Abstract
Aims
Given the high rate of adverse events and high cost of adjuvant chemotherapy, it is optimal to avoid its use when endocrine therapy is equally effective at preventing distant recurrence of early breast cancer. The Oncotype DX test is a predictive and prognostic multigene assay used to guide adjuvant chemotherapy decisions in early breast cancer based on a Recurrence Score (RS) result. A model-based cost-effectiveness analysis compared the Oncotype DX test to clinical risk tools alone for HR+/HER2– node-positive (1–3 axillary lymph nodes) early breast cancer patients based on results from the RxPONDER trial.
Materials and methods
A decision-tree and Markov model was developed in Microsoft Excel. Distributions of patients and distant recurrence probabilities with endocrine and chemo-endocrine therapy were derived from the RxPONDER trial, TransATAC and SWOG-8814. Chemotherapy assignment data were obtained from the Clalit registry. The cost of adjuvant chemotherapy was based on the distribution of treatments used in the UK combined with published drug unit costs in the UK. The cost of distant recurrence and health state utility values were obtained from literature.
Results
The Oncotype DX test was found to be more effective (with an estimated 0.02 additional QALYs) at a lower estimated cost (–£989) compared to clinical risk tools alone. The results did not substantially change with more conservative clinical and cost scenarios. The RxPONDER trial was restricted to RS 0–25, and data synthesis with other studies was required to inform the analysis, which increased uncertainty.
Conclusions
The Oncotype DX test is highly likely to be cost-effective in node-positive early breast cancer. The results were driven by reduction in the use of chemotherapy with consequence avoidance of the costs and harmful effects of chemotherapy. Targeted treatment of a minority (11%) of women with RS 26–100 who benefit from chemotherapy reduced cost and improved survival.
Background
Adjuvant treatment using endocrine therapy or chemo-endocrine therapy is the standard of care for the prevention of metastases after breast-conserving surgery for hormone receptor positive (HR+) and human epidermal growth factor receptor 2 (HER2) negative early breast cancerCitation1,Citation2. The assessment of the clinical profile of breast cancer tumors, including the nodal burden and disease stage supports decisions on the choice of adjuvant treatment. Gene expression profiling of tumors using multigene assays (MGAs) is used in addition to clinical risk tools to guide the use of adjuvant chemotherapy. The Oncotype DX Breast Recurrence Score test (the Oncotype DX test; Exact Sciences Corp., Madison, WI) assesses the expression of 21 genes in tumor tissue and reports a Recurrence Score (RS) result. The ability of the Oncotype DX test to provide prognostic risk assessment for distant recurrence and predict the effect of chemotherapy has been demonstrated for patients with node-positive (N1; 1–3 positive lymph nodes) early breast cancer in the TransATACCitation3 and SWOG-8814 trialsCitation4. These results challenge the accepted practice of considering adjuvant chemotherapy for all patients with involved axillary lymph nodes based on the NHS Predict tool, which is informed by older data from the Early Breast Cancer Trialists’ Collaborative GroupCitation5. The TransATACCitation3 and the Clalit registryCitation6 studies showed that most N1 tumors had RS results 0–18. This patient group has the lowest risk of distant recurrence with no apparent benefit of adjuvant chemotherapy in terms of disease-free recurrenceCitation3,Citation4.
The RxPONDER study was designed to provide more clarity on the value of chemotherapy for N1 patients with HR + and HER2 negative breast cancer with RS results between 0 and 25. Patients entering the study were randomized between chemo-endocrine therapy and endocrine therapy aloneCitation7. The study showed no statistically significant difference in invasive disease-free survival (IDFS), distant relapse-free survival (DRFS), or distant recurrence-free interval (DRFI) at 5 years in post-menopausal patientsCitation8,Citation9. A statistically significant benefit of chemotherapy was observed in the premenopausal patient group, independent of age, tumor grade or nodal burden with an absolute benefit of 4.9% for 5-year IDFS 3.3% for 5-year DRFS, and 2.4% for 5-year DRFI. Two-thirds of patients in the RxPONDER trial were postmenopausal, which suggests that most N1 patients with RS result 0–25 can safely forego adjuvant chemotherapy, reducing the risk of harmful short-term and long-term effects and lowering the cost of adjuvant treatmentCitation8.
The cost-effectiveness of the Oncotype DX test in early breast cancer in the UK was assessed by the National Institute for Health and Care Excellence (NICE) in Diagnostic Guidance 34 (DG34). NICE conducted separate analyses for node-negative low and intermediate risk patients, and an exploratory analysis for N1 patients. In the base case, the NICE model did not assume the predictive value of the Oncotype DX test and informed the analysis using a bespoke analysis of the TransATAC study data, which was restricted to patients assigned to endocrine therapy alone. This analysis assumed that all patients assigned to chemotherapy derive the same benefit by applying a hazard ratio of 0.76 irrespective of underlying clinical or genomic riskCitation10. In a scenario analysis, the NICE model assumed a predictive benefit based on SWOG-8814 and Paik et al.Citation4,Citation11, concluding that the Oncotype DX test was dominant (more effective and cost saving) compared to clinical practice alone. This was in line with the findings from the economic evaluation by Hall et al., which was informed by the OPTIMA Prelim Trial in N1 or high-risk node-negative patientsCitation12. The study outlined in this article aimed to build upon the economic evaluation conducted by NICE with the latest evidence from the RxPONDER phase III trial and updated treatment costs for adjuvant chemotherapy and treatments for distant recurrence.
Methods
Study population, intervention, and comparators
An economic evaluation was conducted to estimate the cost-effectiveness of the Oncotype DX test compared to clinical risk tools alone to guide the use of adjuvant chemotherapy. The model population included patients with ER+/HER2– early breast cancer and one to three positive axillary lymph nodes. The study population was aligned with the NICE model in DG34 and was not restricted by clinical or genomic risk.
Model structure
An executable model was built in Microsoft Excel and Visual Basic for Applications. The modeling approach consisted of a decision-tree model followed by a Markov model. This method is consistent with previous evaluations of tumor profiling in the UK and the NICE appraisal in DG34Citation10,Citation13 ( and ). The costs and consequences of chemotherapy decisions were estimated over a lifetime horizon using 6-month cycles. The analysis was conducted from the perspective of the UK National Health Service (NHS) and personal social services according to the reference case set by NICECitation14. All future costs and outcomes were discounted at a rate of 3.5% per year. Costs were presented in 2020 Pound Sterling, with unit costs published in previous years uplifted using the Hospital and Community Health Services (HCHS) indexCitation15.
Figure 1. Diagram of the decision-tree part of the model. The square node represents the decision whether to use the Oncotype DX test or clinical risk alone to guide chemotherapy decisions. The circle nodes are chance nodes representing the distribution of genomic risk and probability of chemotherapy assignment. The triangle nodes are the terminal nodes for the decision tree and the point at which patients enter the Markov portion of the model.
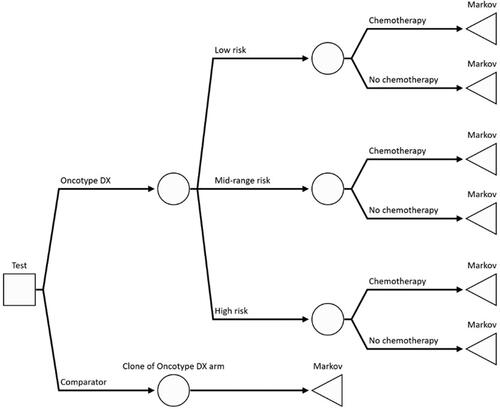
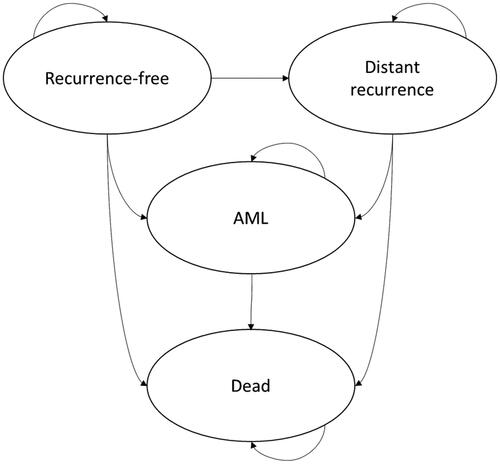
Figure 2. The long-term impact of distant recurrence of breast cancer and acute myeloid leukemia (AML) as a long-term adverse event of chemotherapy was extrapolated beyond the horizon of the clinical study using a Markov model with four mutually exclusive health states: “distant recurrence-free”, “distant recurrence”, “AML” and “dead”. It was assumed that 10.5% of patients entering the distant recurrence state previously experienced a local recurrence.
Clinical inputs
The distribution of patients according to RS result (RS 0–13, RS 14–25, RS 26–100) was based on the RxPONDER studyCitation8. Given that RxPONDER recruited patients with RS result 0–25, the size of the higher-score category was estimated based on the number of patients excluded from the study with RS result 26–100. The probability of being assigned to adjuvant chemotherapy conditional on RS result was obtained from the Clalit registryCitation6. The proportion of patients assigned to chemotherapy according to clinical risk alone was obtained from the National Cancer Registration and Analysis Service data reported in NICE DG34Citation10. The RS result cut-points used in analysis of the Clalit data (RS 0–10, RS 11–25, RS 26–100) were deemed to match closely to the cut points used in RxPONDER. The probability of distant recurrence conditional on the RS result, menopausal status, and the chosen adjuvant treatment was obtained from the RxPONDER study. In the base case, the probability was based on 5-year DRFI estimates presented at the San Antonio Breast Cancer Symposium 2021Citation16. This secondary endpoint was preferred as it allowed for the direct estimation of the probability of distant recurrence, unlike IDFS or DRFS endpoints reported in the study manuscript, which represent combined endpoints of distant or local recurrence, secondary cancer, or death. For patients with RS result 26–100, the probability of distant recurrence was informed by the 10-year DRFI reported in the bespoke analysis of TransATAC patients with 1–3 positive nodes and RS result 31–100 used in NICE DG34Citation10. It was assumed that DRFI in this group is an adequate approximation of the risk of distant recurrence for patients with RS result 26–100, with scenario analyses conducted to test this assumption. The TransATAC study included postmenopausal patients only, and it was assumed that these data apply to the full model population, which included both premenopausal and postmenopausal women. The hazard rate of distant recurrence for patients with RS result 0–25 who were assigned to chemotherapy was obtained directly from the chemotherapy arm of RxPONDER. For patients with RS result 26–100, the treatment effect of chemotherapy was derived from SWOG-8814Citation4, with the assumption that the primary endpoint in SWOG-8814 (disease-free survival) is closely related to freedom from distant recurrence-free, and therefore the same treatment effect applies to both. The baseline probability of distant recurrence in the clinical risk alone arm was based on the distant recurrence data from RxPONDER and TransATAC/SWOG-8814, reflecting the underlying distribution of RS results that these patients would have had if the Oncotype DX test had been used. Local recurrence was modeled implicitly, with 10.5% of patients who enter the distant recurrence health state assumed to have previously experienced local recurrence based on De Bock et al.Citation17 The incidence of short-term adverse events (AEs) linked to chemotherapy was based on grade 3 or 4 AEs reported in the RxPONDER study with an incidence of at least 2% in either treatment arm ()Citation8. The probability of developing AML as a long-term AE of chemotherapy was derived from a meta-analysis of studies reporting the risk of toxicity for chemotherapy treatments in breast cancerCitation18.
Table 1. Grade 3 or 4 adverse event costs based on incidence reported in the RxPONDER trial.
The probability of death without distant recurrence was assumed to be equal to the age-adjusted background mortality derived from UK life tables for females in 2016–2018 (Table A1)Citation19. An adjustment was carried out for death caused directly by chemotherapy (0.3%) informed by the TACT trialCitation20. Probability of death after distant recurrence of breast cancer was based on overall survival in the abemaciclib and fulvestrant arm of the MONARCH 2 trialCitation21. The 6-month probability of death following development of AML was based on the median survival in the CPX-351 treatment group of Study 301 as reported in the NICE technology appraisal of liposomal cytarabine-daunorubicin for acute myeloid leukemia in the UKCitation22.
Table 2. Scenario analysis results.
Health-related quality of life inputs
The impact of breast cancer recurrence and chemotherapy AEs on health-related quality of life (HRQoL) in the model was estimated using health state utility values and utility decrements. The main source of utilities for the recurrence-free and distant recurrence health states was obtained from a study of 361 breast cancer patients in SwedenCitation23. Local recurrence was modeled using a one-off utility decrement based on Campbell et al.Citation24 The utility level in acute myeloid leukemia was obtained from the NICE appraisal of liposomal cytarabine-daunorubicin for untreated acute myeloid leukemiaCitation25. A utility decrement of 0.038 based on Campbell et al. was applied to all patients receiving chemotherapy to reflect the loss in utility associated with treatment administration and AEsCitation24. Health state utilities were adjusted for background morbidity based on cohort age using age-specific general population utilities in the UKCitation26.
Cost inputs
The cost of the Oncotype DX test was based on UK list pricesCitation10. Scenario analyses explored the impact of confidential price discounts for the Oncotype DX test in the UK (). The distribution of chemotherapy regimens was obtained from clinical expert opinion collected by the investigators in the absence of published data. Administration and follow-up costs were based on the method reported in Ward et al.Citation13. For the estimation of per-cycle drug costs, dosage schedules for chemotherapy regimens were sourced from UK guidelinesCitation27–30 and combined with unit costs obtained from eMITCitation31 and BNF OnlineCitation32. Cost assumptions for supportive treatments (G-CSF and aprepitant) were based on Hall et al. and updated based on personal communication with the author of the studyCitation12. Both aprepitant and filgrastim 5 units per chemotherapy cycle were assumed in 20% of all anthracycline cycles. The cost of G-CSF was applied to all patients assigned to taxane chemotherapy or the accelerated epirubicin/cyclophosphamide/paclitaxel (EC/P) regimen. Dosage of G-CSF was modeled based on the mean and distribution of weight of a sample of early breast cancer patients in Edinburgh Cancer Centre and an assumed dose of 0.5 million units per kg (Dr. Peter Hall, personal communication). All patients were assumed to receive ongoing adjuvant endocrine therapy for five years in the recurrence-free health states based on Ward et al.Citation13 A summary of chemotherapy and endocrine therapy cost calculations is reported in and . Drug unit costs are reported in .
Table 3. Early breast cancer chemo-endocrine therapy cost.
Table 4. Details of chemotherapy regimens in early breast cancer.
Table 5. Drug unit costs.
Table 6. Cost of treatment regimens in metastatic breast cancer.
The cost of distant recurrence consisted of the cost of treatments used in metastatic breast cancer and non-drug costs of management (primary care, inpatient and outpatient hospital care) (). The distribution of treatments in metastatic breast cancer was based on a UK observational studyCitation33 and clinical expert opinion. The length of each line of treatment was based on the NICE appraisal of abemaciclib for previously untreated metastatic breast cancer (NICE TA563)Citation35. It was assumed that CDK4/6 inhibitors are used as first-line or second-line treatment options for metastatic breast cancer based on advice from clinical experts and NICE guidelinesCitation34–36. Given that patients with distant recurrence of breast cancer are expected to receive continuous treatment with multiple lines of drug treatment, the costs of distant recurrence were applied in every cycle a patient spent in this health state until death or transition to AML. A scenario analysis was conducted applying the cost of distant recurrence treatment in the first cycle after a recurrence event only, in line with the assumption in the NICE model for DG34Citation10.
Table 7. Base case parameter values, ranges and distributions.
Analytical approach
The base case analysis presented the cost-effectiveness of the Oncotype DX test compared to clinical risk alone in terms of cost per quality-adjusted life-years (QALYs) gained. Disaggregated results were presented in terms of cost by category (cost of Oncotype DX test, adjuvant chemo-endocrine and endocrine therapy, short-term AEs, AML, distant recurrence and end-of-life care cost for breast cancer-related deaths). Life-years and QALYs accumulated by health state were presented for the Oncotype DX test and clinical risk alone. Uncertainty analyses were presented using tornado diagrams to represent the impact of individual parameter values on the model results. One-way analyses were conducted using net monetary benefit, which is easier to interpret than the incremental cost-effectiveness ratio (ICER) in the presence of dominant or dominated comparators. A probabilistic sensitivity analysis (PSA) was conducted by sampling all uncertain parameter values from pre-defined probability distributions using Monte Carlo simulation with 5,000 iterations. The results of the PSA were presented using a scatter plot on the cost-effectiveness plane and the cost-effectiveness acceptability frontier. Acceptability curves plotted the probability of cost-effectiveness of the Oncotype DX test based on a range of willingness-to-pay thresholds per QALY. Plausible ranges and distributions for all parameters included in uncertainty analyses are reported in .
Table 8. Incremental cost-effectiveness of the Oncotype DX test compared to clinical risk alone.
Scenario analyses
Scenario analyses were conducted to explore alternative assumptions related to chemotherapy assignment, probability of distant recurrence, chemotherapy benefit, costs and utility values in the model. The scenario analysis results were presented in terms of incremental cost, QALYs and ICERs.
The treatment effect of chemotherapy from SWOG-8814 was reduced by 50% to test a lower treatment effect for patients with RS result 26–100. Given the uncertainty in the use of chemotherapy for patients with RS 14–25 influenced by the findings from the RxPONDER trial, the proportion of patients assigned to chemotherapy in this subgroup was increased by 20% to gauge the impact on the analysis results. Given that clinico-pathologic factors such as tumor size and grade could be correlated with RS results and influence the use of chemotherapy, scenario analyses tested lower chemotherapy assignment for women with underlying RS 0–25 when chemotherapy decisions were made based on clinical risk alone.
To explore the impact of price discounts on the model results while maintaining confidentiality, an arbitrary discount of 20% was applied to the list price for the Oncotype DX test in the UK. The same discount was applied to the list price of G-CSF, which reduced the total cost of adjuvant chemotherapy in the model. Given that the use of G-CSF in UK clinical practice has changed in response to the coronavirus pandemic, we explored a scenario with increased use of filgrastim with all anthracycline chemotherapy cycles. Based on advice from Dr. Peter Hall, we explored a scenario where FEC-T is replaced by accelerated EC/P, which reflects recent changes in clinical practice in the UK. The cost of distant recurrence was applied in the first cycle only, which reflects the more conservative assumption used in the NICE model in DG34.
Results
Base case cost-effectiveness analysis results
The Oncotype DX test was dominant compared to clinical risk alone, meaning that it generated more QALYs at a lower net cost over a lifetime. In the base case analysis, the Oncotype DX test generated 0.02 additional QALYs and cost savings of £989 (). Breakdown of cost by category is presented in . The main driver of cost savings was reduction in chemotherapy assignment from 75% to 20% of all N1 patients. Reduction in AML and short-term AEs as adverse effects of chemotherapy contributed to cost savings and improvements in life-years and QALYs gained.
Table 9. Breakdown of cost for the Oncotype DX test compared to clinical risk alone.
Table 10. Comparison of model results to NICE DG34, Oncotype DX vs. clinical risk alone.
Comparison to the NICE model
The updated model was compared to the analysis conducted in the NICE appraisal of tumor profiling testsCitation10 (). There were several differences in the structure and assumptions between the model described here and the NICE model, which complicated head-to-head comparisons between the two analyses: the updated model used clinical data sources aligned with the RS result cut-points used in RxPONDER, while the NICE model applied the previous cut-points from the TransATAC study. In addition, the updated model contained revised estimates of the cost of chemotherapy and distant recurrence, which were larger than the values used in the NICE model. This was driven by the inclusion of recently approved treatments for metastatic breast cancer, such as CDK4/6 inhibitors, which were not widely used at the time of the NICE appraisal. The current model applied the cost of distant recurrence in all cycles until death, while the NICE model applied this cost in the first cycle after the distant recurrence event. The results in the two models were broadly aligned if the updated model used clinical data sources aligned with TransATAC/SWOG-8814 RS result cut-points. When the model settings were aligned in terms of RS cut-points and method for distant recurrence cost, the results were similar in terms of expected outcomes. In the aligned model, assuming that the Oncotype DX test is prognostic only resulted in negative incremental QALYs, which reflected the base case conclusion of the NICE model. Updated estimates of cost of chemotherapy and distant recurrence resulted in higher cost savings estimated in the updated model. Assuming predictive ability yielded a dominant outcome for the Oncotype DX test in both the NICE model and the updated model.
Uncertainty analyses
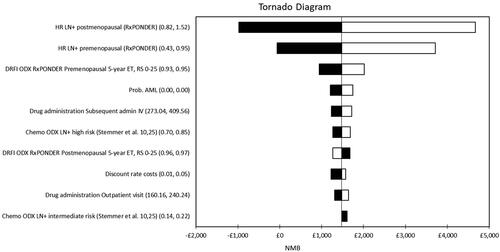
Results of one-way sensitivity analyses are presented in . The treatment effect of chemotherapy, represented as the hazard ratio for distant recurrence with chemotherapy from the RxPONDER study, was the largest contributor to model uncertainty. Distant recurrence rates in the premenopausal subgroup based on RxPONDER also had a large impact on the model results.
Figure 3. Tornado diagram representing the 10 parameters which had the largest impact on the model results. The diagram displays the effect of varying the value of each parameter across a pre-determined range which reflects the confidence interval reported in the original published source or the assumed distribution. The results are presented in terms of net monetary benefit assuming the NICE ceiling willingness-to-pay of £20,000 per QALY: NMB=£20,000 × incremental QALYs – incremental cost.
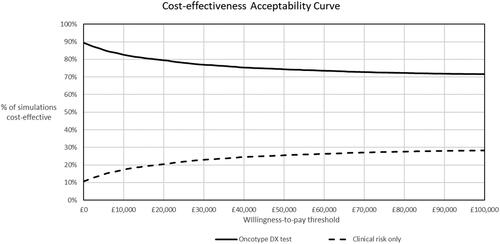
The overall uncertainty from all parameters in the model is illustrated in the cost-effectiveness plane in and cost-effectiveness acceptability curve in .
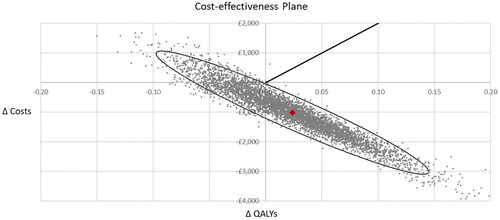
Figure 4. Scatter diagram from the probabilistic sensitivity analysis. The PSA was conducted using Monte Carlo simulation with 5,000 random draws from pre-defined probability distributions for all uncertain parameters. In the probabilistic analysis, the Oncotype DX test generated 0.02 QALYs with a cost saving of £1,017 per patient compared to clinical risk alone, which was consistent with the results of the deterministic cost-effectiveness analysis. Sixty-eight percent of the points in the scatter diagram were in the dominant south-east quadrant. The ellipse represents the area which contains 95% of the simulated points. The line from the origin corresponds to the £20,000 per QALY willingness-to-pay threshold for cost-effectiveness set by NICE.
Scenario analyses
Scenario analysis results are presented in . Overall, the analysis conclusions did not change when alternative assumptions were applied in the model. Increasing the proportion of patients assigned to chemotherapy after using the Oncotype DX test by 20% resulted in an ICER per QALY of £8,568. Relaxing the assumption of a constant 75% chemotherapy assignment for all patients who were not tested with the Oncotype DX test increased the ICER per QALY to £8,224. Applying the cost of distant recurrence as a one-off cost increased the lifetime cost savings from –£989 to –£1,653, which reflects the impact of uncertainty in the cost of subsequent treatments after distant recurrence. Decreasing the assumed chemotherapy benefit in the RS 26–100 subgroup by 50%, and setting more conservative assumptions relating to the long-term probability of distant recurrence did not have a significant impact on the analysis results. Applying an arbitrary price discount of 20% to the list price of the Oncotype DX test increased incremental cost savings from £989 to £1,505. Overall, the incremental QALYs did not change significantly under different scenarios, which mostly tested chemotherapy assignment assumptions and unit costs, which had the largest impact on incremental cost in the model. Using alternative data sources for utility values did not significantly change incremental QALY estimates. The biggest source of uncertainty in terms of incremental QALYs was the hazard ratio estimates for the treatment effect of chemotherapy in RxPONDER, which is reflected in the deterministic and PSA results.
Discussion
Interpretation of results
Adjuvant chemotherapy reduces the risk of distant recurrence for many patients with HR + and HER2 negative breast cancer at the expense of an increased risk of short-term toxicity and long-term AEs including AML. Current guidelines suggest considering adjuvant chemotherapy for all N1 patients, because nodal status is a predictor of recurrence alongside other clinicopathological factors at the population level. Evidence emerging from randomized trials and real-world studies suggest that a large proportion of patients with N1 early breast cancer have very high distant recurrence-free survival with endocrine therapy aloneCitation6,Citation8,Citation40, suggesting there is significant scope to reduce the use of adjuvant chemotherapy within this patient group without worsening outcomes, and thus to reduce the burden of AEs and cost of treatment. The use of MGAs, such as the Oncotype DX test, can be used in combination with clinical risk tools to identify which patients can be safely spared chemotherapy. The study described in this article estimated the cost-effectiveness of the Oncotype DX test in node-positive early breast cancer compared to using clinical risk alone informed by evidence from the RxPONDER phase III trial.
The top-line results from the cost-effectiveness analysis suggest that the Oncotype DX test is less costly and more effective compared to clinical risk alone. This result was driven by reduction in the cost of chemotherapy and the treatment of AEs, and improvements in quality of life from avoiding short and long-term AEs of chemotherapy. The Oncotype DX test is expected to reduce the overall chemotherapy assignment from 75% to 20%.
NICE has previously conducted a cost-effectiveness analysis to compare EPClin, Prosigna, MammaPrint and IHC4 + C against clinical risk alone as part of the Diagnostic Assessment in 2018Citation10. Given that no new clinical studies for the prognostic or predictive ability of the above tests have been published since the NICE appraisal, the addition of these comparators into the current analysis would have contributed little to the evidence base. The External Assessment Group (EAG) analysis in the node-positive subgroup was informed by TransATAC data for EPClin, Prosigna and IHC4 + C. The EAG concluded that EPClin and Prosigna were expected to be a cost-effective option for node-positive patients with an ICER within the range of £20,000 and £30,000 per QALY considered to be cost-effective by NICE. The base-case analysis showed IHC4 + C to be dominant compared to clinical risk alone, although the EAG considered the results highly uncertain due to the design of the validation study and gaps in the clinical evidence base. Given that the outcomes in terms of DRFS were similar for node-negative and node-positive patients in the MINDACT trialCitation41, MammaPrint was unlikely to be considered cost-effective for node-positive patients based on the EAG analysis. Given that the DRFI outcomes from MINDACT were comparable to those of RxPONDER, the results of the EAG analysis were driven by a larger proportion of patients classified as high-risk with MammaPrint (54% for high clinical risk and 18% for low clinical risk, compared to 11% classified as high-risk with the Oncotype DX test for the N1 population in TransATAC and estimates for RxPONDER). This led to a larger proportion of patients assigned chemo-endocrine therapy, which was confirmed by the Bloomfield et al. studyCitation42 and the UKBCG study data in the EAG report. However, NICE did not make a recommendation regarding the use of these MGAs to guide chemotherapy decisions in N1 early breast cancer in the UK due to insufficient data on the prognostic ability of the tests and substantial uncertainty in the EAG economic analysis.
RxPONDER demonstrated that adjuvant chemotherapy is effective across the full RS result range for premenopausal patients. Due to a lack of recently published studies of the impact of the Oncotype DX on chemotherapy decisions after the publication of RxPONDER results, an assessment of its cost-effectiveness analysis by menopausal status is subject to substantial uncertainty and was out of scope for the analysis described in this article and could be the subject of a future study.
Model uncertainty
The results of the cost-effectiveness analysis were subject to substantial uncertainty around some of the clinical input parameters. The results were particularly sensitive to the assumed chemotherapy benefit for women with RS results 0–25 based on the RxPONDER study. The cost-effectiveness of the Oncotype DX test varied according to the probability of chemotherapy assignment without the Oncotype DX test, which was assumed to be constant across the population and based on the NCRAS database in the UK. The incorporation of clinicopathologic information was one of the key methods identified in a systematic review and critique of economic evaluations of the Oncotype DX testCitation43 and remains one of the main uncertainties in the model described here. The PSA results showed a high probability of cost-effectiveness for the Oncotype DX in the presence of uncertainty based on acceptable willingness-to-pay thresholds set by NICE.
Study limitations
The cost-effectiveness analysis relied on a synthesis of evidence which may have contributed to overall model uncertainty. Distant recurrence probabilities for the Oncotype DX test were derived from RxPONDER for patients with RS result 0–25 and TransATAC for patients with RS result 26–100 (by applying data for RS result 30–100). In the absence of protocol-defined adjuvant chemotherapy in TransATAC, the effectiveness of chemotherapy was based on the RS result 30–100 subgroup from SWOG-8814. It was assumed that the treatment effect of chemotherapy for disease-free survival was applicable to distant recurrence rates in the absence of published evidence. The treatment effect from chemotherapy based on SWOG-8814 was robustly tested using conservative scenario analysis, with no substantial changes to study conclusions.
Comparison to published evidence
The cost-effectiveness of the Oncotype DX test in ER+/HER2– early breast cancer in the UK was assessed by NICE in DG34, which included an exploratory analysis for patients with 1–3 positive axillary lymph nodesCitation10. The NICE model was informed by a bespoke analysis of the TransATAC dataset and assumed a 24% reduction in the hazard rate of distant recurrence for all patients assigned to adjuvant chemotherapy in their base case analysis. In a scenario analysis informed by treatment effectiveness data from Paik et al. and SWOG-8814Citation4,Citation11, the Oncotype DX test was shown to be dominant compared to clinical risk alone. In the model outlined in this article, survival and HRQoL were less sensitive to the choice of treatment effect estimates and thus did not have the same impact as in the NICE model. Unlike in the NICE model, a price discount for the Oncotype DX test offered through the Patient Access Scheme was not included for reasons of confidentiality in the current analysis, further limiting the comparison of the updated model to the analysis conducted by NICE. The findings from the current analysis were consistent with the economic analysis of the OPTIMA prelim trial, which demonstrated the Oncotype DX test to be a cost-saving alternative to decisions based on clinical risk alone in the N1 ER + populationCitation12.
Recommendations for policy and future research
The study confirmed previously reported evidence of the economic value of the Oncotype DX test, which complements the clinical value of the test to guide chemotherapy use in ER+/HER2 negative N1 early breast cancerCitation44. It incorporated the latest evidence from the RxPONDER trial, which demonstrated that postmenopausal patients with RS results 0–25, which made up 67% of the trial population, can be safely spared adjuvant chemotherapy. Importantly, the analysis demonstrated that substantial cost savings and reduction in the burden of both short and long-term AEs can be achieved for the overall N1 population, which provides much-needed evidence for clinical decision-makers and could contribute to the upcoming review of the NICE guidance for tumor profiling tests in early breast cancer and future appraisals by HTA bodies in other countries. Chemotherapy sparing for patients at a lower risk of distant recurrence will reduce the strain on oncology units, which is particularly relevant at this challenging time for the health service. Multiple data gaps remain, notably for chemotherapy assignment with and without the Oncotype DX test and the effect of chemotherapy conditional on RS result by menopausal status, which should be the focus of future cost-effectiveness studies (Table A1).
Transparency
Declaration of financial/other relationships
Steve Millen, Andrew Paramore, Sarah Reynia and Nina Fryer are employees of Exact Sciences. Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Authors contributions
VB: conceptualization, formal analysis, methodology, writing – original draft, review and editing; SM: conceptualization, writing – original draft, review and editing; AP: conceptualization, methodology, writing – original draft, review and editing; PH: methodology, writing – original draft, review and editing; TP: methodology, writing – original draft, review and editing; RB: data curation, writing – original draft, review and editing; JG: writing – original draft, review and editing; SR: writing – original draft, review and editing; NF: writing – original draft, review and editing; LL: writing – original draft, review and editing.
Previous presentations
An exploratory analysis for postmenopausal women with node-positive early breast cancer based on the model described in this article was presented at the 2021 ASCO Annual Meeting: J Clin Oncol. 2021;39(15_suppl):534. DOI: 10.1200/JCO.2021.39.15_suppl.534.
Acknowledgements
We would like to acknowledge the expert input of Dr. Mark Verrill, Prof. Robert Coleman, Prof. Carlo Palmieri and Dr. Richard Simcock to validate model assumptions and identify model inputs.
Data availability statement
The parameter inputs used in the model were identified from sources in the open domain or peer-reviewed journal articles. All parameter values and their sources are reported in the article and appendix. The Excel data traces used to generate the results of the cost-effectiveness model can be obtained upon written request to the corresponding author.
Additional information
Funding
References
- Biganzoli L, Wildiers H, Oakman C, et al. Management of elderly patients with breast cancer: updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol. 2012;13(4):e148–e160.
- Early and locally advanced breast cancer: diagnosis and management NICE guideline [Internet]; 2018; [cited 2020 Aug 14]. Available from: www.nice.org.uk/guidance/ng101
- Sestak I, Buus R, Cuzick J, et al. Comparison of the performance of 6 prognostic signatures for estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4(4):591–553.
- Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11(1):55–65.
- Wishart GC, Azzato EM, Greenberg DC, et al. PREDICT: a new UK prognostic model that predicts survival following surgery for invasive breast cancer. Breast Cancer Res. 2010;12(1):1–10.
- Stemmer SM, Steiner M, Rizel S, et al. Clinical outcomes in ER + HER2 -node-positive breast cancer patients who were treated according to the recurrence score results: evidence from a large prospectively designed registry. NPJ Breast Cancer. 2017;3(1):1–7.
- Ramsey SD, Barlow WE, Gonzalez-Angulo AM, et al. Integrating comparative effectiveness design elements and endpoints into a phase III, randomized clinical trial (SWOG S1007) evaluating oncotypeDX-guided management for women with breast cancer involving lymph nodes. Contemp Clin Trials. 2013;34(1):1–9.
- Kalinsky K, Barlow WE, Gralow JR, et al. 21-gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med. 2021;385(25):2336–2347.
- Kalinsky K. Predicting chemotherapy benefit in node-positive breast cancer with the 21-gene test: the Oncotype DX Breast Recurrence Score® Test. San Antonio Breast Cancer Conference; 2021.
- National Institute for Health and Care Excellence. Tumour profiling tests to guide adjuvant chemotherapy decisions in early breast cancer. London (UK); 2018.
- Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726–3734.
- Hall PS, Smith A, Hulme C, et al. Value of information analysis of multiparameter tests for chemotherapy in early breast cancer: the OPTIMA prelim trial. Value Health. 2017;20(10):1311–1318.
- Ward S, Scope A, Rafia R, et al. Gene expression profiling and expanded immunohistochemistry tests to guide the use of adjuvant chemotherapy in breast cancer management: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2013;17(44):1–302.
- National Institute for Health and Care Excellence. Guide to the methods of technology appraisal [Internet]; 2013. Available from: https://www.nice.org.uk/process/pmg9/chapter/foreword
- Curtis Lesley A, Burns A. Unit costs of health and social care 2018. Project report. Canterbury (UK): University of Kent; 2018.
- Kalinsky K, Barlow WE, Gralow JR. Distant-disease free interval in participants with 1–3 positive lymph nodes, hormone receptor-positive and HER2-negative breast cancer with recurrence score < or = 25 randomized to endocrine therapy +/- chemotherapy: SWOG S1007 (RxPONDER). San Antonio (TX): San Antonio Breast Cancer Symposium; 2021.
- De Bock GH, Putter H, Bonnema J, et al. The impact of loco-regional recurrences on metastatic progression in early-stage breast cancer: a multistate model. Breast Cancer Res Treat. 2009;117(2):401–408.
- Petrelli F, Borgonovo K, Cabiddu M, et al. Mortality, leukemic risk, and cardiovascular toxicity of adjuvant anthracycline and taxane chemotherapy in breast cancer: a meta-analysis. Breast Cancer Res Treat. 2012;135(2):335–346.
- Office for National Statistics. National life tables 2016–2018, United Kingdom [Internet]; 2019; [cited 2021 Feb 3]. Available from: https://www.ons.gov.uk/releases/nationallifetablesuk2016to2018
- Ellis P, Barrett-Lee P, Johnson L, et al. Sequential docetaxel as adjuvant chemotherapy for early breast cancer (TACT): an open-label, phase III, randomised controlled trial. Lancet. 2009;373(9676):1681–1692.
- Sledge GW, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6(1):116–124.
- National Institute for Health and Care Excellence. Liposomal cytarabine–daunorubicin for untreated acute myeloid leukaemia. London (UK); 2018.
- Lidgren M, Wilking N, Jönsson B, et al. Health related quality of life in different states of breast cancer. Qual Life Res. 2007;16(6):1073–1081.
- Campbell HE, Epstein D, Bloomfield D, et al. The cost-effectiveness of adjuvant chemotherapy for early breast cancer: a comparison of no chemotherapy and first, second, and third generation regimens for patients with differing prognoses. Eur J Cancer. 2011;47(17):2517–2530.
- National Institute for Health and Care Excellence. Azacitidine for the treatment of myelodysplastic syndromes, chronic myelomonocytic leukaemia and acute myeloid leukaemia. London; 2011.
- Ara R, Brazier J. Deriving an algorithm to convert the eight mean SF-36 dimension scores into a mean EQ-5D preference-based score from published studies (where patient level data are not available). Value Health. 2008;11(7):1131–1143.
- London Cancer Alliance West and South. LCA breast cancer clinical guidelines [Internet]; 2016; [cited 2022 Feb 18]. Available from: http://rmpartners.cancervanguard.nhs.uk/wp-content/uploads/2017/03/lca-breast-cancer-clinical-guidelines-october-2013-updated-march-2016-.pdf
- NHS Thames Valley. Chemotherapy regimens breast cancer [Internet]; 2022; [cited 2022 Feb 18]. Available from: http://tvscn.nhs.uk/wp-content/uploads/2018/12/Breast-4.2-November-2018.pdf
- London Cancer Alliance West and South. Breast pathway group FEC75 in early breast cancer [Internet]; 2016; [cited 2022 Feb 18]. Available from: https://rmpartners.nhs.uk/wp-content/uploads/2017/03/LCA-Breast-FEC75-EBC-November-2014.pdf
- London Cancer Alliance West and South. Breast pathway group – EC × 4 – Docetaxel × 4: epirubicin & cyclophosphamide followed by docetaxel in early breast cancer [Internet]; 2016; [cited 2022 Feb 18]. Available from: https://rmpartners.nhs.uk/wp-content/uploads/2017/03/LCA-Breast-EC-Docetaxel-EBC-November-2014.pdf
- Department of Health and Social Care. Drugs and pharmaceutical electronic market information tool (eMIT) [Internet]; 2021; [cited 2022 Jan 7]. Available from: https://www.gov.uk/government/publications/drugs-and-pharmaceutical-electronic-market-information-emit
- National Institute for Health and Care Excellence. British National Formulary [Internet]; 2020; [cited 2022 Jan 7]. Available from: https://bnf.nice.org.uk/
- Kurosky SK, Mitra D, Zanotti G, et al. Treatment patterns and outcomes of patients with metastatic ER+/HER-2– breast cancer: a multicountry retrospective medical record review. Clin Breast Cancer. 2018;18(4):e529–e538.
- National Institute for Health and Care Excellence. Ribociclib with fulvestrant for treating hormone receptor-positive, HER2-negative, advanced breast cancer [Internet]. London (UK); 2019. Available from: https://www.nice.org.uk/guidance/ta593
- National Institute for Health and Care Excellence. Abemaciclib with an aromatase inhibitor for previously untreated, hormone receptor-positive, HER2-negative, locally advanced or metastatic breast cancer; 2019.
- National Institute for Health and Care Excellence. Palbociclib with an aromatase inhibitor for previously untreated, hormone receptor-positive, HER2-negative, locally advanced or metastatic breast cancer [Internet]; 2017. Available from: https://www.nice.org.uk/guidance/ta495
- Karnon J, Kerr GR, Jack W, et al. Health care costs for the treatment of breast cancer recurrent events: Estimates from a UK-based patient level analysis. Br J Cancer. 2007;97(4):479–485.
- Zeidan AM, Mahmoud D, Kucmin-Bemelmans IT, et al. Economic burden associated with acute myeloid leukemia treatment. Expert Rev Hematol. 2015;9(1):79–89.
- Hinde S, Theriou C, May S, et al. The cost-effectiveness of EndoPredict to inform adjuvant chemotherapy decisions in early breast cancer. Heal Policy Technol. 2019;8(1):75–83.
- Roberts MC, Miller DP, Shak S, et al. Breast cancer-specific survival in patients with lymph node-positive hormone receptor-positive invasive breast cancer and oncotype DX recurrence score results in the SEER database. Breast Cancer Res Treat. 2017;163(2):303–310.
- Cardoso F, van 't Veer L, Poncet C, et al. MINDACT: long-term results of the large prospective trial testing the 70-gene signature MammaPrint as guidance for adjuvant chemotherapy in breast cancer patients. J Clin Oncol. 2020;38(15 Suppl.):506.
- Bloomfield DJ, Arbon A, Cox J, et al. Patient/oncologist decisions about adjuvant chemotherapy in ER + ve, HER2-ve early breast cancer following endopredict testing. J Clin Oncol. 2017;35(15 Suppl.):e12002.
- Wang SY, Dang W, Richman I, et al. Cost-effectiveness analyses of the 21-gene assay in breast cancer: systematic review and critical appraisal. J Clin Oncol. 2018;36(16):1619–1627.
- National Comprehensive Cancer Network. NCCN guidelines: breast cancer, version 2.2021. Plymouth Meeting (PA); 2021.