Abstract
Aim
Atopic dermatitis (AD) is a chronic inflammatory skin disorder characterized by severe itching, erythema and scaling, causing pain, stigmatization and social isolation. Despite the growing availability of treatment options, unmet care needs remain. This research aimed to assess the cost-effectiveness of a novel JAK inhibitor (JAKi) compared to a monoclonal antibody and to identify key drivers of cost-effectiveness.
Materials and methods
A de novo economic model was developed to assess the cost-effectiveness of a novel JAKi compared to an established monoclonal antibody for the treatment of moderate-to-severe AD patients from a UK perspective. A targeted literature review was conducted to inform the development of the economic model with an advanced model structure. Various scenario and sensitivity analyses were performed to account for parameter- and structural uncertainty and to identify key drivers of cost-effectiveness.
Results
The JAKi was not cost-effective compared to the monoclonal antibody (£219,733.88 per quality-adjusted life year (QALY) gained) at selected price levels when applying the UK willingness-to-pay threshold of £30,000 per QALY gained. Key drivers of cost-effectiveness were utility values, intervention efficacy and drug acquisition costs. A decrease in JAKi’s dose costs, as well as a lower dose, lead to cost-effectiveness.
Limitations
Assumptions regarding parameter inputs were necessary, therefore a considerable level of uncertainty regarding efficacy and cost data is to be accounted for in the interpretation of the results. In particular, the efficacy data were based on single clinical studies.
Conclusions
This research revealed the cost-effectiveness of a JAKi compared to a monoclonal antibody for the treatment of moderate-to-severe AD to be highly sensitive to the costs and effectiveness inputs and identified further cost-effectiveness drivers. It demonstrated that the JAKi could be cost-effective compared to an established monoclonal antibody with a lower dose or a reduced price.
Introduction
Atopic dermatitis (AD), which is also referred to as atopic eczema, is a chronic inflammatory skin disorderCitation1. Displaying point prevalence in adults of 4.4% in the EU (including UK) and 4.9% in the USCitation2, AD belongs to the most common skin diseasesCitation3. It is characterized by severe itching, erythema, scaling and sometimes vesiculation and crustingCitation4. Patients not only experience skin painCitation5, they are also faced with stigmatization, lower self-esteem and social isolation which can cause sleep, depressive or anxiety disordersCitation6–8. This stress which is caused by AD reinforces its symptoms, resulting in a vicious cycleCitation8. Additionally, AD patients often suffer from further atopic diseases like asthma or allergic rhinitisCitation7. This high burden not only decreases AD patients’ quality of lifeCitation9, it also causes absenteeism and productivity lossesCitation10. No laboratory test for the diagnosis of AD existsCitation11. Instead, AD is diagnosed by clinical examination and its severity is classified with validated clinical tools like the eczema area and severity index (EASI)Citation11. For most patients, AD symptoms last their whole life even though good management can in phases mitigate severityCitation12.
Several options for treating AD exists. Over the counter (OTC) skin emollients and prescribed topical corticosteroids (TCS) are first-line treatment options in the UKCitation13, followed by topical calcineurin inhibitors (TCI) in second-lineCitation13 and phototherapy as a third-line therapyCitation13. If limited effectiveness is observed or the patient shows more severe symptoms, systemic pharmacotherapy (i.e. oral immunosuppressants) can be prescribedCitation13. For moderate-to-severe AD patients, monoclonal antibodies such as dupilumab or Janus kinase inhibitors (JAKi) like baricitinib have represented fifth-line therapy options to dateCitation7,Citation13. Despite the availability of these different treatment options for AD patients with diverse severity levels, optimal treatment for all patients does not exist yet. Current treatments lack practicability as the application requires time, is uncomfortable or not all patients fully respond to themCitation7,Citation13. Considering this treatment gap and disease burden, it is of clinical and societal importance that new treatments aiming to fulfill unmet care needs are constantly being developedCitation7. Upadacitinib is a novel JAKi and was recently approved for the treatment of moderate-to-severe AD by the European CommissionCitation14 and the Medicines and Healthcare products Regulatory Agency (MHRA)Citation15; it has not yet been recommended for reimbursement by NICECitation16. Abrocitinib which recently received marketing authorization by the European CommissionCitation17 and the investigational therapy tralokinumabCitation18 may also contribute to reduce the currently high unmet needs in AD in the future.
In order for patients to be able to benefit from a developed pharmacological therapy, it must not only be clinically effective to receive marketing authorizationCitation19; it additionally needs a positive reimbursement decisionCitation19. In several countries such as the UK, the relationship between costs and consequences of new and established therapies in terms of an economic evaluation is a critical element of the Health Technology Assessment (HTA) to decide on the reimbursement of novel therapiesCitation19.
Economic models can further reveal the most influential circumstances under which a treatment option can meet the established cost-effectiveness thresholdCitation20. Currently published economic models for AD treatments did not provide these insights. These economic models further either did not include JAKi, did not take the UK National Health Service (NHS) and Personal Social Services (PSS) perspective or used a model structure that could not depict the costs and consequences of a JAKi treatment compared to standard of care adequately. Furthermore, new trials that investigated higher treatment responses recently became available, suggesting the need for a new economic model. Therefore, the development of a de novo economic model that considers recent developments in AD treatment, as well as the specific recommendations and reimbursement conditions of the country of interest, motivated the aims of this research.
The aim of this research was to develop a de novo economic model for moderate-to-severe AD to conduct an economic evaluation that compares the JAKi upadacitinib to the monoclonal antibody dupilumab from the UK NHS and PSS perspective. It further aimed to identify the key drivers of cost-effectiveness. Such findings were expected to aid decision-makers in the reimbursement deliberations of future treatment options for AD.
Methods
Targeted literature review
A targeted literature review (TLR) was conducted to acquire information about the treatment of moderate-to-severe AD patients with upadacitinib and dupilumab regarding (a) treatment efficacy, (b) healthcare resource use and costs, (c) health-related utilities, and (d) existing economic evaluations to develop a de novo economic model. First, relevant HTA documents like reports by the Institute for Clinical and Economic Review in the US and, guidelines and technology appraisals (TA) by NICE were searched. Based on the findings, keywords and medical subject heading (MeSH) terms were predefined and connected with Boolean operators to make the subsequent TLR in PubMed more efficient. Only articles published in English no later than September 23, 2021 were considered. Appendix 1 contains the search strategy. The inclusion and exclusion criteria followed the PICTOS (Population, Intervention, Comparator, Outcome, Timing, Setting/Study Design) frameworkCitation21 and are presented in Appendix 2. Appendix 3 shows the corresponding Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chartCitation22.
Economic evaluation
A Markov cohort model was the preferred modelling technique because it allowed patients to switch between health states, return to, or stay within them for several cycles which were suitable for modeling longer time horizonsCitation19. This was in line with existing economic models concerned with AD that was published by NICE in TA534 (dupilumab) and TA681 (baricitinib) and by the Institute for Clinical and Economic Review (dupilumab)Citation13,Citation23,Citation24. These economic models served as reference points during the development of a novel version that included upadacitinib as an intervention and used an improved model structure that represented reality more accurately. The economic model took the perspective of UK NHS and PSS and followed therefore NICE’s reference caseCitation25. The economic model was developed using the programming language R.
Target population
The characteristics of the target population were obtained from the dupilumab economic model published by NICE in TA534 as this evaluation took the UK perspectiveCitation13. The target population thus consisted of adults, i.e. 18 years or older, with moderate-to-severe AD who have exhausted all previous lines of therapies due to loss of responseCitation13. In line with the dupilumab economic model by NICE in TA534, patients had suffered on average 29 years from the disease at the start of the modelCitation13. The base case population was 38 years old at the start of the economic modelCitation13. 60% of the population were males, 91% were ‘white’, 50% of the patients suffered from moderate and 50% from severe ADCitation13.
Intervention and comparators
Upadacitinib was selected as an intervention and compared to the established standard of care in the UK dupilumabCitation13. Although upadacitinib has not yet been recommended by NICE for the treatment of AD, it was chosen as the intervention because it is currently the most promising candidate of fifth-line treatments for moderate-to-severe AD, showing higher efficacy than abrocitinib and tralokinumabCitation26–28. Upadacitinib is a novel JAKi and therefore operates differently than dupilumabCitation29. With upadacitinib as the intervention, the evaluation uses frontier treatments of two available treatment classes.
It is unclear whether a daily oral dosage of upadacitinib 15 mg or 30 mg will be recommended by NICE. However, upadacitinib 30 mg showed the highest efficacy in a randomized controlled trial (RCT) and was therefore chosen in the base caseCitation28. The potential cost-effectiveness of upadacitinib 15 mg was tested in a scenario analysis. The comparator dupilumab is a fully-humanized monoclonal antibodyCitation30 and is prescribed in the UK as a fifth-line therapy option to moderate-to-severe AD patients since August 2018Citation13. According to NICE’s recommendations, dupilumab is injected subcutaneously, initially with a loading dose of 600 mg, followed by 300 mg every other weekCitation13. As dupilumab should be combined with TCS and TCICitation13, it was assumed that both, upadacitinib 30 mg and dupilumab, were administered as combined therapies. Best supportive care (BSC), which was included as a second line of treatment in this economic model, consisted of phototherapy, psychological support, TCS and TCICitation13. All patients, independent of the intervention, were allowed to receive emollients, treatments for flares and seek medical appointmentsCitation24.
Model structure
depicts this de novo economic model structure. All patients start in the induction phase and receive either dupilumab or upadacitinib 30 mg. Patients with treatment response, i.e. an improved skin condition of 50-74%, 75-89% or 90-100% after the first cycle, transition to the respective maintenance health state EASI 50, 75 or 90 and receive treatment as long as they maintain this level of response. Patients without response during the induction phase or loss of response in the maintenance health state, stop the intervention and transition to induction of BSC. Patients that achieve at least an EASI 50 after one cycle with BSC transition to BSC EASI 50. Patients without a response or loss of response to BSC transition to no response and remain in their health state until death. All patients can die after each cycle.
Figure 1. Model structure. Note. All patients start in the induction phase. Patients can transition to absorbing death health state from any health state. Abbreviations. BSC, best supportive care; EASI, eczema area and severity index.
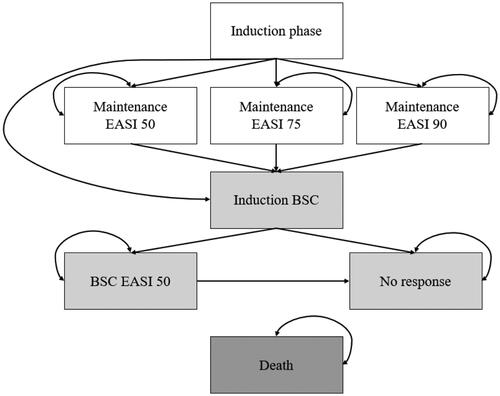
The rationales of the underlying assumptions were as follows: First, this de novo economic model incorporated EASI 50, 75 and 90 as three individual response health states because it was difficult to derive efficacy data for combined endpoints as RCTs usually do not report those. Furthermore, the inclusion of a single response health state might not be an appropriate depiction of clinical reality because it could neglect the quality of life and economic benefits that patients with higher response attain. Thus, comparative benefits of a new therapy could be captured probably more accurately by implementing several response health states with response-specific utilities and costs. This approach followed the Institute for Clinical and Economic ReviewCitation23. Both NICE models defined response to treatment as the combined endpoint, consisting of an EASI 50 and an improvement of the dermatology life quality index (DLQI) of 4Citation13,Citation24. Second, patients could not transition between response health states because it was challenging to obtain probabilities for transitions between response health states. This was in line with the dupilumab economic model published by the Institute for Clinical and Economic ReviewCitation23. Third, by implementing an induction health state and a cycle length of 16 weeks, patients could stop receiving the intervention in case of loss of response every 16 weeks which was in line with NICE’s recommendations for the treatment with dupilumabCitation13. The baricitinib economic model in TA681 also included an induction state but opted for a shorter cycle length of four weeksCitation24. To model the stopping rule, TA534 prefixed a decision tree before its Markov model which simultaneously increased complexityCitation13. Fourth, BSC was implemented as a second line of treatment because this approach was taken by the two economic models published by NICE also using the UK perspectiveCitation13. The dupilumab economic model by the Institute for Clinical and Economic Review on the other hand included only a no response health state with usual careCitation23. Fifth, this economic model implemented an absorbing death health state and assumed that neither the disease nor the treatments were associated with a change in mortality. Thus, the chance to die was assumed to be the same in all health states and mortality rates were derived from national life tables. These assumptions were based on the baricitinib economic model by NICE and the dupilumab economic model by the Institute for Clinical and Economic ReviewCitation23,Citation24. Sixth, a lifetime horizon was implemented in this economic model as AD is a chronic conditionCitation12 in line with all three existing economic modelsCitation13,Citation23,Citation24.
Transition probabilities
The transition probabilities were estimated based on the HeadsUp RCT that was identified during the TLRCitation31. This head-to-head trial between upadacitinib 30 mg and dupilumab provided the latest efficacy data after 16 and after 24 weeksCitation31. Other studies that were found during the TLR reported results of either of the two therapies individually against placebo, which were considered less relevant for this economic evaluationCitation28,Citation30.
The response rates after 24 weeks were linearly adjusted for the cycle length of 16 weeks. These transformed numbers served as probabilities for maintaining treatment response. The transition probabilities for the EASI 50 health states could not be derived directly from the HeadsUp trial as they were not reportedCitation31. Therefore, it was assumed that every patient that did not have a high response, needed rescue therapy, discontinued treatment for any reason or died, achieved an EASI 50. This assumption may, however, overestimate the proportion of patients that achieve an EASI 50. The HeadsUp study investigated upadacitinib 30 mg and dupilumab as monotherapies which was not in line with NICE’s recommendations regarding the treatment with dupilumab suggesting concomitant TCS and TCICitation13,Citation31.
The EASI 50 response rates for BSC following all interventions were derived from the LIBERTY AD CHRONOS study by taking the placebo EASI 50 response rates as a reference pointCitation30. This study investigated the efficacy of dupilumab as combined therapy and included the use of TCS and TCI in all groups, including the placebo groupCitation30.
Similar to previous economic models, neither AD nor the treatments investigated were assumed to be related to mortalityCitation13,Citation23,Citation24 and thus general mortality rates depending on age and gender were taken from the UK national life tableCitation32. Patients had to stop active treatment or BSC when they did not respond or lost response after 16 weeks, discontinued treatment, had severe side effects that forced a stop, or for which rescue therapy was neededCitation13,Citation28,Citation30. It was assumed that there was no difference in transition probabilities between moderately and severely affected AD patients due to a lack of separate numbers. The response rates of dupilumab and upadacitinib 30 mg are presented in .
Table 1. Transition probabilities.
Utilities
The utility values for each health state are presented in . All utilities except for the utilities in the BSC induction and in the no response health state were taken from the dupilumab economic model by the Institute for Clinical and Economic Review that included separated utilities for moderate and severe patientsCitation23. In the induction phase, patients had baseline utilityCitation23. The maintenance EASI 50 and the BSC EASI 50 health state were assumed to have the same utility. Patients that transitioned from intervention to induction of BSC or from BSC to no response were assumed to not immediately return to baseline utility but to have an intermediate utility insteadCitation13. Furthermore, a loss of benefit over time despite maintenance of response was assumedCitation13. Both assumptions were in line with the dupilumab economic model by NICE in TA534Citation13. In particular, utility benefit loss started from year 2 with 2%, 5% in year 3, 7% in year 4 and 8% from year 5 onwards in the intervention maintenance health statesCitation13. In the remaining health states, 25% of the benefit was lost in year 2, 50% in year 3, 75% in year 4 and from year 5 onwards, the patient returned to baseline utilityCitation13.
Table 2. Utilities.
Resource use and cost data
The resource utilization inputs were estimated based on TA534 and TA681Citation13,Citation24. Productivity losses were excluded as those costs are not relevant from an NHS and PSS perspectiveCitation33. Unit prices that were relevant for NHS and PSS were assignedCitation33. Costs were considered in 2020 pound sterling and no conversions were necessaryCitation33. All costs were adjusted according to the NHS cost inflation index (NHSCII) when appropriateCitation34,Citation35. Both costs and benefits were discounted by 3.5% which was in line with NICE’s reference caseCitation33. A half-cycle correction was applied.
provides an overview of the resource use and costs per health state and intervention. These could be categorized in (1) intervention costs, (2) other healthcare costs including BSC when applicable and (3) costs for treating adverse events. Although the HeadsUp trial did only present efficacy data for dupilumab and upadacitinib 30 mg as monotherapiesCitation31, this economic model assumed the occurrence of TCS and TCI costs during the intervention. Combined therapy was deemed more realistic and was recommended by NICE for the treatment with dupilumabCitation13. Intervention-specific resources for dupilumab, therefore, included dupilumab injections, injection training, TCS and TCI and for upadacitinib 30 mg included the medication itself and TCS and TCICitation24. Prices for medications were derived from the British National Formulary (BNF)Citation36. The 30 mg dosage was not approved yet and thus not listed in the BNFCitation36. Therefore, it was assumed that patients took double the dose of upadacitinib 15 mgCitation36. Other healthcare costs included costs for emollients, medical appointments, the treatment of flares, phototherapy, psychological support and blood monitoringCitation24. Costs for the treatment of adverse events included the treatment of allergic and infectious conjunctivitis and oral herpes in non-intervention statesCitation24. Dupilumab’s adverse event resource use consisted of the treatment of injection site reaction, allergic and infectious conjunctivitis and oral herpesCitation24. JAKi’s safety profile was characterized by an immunosuppressive effectCitation7. Therefore, upadacitinib 30 mg patients were assumed to be at risk for upper respiratory tract infections (URTI)Citation31. It was assumed that there was no difference in costs between moderate and severe AD patients due to the lack of separate volume data.
Table 3. Resource use and costs.
Results and analyses
Quality adjusted life years (QALY) gained and costs that occurred over the length of the economic model were summed up per intervention and used to calculate the incremental cost-effectiveness ratio (ICER) expressed in costs per QALY gainedCitation33. The WTP threshold set by NICE (£30,000) was used to define cost-effectivenessCitation33.
To account for uncertainty and to identify key drivers of cost-effectiveness, different sensitivity, scenario and threshold analyses were conducted. Several deterministic sensitivity analyses (SA) were performed to reveal to what extent single parameters (including start age, discount rates, time horizon, utilities, costs, efficacy) influenced the cost-effectiveness of the novel JAKi upadacitinib 30 mgCitation19. Results were depicted in tornado diagrams as recommended by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR)Citation35. To further account for the structural uncertainty, six alternative scenarios were constructed to assess the impact of different structural assumptions on cost-effectiveness. Firstly, a scenario was created where patients in higher response health states, i.e. EASI 75 and EASI 90 were assumed to have lower other healthcare costs due to their improved skin conditions and patients in the no response health state were assumed to have higher other healthcare costs due to their worsened skin condition. Secondly, it was assumed that there was no utility loss over time. Thirdly, an alternative model structure was created that included EASI 50 as the only response option similar to the dupilumab and baricitinib economic models published by NICECitation13,Citation24. Fourthly, upadacitinib 15 mg instead of upadacitinib 30 mg was compared to dupilumab. Efficacy data for this scenario was derived from Guttman-Yassky et al.Citation28. The impact of disease severity on the cost-effectiveness of upadacitinib 30 mg was assessed in a subgroup analysis in scenarios five and sixCitation19,Citation35. The subgroup analysis was difficult to conduct due to the lack of separate data. Thus, only moderately and severely affected patients in terms of utilities perceived in different health states could be assessed separately. As part of the threshold analysis, the value-based price (VBP), i.e. the price for upadacitinib 30 mg to be cost-effective at a certain WTP threshold was calculated for a WTP of £20,000 and £30,000.
Model validation
A TLR was conducted to ensure that relevant data sources were identified. A cross-validation based on existing economic dupilumab and upadacitinib 30 mg modelsCitation23,Citation39 published by the Institute for Clinical and Economic Review was conducted as recommended by the ISPOR-SMDM guidelinesCitation40. Time horizon and discount rates were adjusted when necessary to increase comparability. The costs of the economic models by the Institute for Clinical and Economic Review were not relevant as these were U.S. specific. A comparison with TA534 and TA681 was not possible due to censored dataCitation13.
Results
Base case results
In the base case, upadacitinib 30 mg had higher total QALYs (+0.023) and higher total costs (+£5,103.78) than dupilumab. This yielded an ICER of £219,733.88 (costs per QALY gained) for upadacitinib 30 mg compared to dupilumab, assuming a price of £57.54 per day for upadacitinib 30 mgCitation36. Considering NICE’s WTP threshold of £30,000 per additional QALY gainedCitation33, the JAKi updacitinib 30 mg was not cost-effective compared to the monoclonal antibody dupilumab. contains the detailed base-case results.
Table 4. Base case results.
Deterministic sensitivity analyses
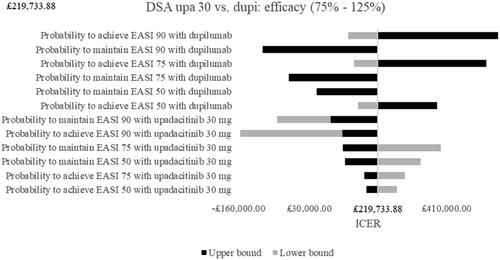
Results of the deterministic SA for upadacitinib 30 mg vs. dupilumab are depicted as tornado diagrams in and . Only the most influential parameters were presented. The analyses showed that utility values in the no response health state and in the maintenance health states had a high impact on the ICER. Higher utilities in the no response health state thereby led to a decreased and negative ICER and upadacitinib 30 mg became dominated by dupilumab. Higher utilities in the maintenance health state decreased the ICER as well but simultaneously improved the cost-effectiveness of upadacitinib 30 mg. Looking at costs, the most influential parameters were the drug costs of upadacitinib 30 mg and dupilumab. Whereas higher dose costs of upadacitinib 30 mg increased the ICER, higher dose costs of the comparator dupilumab lowered it and led almost to an ICER below the WTP threshold of £30,000. Lower upadacitinib 30 mg drug costs improved cost-effectiveness and upadacitinib 30 mg became dominant compared to dupilumab. The efficacy tornado diagram in shows that the probabilities to achieve or maintain a certain response with both drugs had remarkable impact. The increase of the probability to achieve an EASI 90 with dupilumab thereby had the highest impact and led to an increased ICER. An increased probability to maintain an EASI 90 with dupilumab, however, led to a lower and negative ICER. This pattern could be observed for the following dupilumab efficacy values as well. When the efficacy values of upadacitinib 30 mg were increased, the ICER decreased for all parameters while a decrease led to a higher ICER with the exception of the probabilities to achieve or maintain an EASI 90. Here, lower efficacy numbers led to negative ICERs, i.e. dupilumab dominated upadacitinib 30 mg.
Figure 2. Deterministic sensitivity analyses of selected parameters. Note. Upper bound of utility in no response was -£35,198,512.55. Abbreviations. DSA, deterministic sensitivity analyses; dupi, dupilumab; EASI, eczema area and severity index; ICER, incremental cost-effectiveness ratio; upa, upadacitinib.
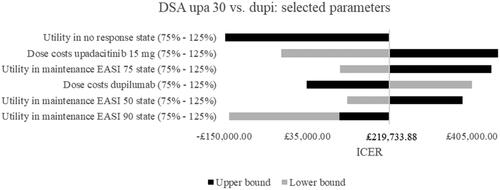
Figure 3. Deterministic sensitivity analyses of efficacy parameters. Note. Upper bound of probability to achieve EASI 90 with dupilumab was £1,735,169.38. Abbreviations. DSA, deterministic sensitivity analyses; dupi, dupilumab; EASI, eczema area and severity index; ICER, incremental cost-effectiveness ratio; upa, upadacitinib.
Scenario analyses
presents the results of the six alternative scenarios. When reduced other healthcare costs in higher response health states and higher other healthcare costs in the no response health state were assumed, the ICER decreased slightly. Even though cost-effectiveness did not change in this case, the results showed that implementing several response levels with differing costs instead of only one response level could increase precision. The second scenario assumed that there was no utility loss over time. Consequently, the no response health state became relatively better and the benefit between intervention and comparator decreased, leading to an increased ICER. The next scenario analysis where only one endpoint, i.e. EASI 50 was implemented instead of three led to a negative ICER, i.e. upadacitinib 30 mg was dominated by dupilumab and showed that the number of included response health states could impact the results. The fourth scenario compared a lower dose of upadacitinib to dupilumab. This resulted in lower costs and QALYs than dupilumab and a decreased but positive ICER. Nevertheless, upadacitinib 15 mg could be regarded as cost-effective compared to dupilumab when a willingness to accept threshold of £30,000 was assumed because the savings per QALY sacrificed were above that threshold. When only severe AD patients were considered, the cost-effectiveness improved as severely affected patients were expected to achieve a relatively higher benefit from successful treatment. However, the ICER was still above the WTP threshold of £30,000/QALY. When only moderately affected AD patients were included, the ICER increased.
Table 5. Scenario analyses.
Threshold analysis
In the base case, it was assumed that upadacitinib 30 mg costs £57.54 per dayCitation36. The VBP presented in revealed that the drug acquisition costs per day may not exceed £46.35 to reach cost-effectiveness, considering a WTP threshold of £30,000. Conversely, the price per day for upadacitinib 30 mg needs to be reduced by 19.5% in order for upadacitinib 30 mg to be cost-effective compared to dupilumab, considering a WTP threshold of £30,000.
Table 6. Threshold analysis.
Model validation
The economic model published by the Institute for Clinical and Economic Review yielded total QALYs of 16.28 for the treatment with dupilumab, applying a discount rate of 3%Citation23. This de novo economic model resulted in a total of 14.12 QALYs for dupilumab when the same discount rate was considered. The difference could be explained by the utility loss which was not assumed in the economic model by the Institute for Clinical and Economic ReviewCitation23. An updated economic evaluation published by the Institute for Clinical and Economic Review yielded total QALYs of 3.43 for dupilumab when the economic model run for five years and a 3% discount rate was appliedCitation39. Under these circumstances, the presented economic model resulted in 3.39 QALYs and thus was similar to the existing economic model. The same updated economic model resulted in 3.35 QALYs for upadacitinib 30 mgCitation39 while the de novo economic model yielded a similar value of 3.41 QALYs.
Discussion
This economic evaluation revealed that the JAKi upadacitinib 30 mg led to slightly higher QALYs than the biological drug dupilumab while causing higher costs. These higher costs were not caused by a higher price per dose but by a much higher administration frequency of upadacitinib compared to dupilumab. At the assumed price, upadacitinib 30 mg was found not to be cost-effective compared to dupilumab when a WTP threshold of £30,000 was applied. The analyses further showed that the key drivers of cost-effectiveness were utility values of the no response and the maintenance health states, drug costs of upadacitinib 30 mg and dupilumab, and efficacy of both interventions, in particular, the probability to achieve an EASI 90 response with dupilumab. With a decrease of upadacitinib 30 mg’s dose costs by approximately 20%, cost-effectiveness could be demonstrated. Efficacy data was based on a study where upadacitinib 30 mg was administered as monotherapy. The real efficacy of a combined therapy could be higher and, as revealed by the SAs, would improve the cost-effectiveness. This is the case for dupilumab as well but the SAs showed that an increase in the probability to maintain a low response with dupilumab could also decrease the ICER and increase cost-effectiveness of the JAKi. This unexpected finding probably occurred because the costs of staying in the dupilumab EASI 50 health state were relatively greater than the QALYs gained in this low-response health state. Exclusion of the EASI 75 and 90 response health states for both interventions led to a negative ICER as well and thus to dominance of dupilumab compared to upadacitinib 30 mg. This might be the case because the efficacy of dupilumab for a low response was higher as the dropout rate was lower. The scenarios showed that the inclusion and exclusion of response health states could have a huge impact on the results. Interestingly, the comparison of a lower dose of the JAKi (15 mg) to the biological drug led to cost-effectiveness of the JAKi. The QALYs gained with upadacitinib 15 mg were lower but the costs were lower as well.
This economic evaluation had several strengths. The model validation showed that the QALYs of this de novo economic model were similar to other economic models’ QALYs. Due to the inclusion of the relatively new JAKi upadacitinib 30 mg and the monoclonal antibody dupilumab which is the current standard of care, the economic model can be considered as being up to date. Furthermore, the hybrid model structure combined the advantages of several economic models and therefore a more precise and realistic analysis was possible. Three instead of one endpoint were incorporated into the economic model. Thus, the economic model accounted for higher quality of life benefits that occurred in higher response health states allowing a more accurate prediction of the costs and health benefits of both treatments. The need to implement more than one response health state was supported by the third scenario analysis which included only one EASI 50 maintenance health state and was similar to AD economic models published by NICECitation13,Citation24. The analysis showed that this approach could lead to an underestimation of the cost-effectiveness of the intervention (here upadacitinib 30 mg) which in turn can incorrectly inform research and development (R&D) or reimbursement decisions. Therefore, it was probably correct to include three response health states like the dupilumab economic model by the Institute for Clinical and Economic Review did instead of following NICE’s opinion that one response level would be sufficientCitation13,Citation23,Citation24. The cycle length of 16 weeks combined with the two induction health states increased precision and could consider NICE’s recommendation to stop the intervention in case of no response after 16 weeksCitation13. At the same time, it decreased complexity as a pre-fixed decision tree-like in TA534 was not neededCitation13. The use of a replicated dupilumab economic model published either by NICE in TA534 or by the Institute for Clinical and Economic Review would have probably led to a false ICER, the true key drivers of cost-effectiveness not being identified and in the case of the second economic model, the UK NHS and PSS perspective not being represented appropriately. Therefore, the combination of the existing economic models increased the reliability of the analyses and their results. It was in general difficult to find input data for the economic model but due to the TLR probably all data sources available could be identified. The inclusion of the second line of treatment with BSC before a final no response health state made the economic model and its results more realistic from a clinical practice perspective. Various analyses addressed the structural uncertainty and although it was difficult to agree on input data and to make reasonable structural model assumptions where no data was available, important insights regarding the cost-effectiveness of two drugs with different modes of operations were revealed.
Nevertheless, this economic evaluation also had limitations. The model structure did not allow patients to switch between response health states. It therefore indirectly assumed that a patient immediately falls below an EASI 50 when not achieving EASI 90. This may, however, not represent clinical reality correctly. Furthermore, the available data to develop the economic model was limited. TA534 and TA681 were censored which also impeded the external model result validationCitation13,Citation24. Efficacy data for dupilumab, upadacitinib 30 mg and BSC were based on single studiesCitation30,Citation31. Those studies did not consider the stopping rule that was assumed in the economic model and led to discontinuation when the patient did not respond or lost response after 16 weeksCitation30,Citation31. Moreover, the dupilumab and upadacitinib 30 mg response rates were available for 16 and 24 weeksCitation31 whereas the BSC response rates were available for 16 and 52 weeksCitation30. Additionally, the HeadsUp trial that was used for the transition probabilities of dupilumab and upadacitinib 30 mg did not report EASI 50Citation31. The necessary assumptions to obtain the respective transition probabilities anyway might have led to an overestimation of the proportion of patients that achieve and maintain an EASI 50 while being treated with either dupilumab or upadacitinib 30 mg. Furthermore, the HeadsUp trial investigated dupilumab and upadacitinib 30 mg as monotherapiesCitation31. However, NICE recommends dupilumab to be administered as a combined therapy with TCS and TCICitation13. The use of TCS and TCI during the intervention were included in the costs of the respective health states to at least depict this part in a more realistic way. The types of adverse events and their rates of occurrence were mainly derived from TA681Citation24. Those were, however, not in line with the study resultsCitation30,Citation31. As a result, costs and consequences caused by the occurrence of adverse events might be underestimated for all interventions. Furthermore, the study only incorporated the effects of upadacitinib and dupilumab on AD. As many patients have comorbidities such as asthma or allergic rhinitisCitation7, and both drugs could alleviate the symptoms of these comorbidities, this might impact the cost-effectiveness of the respective drug. No data for resource use of different response levels were available and thus it was assumed that costs for maintenance EASI 50, 75 and 90 were the same but this assumption could be incorrect. Due to a lack of distributions, no probabilistic sensitivity analysis (PSA) could be conducted. Finally, most data were not available separately for subgroups. Performing subgroup analyses was therefore restricted.
This research suggested that the existing NICE-approved AD economic models are no longer sufficient for the evaluation of new interventions. Novel treatment options for AD like upadacitinib aim for higher treatment responses than EASI 50. This is reflected by newer trials that report newer endpoints. Additionally, Silverberg et al. (2021) concluded that higher EASI improvements lead to higher improvements in patient-reported outcome measuresCitation41. Economic models need to accommodate these changes and new findings. Therefore, the use of only one combined endpoint does not meet the requirements of new interventions anymore, a fact that U.S. specific economic models already considerCitation23,Citation37. On the other hand, it is needed to stick closely to the existing AD economic models published by NICE to account for the UK NHS and PSS perspective. Therefore, complete replication of U.S. economic models is not feasible either. However, the combination of the advantages of existing economic models led to a complex model structure. While this probably depicts clinical reality more accurately, it might at the same time not live up to the purpose of a model which is to simplify reality. This is accompanied by the difficulty to obtain input data as data for a total of eight health states and corresponding transitions is needed, the cycle length of 16 weeks is relatively short and the stopping rule is a feature that is not commonly accounted for in RCTs. While further data generation of AD treatments, especially on long-term treatment response and compliance, may reduce treatment-specific parameter uncertainty, the patient and clinical expert opinion must be considered to address uncertainties on structural model parameters and assumptions for future economic analyses. Additionally, the data censoring of important HTA evidence hampered replication and cross-validation and will continue to do so in the future. By reporting in greater detail input data and results, future economic models could build upon existing models and external validation could be simplified. This would result in improved quality of economic models and more accurate results that can better inform decision-making.
Conclusions
While this de novo economic model demonstrated that the JAKi upadacitinib 30 mg was not cost-effective compared to the standard of care dupilumab under base-case assumptions, key cost and health effect drives were highlighted in various sensitivity analyses. Utility values, intervention efficacy and drug acquisition costs were most influential for upadacitinib 30 mg to be cost-effective compared to dupilumab. Furthermore, the scenario and threshold analyses demonstrated that using half of the dose of upadacitinib or reducing the daily drug acquisition costs of upadacitinib 30 mg by 20% led to cost-effectiveness of the JAKi. This research additionally exposed a critical limitation of replicating pre-existing models for AD. The improved AD economic model and the gained insights could help the industry to make informed R&D decisions in order to develop the required evidence to allow investigational products to achieve future reimbursement to reduce AD patients’ currently high unmet care needs. More robust clinical, cost and quality of life data in the future will allow more accurate simulation of the cost-effectiveness of therapies in AD and will enable suitable differentiation strategies.
Transparency
Declaration of funding
This research did not receive funding.
Declaration of financial/other relationships
DW is a registered Ph.D. student at Maastricht University and an employee of UCB Pharma, UCB Pharma had no role in the writing or reviewing of this manuscript. All other authors declare no conflict of interest. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors contributed to the study's conception and design. Material preparation, data collection, and analysis were performed by KH and DW. The manuscript was written by KH and all authors provided feedback throughout the development of the manuscript. All authors read and approved the final manuscript. All authors sufficiently contributed to this research according to ICMJE criteria to qualify as listed authors.
Acknowledgements
The authors would like to thank UCB Pharma for the knowledge support on the understanding of the disease.
References
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132(5):1132–1138.
- Barbarot S, Auziere S, Gadkari A, et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy. 2018;73(6):1284–1293.
- Avail Dermatology. Top 5 Most Common Skin Conditions. 2018. [cited 2021 Jun 10]. Available from: https://availdermatology.com/common-skin-conditions/.
- Williams HC, editor. Atopic dermatitis: the epidemiology, causes, and prevention of atopic eczema. Cambridge: Cambridge University Press; 2000.
- Vakharia PP, Chopra R, Sacotte R, et al. Burden of skin pain in atopic dermatitis. Ann Allergy Asthma Immunol. 2017;119(6):548–552. e3.
- Cheng C-M, Hsu J-W, Huang K-L, et al. Risk of developing major depressive disorder and anxiety disorders among adolescents and adults with atopic dermatitis: a nationwide longitudinal study. J Affect Disord. 2015;178:60–65.
- Love I, White K. Atopic dermatitis/atopic eczema: disease landscape & forecast. Decision Resources Group. 2020.
- Senra MS, Wollenberg A. Psychodermatological aspects of atopic dermatitis. Br J Dermatol. 2014;170(Suppl 1):38–43.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–347.
- Ariëns LFM, van Nimwegen KJM, Shams M, et al. Economic burden of adult patients with moderate to severe atopic dermatitis indicated for systemic treatment. Acta Derm Venereol. 2019;99(9):762–768.
- Frazier W, Bhardwaj N. Atopic dermatitis: diagnosis and treatment. Am Fam Physician. 2020;101(10):590–598.
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):345–360.
- National Institute for Health and Care Excellence. Dupilumab for treating moderate to severe atopic dermatitis: Technology appraisal guidance [TA534]. 2018. [cited 2021 Jun 10]. Available from: https://www.nice.org.uk/guidance/TA534.
- European Medicines Agency. Rinvoq: upadacitinib. 2021. [cited 2021 Dec 19]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq.
- Medicines and Healthcare products Regulatory Agency. SUMMARY OF PRODUCT CHARACTERISTICS. 2021. [cited 2021 Oct 23]. Available from: https://mhraproducts4853.blob.core.windows.net/docs/9890d20041d9d6b42d1d098c89bfe58333260ad7.
- National Institute for Health and Care Excellence. Upadacitinib for treating moderate to severe atopic dermatitis in people aged 12 and over [ID3733]: In development [GID-TA10597]. 2021. [cited 2021 Jun 10]. Available from: https://www.nice.org.uk/guidance/indevelopment/gid-ta10597.
- European Medicines Agency. Cibinqo: abrocitinib. 2021. [cited 2021 Dec 19]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/cibinqo.
- National Institute for Health and Care Excellence. Tralokinumab for treating moderate to severe atopic dermatitis [ID3734]: In development [GID-TA10596]. 2021. [cited 2021 Jun 28]. Available from: https://www.nice.org.uk/guidance/indevelopment/gid-ta10596.
- Drummond MF, Sculpher M, Claxton K, et al. Methods for the economic evaluation of health care programmes. 4th edn. Oxford: Oxford University Press; 2015.
- Ling DI, Lynd LD, Harrison M, et al. Early cost-effectiveness modeling for better decisions in public research investment of personalized medicine technologies. J Comp Eff Res. 2019;8(1):7–19.
- Nelson HD. Systematic reviews to answer health care questions. Philadelphia: Wolters Kluwer Health; 2014.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- Institute for clinical and economic review. Dupilumab and crisaborole for atopic dermatitis: effectiveness and value. 2017. [cited 2021 Jul 4]. Available from: https://icer.org/wp-content/uploads/2020/10/MWCEPAC_ATOPIC_EVIDENCE_REPORT_051217.pdf.
- National Institute for Health and Care Excellence. Baricitinib for treating moderate to severe atopic dermatitis: technology appraisal guidance [TA681]. 2021. [cited 2021 Jun 10]. Available from: https://www.nice.org.uk/guidance/ta681.
- Mauskopf J, Chirila C, Birt J, et al. Drug reimbursement recommendations by the national institute for health and clinical excellence: have they impacted the national health service budget? Health Policy. 2013;110(1):49–59.
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE Mono-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396(10246):255–266.
- Wollenberg A, Blauvelt A, Guttman-Yassky E, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021;184(3):437–449.
- Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145(3):877–884.
- Parmentier JM, Voss J, Graff C, et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494). BMC Rheumatol. 2018;2:23.
- Blauvelt A, M de B-W, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287–2303.
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157(9):1047–1055.
- Office for National Statistics. National life tables: UK. 2021. [cited 2021 May 6]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables.
- National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. 2013. [cited 2021 Jun 10]. Available from: https://www.nice.org.uk/process/pmg9/chapter/foreword.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019 | PSSRU. 2019. [cited 2021 Jun 6]. Available from: https://www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2019/.
- Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS)-explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines good reporting practices task force. Value Health. 2013;16(2):231–250.
- National Institute for Health and Care Excellence. BNF British National Formulary. 2021. [cited 2021 May 6]. Available from: https://bnf.nice.org.uk/
- National Health Service. National tariff payment system documents, annexes and supporting documents. 2020. [cited 2021 Jun 10]. Available from: https://www.england.nhs.uk/publication/national-tariff-payment-system-documents-annexes-and-supporting-documents/
- National Health Service. National cost collection for the NHS. 2019. [cited 2021 Jun 10]. Available from: https://www.england.nhs.uk/national-cost-collection/
- Institute for clinical and economic review. JAK inhibitors and monoclonal antibodies for the treatment of atopic dermatitis: effectiveness and value. 2021. [cited 2021 Jul 4]. Available from: https://icer.org/wp-content/uploads/2020/12/ICER_Atopic-Dermatitis_Draft-Evidence-Report_051421.pdf
- Eddy DM, Hollingworth W, Caro JJ, et al. Model transparency and validation: a report of the ISPOR-SMDM modeling good research practices task force-7. Med Decis Making. 2012;32(5):733–743.
- Silverberg JI, Lei D, Yousaf M, et al. What are the best endpoints for eczema area and severity index and scoring atopic dermatitis in clinical practice? A prospective observational study. Br J Dermatol. 2021;184(5):888–895.
Appendix 1. Search strategy
Appendix 2. Inclusion and exclusion criteria
Appendix 3. PRISMA flowchart
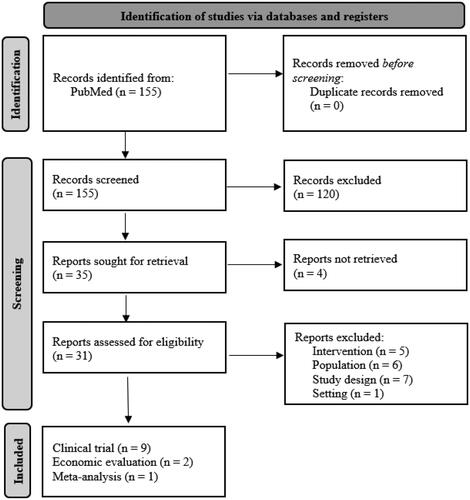
Note. Based on Page et al.Citation22.
