life. Health Qual Life Outcomes. 2018;16(1):84.
- Witkop M, Neff A, Buckner TW, et al. Self-reported prevalence, description and management of pain in adults with haemophilia: methods, demographics and results from the pain, functional impairment, and quality of life (P-FiQ) study. Haemophilia. 2017;23(4):556–565.
- Witkop ML, Lambing A, Nichols CD, et al. Interrelationship between depression, anxiety, pain, and treatment adherence in hemophilia: results from a US cross-sectional survey. PPA. 2019;13:1577–1587.
- O'Hara J, Walsh S, Camp C, et al. The relationship between target joints and direct resource use in severe haemophilia. Health Econ Rev. 2018;8(1):1.
- Spadarella G, Minno AD, Milan G, et al. Paradigm shift for the treatment of hereditary haemophilia: towards precision medicine. Blood Rev. 2020;39:100618.
- Dolan G, Benson G, Duffy A, et al. Haemophilia B: where are we now and what does the future hold? Blood Rev. 2018;32(1):52–60.
- den Uijl IEM, Fischer K, Van Der Bom JG, et al. Analysis of low frequency bleeding data: the association of joint bleeds according to baseline FVIII activity levels. Haemophilia. 2011;17(1):41–44.
- Nathwani A. Gene therapy for hemophilia. Hematology Am Soc Hematol Educ Program. 2019;2019(1):1–8.
- Burke T, Shaikh A, Ali TM, et al. Bleeding data across baseline FIX expression level in people with hemophilia B: an analysis using the ‘factor expression study. Blood. 2021;138(Supplement 1):592–592.
- Burke T, Asghar S, O'Hara J, et al. Clinical, humanistic, and economic burden of severe hemophilia B in the United States: results from the CHESS US and CHESS US + population surveys. Orphanet J Rare Dis. 2021;16(1):143.
- O'Hara J, Hughes D, Camp C, et al. The cost of severe haemophilia in Europe: the CHESS study. Orphanet J Rare Dis. 2017;12(1):106.
- Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343.
- O'Hara J, Martin AP, Nugent D, et al. Evidence of a disability paradox in patient-reported outcomes in haemophilia. Haemophilia. 2021;27(2):245–252.
- Harrell FE, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1988;80(15):1198–1202.
- Pierce GF, Ragni MV, van den Berg HM, et al. Establishing the appropriate primary endpoint in haemophilia gene therapy pivotal studies. Haemophilia. 2017;23(5):643–644.
- Orio A, Skinner MW, Clearfield E, et al. Core outcome set for gene therapy in haemophilia: results of the coreHEM multistakeholder project. Haemophilia. 2018;24(4):e167–e172.