Abstract
Aims
There are no direct comparisons of the relative cost-effectiveness of second-generation anti-androgens (enzalutamide and apalutamide) used in managing metastatic castration-sensitive prostate cancer (mCSPC) in Canada. This study compared the cost-effectiveness of enzalutamide versus apalutamide versus androgen deprivation therapy (ADT) alone (standard of care) in patients with mCSPC from the Canadian public payer perspective using a Markov model with a 15-year time horizon.
Materials and methods
Efficacy data for enzalutamide and ADT alone were informed by the ARCHES and ENZAMET clinical trials, while a Bayesian network meta-analysis enabled comparison with apalutamide and ADT alone.
Results
Over the 15-year period, enzalutamide achieved the highest number of life-years (LY, 7.6) and quality-adjusted life-years (QALY, 5.62) compared with apalutamide (LY, 6.1; QALY, 4.59) and ADTs (LY, 4.9; QALY, 3.61). Enzalutamide incurred the most costs ($349,345) compared with apalutamide ($294,349) and ADT ($162,550). Sequential analysis showed that enzalutamide lies on the cost-effectiveness frontier with ADT alone (incremental cost-effectiveness ratio: $92,868/QALY), with apalutamide extendedly dominated through enzalutamide and ADT alone.
Limitations
Limitations include the heterogeneity of the studies included in the network meta-analysis and the validations for the treatment sequencing assumptions in the modeling.
Conclusions
Enzalutamide was the most effective treatment option for mCSPC in the Canadian market, with the greatest LYs and QALYs, and incurred the most costs.
Introduction
Prostate cancer is the most common type of cancer in Canadian men. It is estimated that one in seven men in Canada will be diagnosed with some form of prostate cancer during his lifetime and that one in 29 will die from the diseaseCitation1. In 2017, there were 21,300 new cases diagnosed among Canadian men, accounting for approximately 21% of new cancer casesCitation2, with total healthcare costs in the 12 months following diagnosis estimated at $15,170 per patient aged ≥45 yearsCitation3.
It is estimated that 8.6% of patients with prostate cancer in Canada are diagnosed with metastatic disease each yearCitation2, exhibiting disseminated disease at initial presentation (de novo metastases). At this stage, described as metastatic hormone-sensitive prostate cancer or metastatic castration-sensitive prostate cancer (mCSPC), patients are typically sensitive to, or have not received, androgen deprivation therapy (ADT). Although ADT alone is initially effective in almost all patients with mCSPC and is recommended therapy for de novo metastatic disease in CanadaCitation2,Citation4, the majority of patients who receive ADT alone progress to metastatic castration-resistant prostate cancer (mCRPC)Citation5–7, which is associated with increased morbidity and mortality, declining quality-of-life (QoL), and higher costsCitation8–10.
The second-generation anti-androgen abiraterone acetate plus prednisone is indicated in different countries for patients with newly diagnosed high-risk mCSPCCitation2,Citation4,Citation11, while docetaxel (chemohormonal therapy) is commonly used in the management of patients with mCSPC who have high-volume diseaseCitation12 and are able to tolerate docetaxelCitation11,Citation13–15. Currently, enzalutamide and apalutamide are indicated and used in the management of mCSPC, regardless of volume of disease, and have received health technology assessment recommendations for reimbursement in CanadaCitation4, while abiraterone acetate plus prednisone is recommended only for patients with high-risk mCSPCCitation4,Citation12.
The ARCHESCitation16 and ENZAMETCitation17 phase 3 clinical trials have demonstrated significant treatment benefits with enzalutamide in patients with mCSPC, regardless of volume of disease. In men with mCSPC, enzalutamide in combination with ADT significantly reduced the risk of radiographic progression or death versus placebo plus ADT (hazard ratio (HR) = 0.39; 95% confidence interval (CI) = 0.30‒0.50%; p < 0.001) while maintaining the QoL reported at baseline in ARCHESCitation18; in men with mCSPC receiving testosterone suppression in the ENZAMET study, the combination was associated with significantly longer progression-free survival (PFS; HR = 0.39; 95% CI = 0.33‒0.47%; p < 0.001) and overall survival (OS; HR for death = 0.67; 95% CI = 0.52‒0.86%; p = 0.002) than the standard of care (non-steroidal anti-androgen)Citation17. Enzalutamide is also indicated for the treatment of castration-resistant prostate cancer (CRPC), irrespective of the presence of metastasis. There were several important differences between the ENZAMET and ARCHES clinical trials. In ENZAMET, the primary outcome measure was OS, while in ARCHES it was radiographic PFS (rPFS). Although PFS was also measured in ENZAMET, clinical PFS was used rather than rPFS as in ARCHES. In addition, different comparators were used in the two studies – in ENZAMET the comparator was a non-steroidal anti-androgen, while in ARCHES it was ADT plus placebo. Finally, although both studies allowed docetaxel use, in ENZAMET, concomitant administration was permitted, while in ARCHES, prior docetaxel therapy was permitted before the administration of enzalutamide.
The TITAN phase 3 trial demonstrated the significant treatment benefits of apalutamide in patients with mCSPC, regardless of volume of disease. Apalutamide in combination with ADT was associated with significantly longer rPFS (HR = 0.48; 95% CI = 0.39‒0.60%; p < 0.001) and improved OS (HR for death = 0.67; 95% CI = 0.51‒0.89%; p < 0.0001) versus placebo plus ADT while maintaining QoLCitation19. Apalutamide is also indicated for the treatment of non-metastatic CRPC.
These clinical trials have separately demonstrated the therapeutic benefits of enzalutamide, including a 61% reduction in the risk of radiographic progression or death, and apalutamide, with a 33% reduction in the risk of mortality, in patients with mCSPC, regardless of volume of disease. However, currently there are no direct comparisons of the relative cost-effectiveness of these treatments in the Canadian market. The objective of this analysis was to develop a health economic model that assesses the cost-effectiveness of enzalutamide versus apalutamide versus ADT alone in patients with mCSPC, irrespective of risk category, from the Canadian public payer perspective.
Methods
Model overview
A Markov state transition model was developed to estimate the cost-effectiveness of treating patients with mCSPC in Canada. The model structure, input parameters, and key assumptions were aligned with Canadian Agency for Drugs and Technologies in Health guidelinesCitation17 and validated by clinical and health economics experts in a series of individual interviews to ensure this information reflected Canadian clinical practice.
The modeled population reflected local clinical practice in Canada and comprised adult men with mCSPC, including those with de novo metastasis, metastasis developed following local therapy, prior exposure to ADT, and high or low disease burden. Based on consultation with clinical experts, the assumption that treatment of de novo metastatic patients was equivalent to those who had received prior therapy was validated. All analyses were performed on the intent-to-treat analysis populationCitation20. The intervention with enzalutamide plus ADT was compared with (i) apalutamide plus ADT and (ii) ADT alone.
Model structure
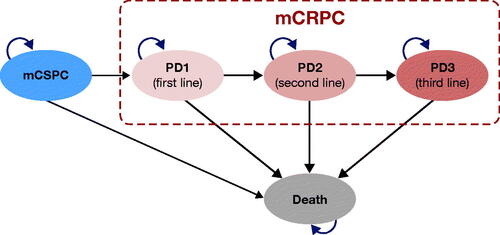
The model was based on the standard three-state structure commonly used in oncology (stable disease, progressed disease (PD), and death)Citation21 that reflects the natural progression of the disease from mCSPC to mCRPC and death. Considering the progressive nature of the disease, mCRPC was further split into three subhealth states (PD1–3) (), allowing for the incorporation of different treatment sequences and gradually declining health state utility values. Each disease state within the model was mutually exclusive (patients could only be in a particular subhealth state at any given point) and progression to the next disease state was deemed irreversible. A time horizon of 15 years was set to ensure that all relevant differences in treatment-related future costs and outcomes were captured for >99% of the patients in the model. This time horizon reflects the majority of costs and consequences resulting from the median age at diagnosis (70 years in ARCHESCitation16; 69 years in ENZAMETCitation17), impact of interventions on survival of patients with mCSPC, and >10% survival rate 10 years after initiating treatment and has also been used in a prior cost-effectiveness study in patients with mCSPC in a Canadian settingCitation22. A cycle length of 1 month was set to represent the frequency of key clinical events and interventions.
Clinical inputs
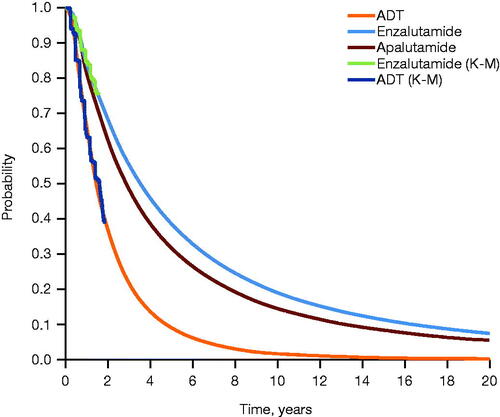
A time-to-event analysis was conducted to inform OS for enzalutamide and ADT, pooling data from both ARCHESCitation16 and ENZAMETCitation17 (patients receiving concomitant docetaxel in ENZAMET were excluded from the pooled group) (). rPFS for enzalutamide and ADT was informed by data from ARCHES only, as the ENZAMET study measured clinical PFS (). To generate life-years gained (progression-free life-years (PFLYs) and total life-years gained), long-term treatment effects were extrapolated using parametric survival analysis, with Weibull and log-normal distributions selected as the best fits to model OS and rPFS, respectively. PFLYs refer to time in the mCSPC health state. There was no definitive difference in survival between ADT and non-steroidal anti-androgen plus ADTCitation23, which were the control arms for ARCHES and ENZAMET, respectively, thus allowing for the pooling of data. The transition between the various mCRPC PD states was informed by treatment durations reported for pivotal clinical trials in mCRPCCitation24–29. Treatment duration in the progression-free mCSPC health state was assumed to be equivalent to rPFS.
Figure 2. Extrapolated OS across treatment arms. Extrapolation of OS was performed using parametric survival analysis, with Weibull distribution selected as the best fit. Abbreviations. ADT, androgen deprivation therapy; K-M, Kaplan–Meier; OS, overall survival.
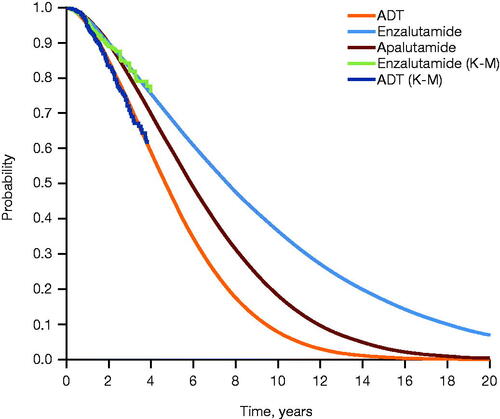
Figure 3. Extrapolated rPFS across treatment arms. Extrapolation of PFS was performed using parametric survival analysis, with log-normal distribution selected as the best fit. Abbreviations. ADT, androgen deprivation therapy; K-M, Kaplan–Meier; PFS, progression-free survival; rPFS, radiographic progression-free survival.
While OS and rPFS for enzalutamide and ADT were based on trial data, a Bayesian network meta-analysis (NMA) was conducted to provide indirect evidence on the relative efficacy of enzalutamide versus apalutamide versus ADT (standard of care). Details of the methodology and results from the NMA and the systematic literature review (SLR) used to identify the studies in the NMA are outlined in the Supplementary materials (pages 1–4; Supplementary Tables S1–S3, Supplementary Figures S1 and S2). The master evidence network included 21 studies conducted in patients with mCSPC and was built through an SLR (Supplementary Figures S1 and S2), whose eligibility criteria are shown in Supplementary Table S1. Results from the NMA showed a statistically significant benefit in OS for enzalutamide versus ADT alone (HR = 0.61; 95% credible interval (CrI) = 0.43–0.91) and a numerically greater, but not statistically significant, benefit versus apalutamide (HR = 0.91; 95% CrI = 0.52–1.16). A statistically significant rPFS benefit was shown for enzalutamide versus ADT alone (HR = 0.39; 95% CrI = 0.30–0.50) and a numerically greater, but not statistically significant, benefit versus apalutamide (HR = 0.81; 95% CrI = 0.58–1.14). Outcomes were analyzed on the log-HR scale using the normal likelihood and identity link.
Valuation of health (health state utilities)
All health states in the model were based on the EuroQol 5-Dimensions, 3-Levels questionnaire (EQ-5D-3L) and originated from two sources: (i) ARCHES and (ii) AFFIRM, a phase 3 clinical trial evaluating survival in patients with mCRPC receiving enzalutamide or placebo following chemotherapyCitation29. As such, utility values for mCSPC, PD1, and end of life were converted from ARCHES EQ-5D-5LCitation16 using the van Hout algorithm and the UK tariffCitation30. Utilities for PD3 were derived from EQ-5D-3L data from AFFIRM using the UK tariffCitation30. Utility for PD2 was assumed to be a mean of PD1 and PD3 (Supplementary Table S4). To account for the potential impact of country-specific EQ-5D data on the cost-effectiveness results, we also conducted a utility analysis applying the Canadian EQ-5D-5L tariff to the ARCHES quality-of-life data as part of the scenario analyses (Supplementary Table S4).
Utility decrements were assigned to adverse events (AEs) using data from published literature. Information on AEs was obtained from ARCHES and ENZAMET. The model included treatment-emergent AEs of grades 3–4 reported in ≥2% of patients and AEs of special interest (those with a high impact on costs and health) in patients with mCSPC and mCRPC.
Resource use and costs
Given the public payer perspective, only direct medical costs were used in the model. These included drug acquisition, concomitant best supportive care, outpatient physician visits, monitoring and laboratory testing, imaging, hospital care, and terminal care costs. Treatment costs were derived using list prices, and all costs were measured in 2019 Canadian dollars. Unit costs were predominantly Ontario-based, including Ontario Schedule of Benefits for Physician ServicesCitation31, Ontario Health Insurance Plan Schedule for Laboratory ServicesCitation32, Ontario Case Costing InitiativeCitation33, and Ontario Drug Benefit FormularyCitation34. These were supplemented with published data where needed. Data on healthcare resource utilization were derived from product monographs and a survey of Canadian clinical experts, complemented with information published in scientific literature.
Outcomes: base-case analysis
Aligned with the Canadian Agency for Drugs and Technologies in Health economic evaluations guidelines, we conducted a cost-utility analysis to compare the costs and quality-adjusted life-years (QALYs) across the comparators. Expected costs and QALYs for each comparator were derived through probabilistic analysis performed in Excel using a Monte Carlo simulation with 5,000 iterations. LYs, QALYs, and costs were discounted at 1.5% per year as per best-practice norms recommended by the International Society for Pharmacoeconomics and Outcomes ResearchCitation35,Citation36 and the National Institute for Health and Care ExcellenceCitation37. Uncertainty was presented using a cost-effectiveness acceptability curve (Supplementary Figure S2) demonstrating the probability of interventions to be cost-effective at different willingness-to-pay (WTP) thresholds. As the model had more than one comparator, sequential analysis was used to exclude interventions that were either dominated or subject to extended dominance. The results of sequential analysis were presented on a cost-effectiveness frontier. A full description of model parameters and settings used for the base-case analysis is shown in Supplementary Table S5. Key inputs in the model base case are shown in Supplementary Table S6.
Outcomes: sensitivity analyses
Parameter uncertainty was accounted for through probabilistic analysis. Scenario analyses run probabilistically (5,000 Monte Carlo iterations for each scenario) were conducted to explore methodological and structural uncertainty. The specific scenarios investigated in the scenario analyses are listed in in the Results section.
Results
Patients in the enzalutamide arm achieved the highest number of LYs (7.6) over the 15-year time horizon versus 6.1 and 4.9 years in the apalutamide and ADT arms, respectively. Patients receiving enzalutamide remained in the mCSPC health state for longer compared to the other treatments (especially ADT alone), resulting in considerably higher PFLYs: 4.95 (enzalutamide), 3.98 (apalutamide), and 2.11 (ADT), respectively. They also progressed faster through the PD1 and PD2 health states and remained in the PD3 health state longer. In contrast, patients on ADT alone progressed faster to PD1 and remained in this health state longer due to treatment sequencing; most patients received an androgen receptor axis-targeted agent in PD1 ().
Table 1. Base-case LYG in each disease state across treatment arms over a 15-year time horizon.
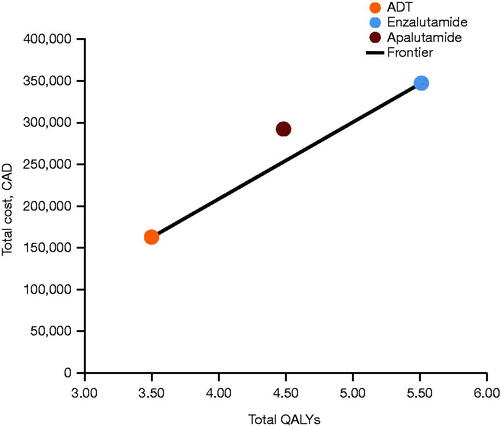
Based on probabilistic analysis, enzalutamide accrued costs of $349,345 with 5.62 QALYs over the 15-year period (). Conversely, ADT alone was the least costly treatment ($162,550), but it provided the lowest QALY gains (3.61 QALYs; ). Apalutamide accrued costs of $294,349 and 4.59 QALYs. The probability of the treatments being cost-effective at different WTP thresholds ($0–300,000) is shown in Supplementary Figure S2. Beyond a WTP threshold of $90,000, the probability of enzalutamide being the optimal treatment option begins to increase, with a > 50% probability beyond a WTP threshold of approximately $92,000. The probability of apalutamide being the optimal treatment within the explored range of WTP thresholds does not exceed 5.8%.
Table 2. Base-case cost-effectiveness outcomes (discounted) across treatment arms over a 15-year time horizon.
Table 3. Summary of ICERs for enzalutamide versus ADT alone for each scenario analysis.
Based on sequential analysis, enzalutamide lies on the cost-effectiveness frontier along with ADT alone at an incremental cost-effectiveness ratio (ICER) of $92,868 per QALY, with apalutamide extendedly dominated through ADT alone and enzalutamide ().
Figure 4. Base-case results: cost-effectiveness frontier. Abbreviations. ADT, androgen deprivation therapy; CAD, Canadian dollars; QALY, quality-adjusted life-year.
Results from the probabilistically run scenario analyses confirmed the base-case results, with sequential ICERs ranging from $82,196 to $117,938 ().
Discussion
To our knowledge, these results represent the first cost-effectiveness analysis to compare two second-generation anti-androgen therapies (enzalutamide and apalutamide, both approved for treatment of patients with CRPC in Canada) with previous standard of care (ADT alone) in patients with mCSPC from the Canadian public payer perspective.
Enzalutamide lies on the cost-effectiveness frontier along with ADT alone at an ICER of $92,868 per QALY, with apalutamide extendedly dominated through enzalutamide and ADT alone. The robustness of these findings was confirmed by sensitivity analyses in which the ICER ranged from $82,196 to $117,938 (sequential analysis results). Taken together, these results indicate that enzalutamide has the highest probability of being the most cost-effective second-generation anti-androgen.
Patients in the enzalutamide arm gained more QALYs than patients who received either ADT alone or apalutamide, which makes enzalutamide the most effective therapy for mCSPC in the overall mCSPC population. This gain in QALYs reflects greater PFLY and LY gains accumulated over the 15-year time horizon by patients in the enzalutamide arm versus those receiving either ADT or apalutamide.
The fact that enzalutamide is the most costly therapy in the model is driven by the greater PFLYs, resulting in a longer duration of treatment in the mCSPC health state, and greater LYs. However, the drug acquisition costs may be overestimated with the assumption of full treatment compliance and no changes such as dose reductions or interruptions.
To establish relative cost-effectiveness of apalutamide, enzalutamide, and ADT, it was necessary to obtain comparisons of their relative efficacy. Although there are multiple clinical trials describing the efficacy of these treatments versus current standard of care, direct comparative data from head-to-head studies are lacking. In these instances, indirect comparisons can prove helpful in determining relative treatment effects. NMAs allow data from multiple clinical trials to be compared either directly or indirectly by including multiple pairwise comparisons across a range of interventions to generate estimates of relative treatment effects. NMAs are a valuable tool in healthcare decision-making and are gaining increasing acceptance with decision-making bodiesCitation38.
The comparative efficacy data used in this comparison were derived from part of a wider SLR and NMA, which has been detailed in the Supplementary materials, and focused on those treatments recommended for use in the wider population of patients with mCSPC in Canada – enzalutamide and apalutamide in combination with ADT and ADT alone. The results of our NMA favored enzalutamide over ADT alone for both OS and rPFS, but showed no significant difference in efficacy between enzalutamide and apalutamide for either outcome. These results are similar to those obtained from other NMAs that include comparisons of enzalutamide and apalutamide in the treatment of men with mCSPC. Both enzalutamide (HR = 0.64; 95% CrI = 0.46–0.88) and apalutamide (HR = 0.63; 95% CrI = 0.43–0.92) showed significant improvement in OS compared to ADT alone, but there was no difference in efficacy between the two treatments (HR = 1.00; 95% CrI = 0.61–1.70)Citation39. Similarly, Mori et al. showed that enzalutamide significantly improved OS (HR = 0.85; 95% CrI = 0.73–0.99) compared with ADT alone, and apalutamide provided a numerical, but non-significant, improvement versus ADT (HR = 0.84; 95% CrI = 0.69–1.02). Both treatments significantly improved PFS, while enzalutamide seemed to be the most likely best treatment option in terms of OS and PFS in patients with low-volume mCSPCCitation40. An SLR and NMA comparing docetaxel, abiraterone acetate, enzalutamide, or apalutamide in combination with ADT as first-line therapy in men with mCSPC reported that enzalutamide with ADT had the lowest absolute HR compared with ADT only (HR = 0.53; 95% CrI = 0.37–0.75) and an estimated 76.9% probability of the combination being the preferred treatment to prolong OS versus other combination treatments, including apalutamide, or ADT aloneCitation41.
This study represents, to the best of our knowledge, the only extensive analysis that investigates relative cost-effectiveness among the newer anti-androgen therapies used for the management of patients with mCSPC in Canada. The analysis makes use of a number of data sources, including patient-level data from ARCHES and ENZAMET, published clinical trial data, and other published literature to inform both the NMA and the models used to establish relative cost effectiveness. Furthermore, we include a sensitivity analysis that considers a number of potential different scenarios. These results, which show a variation in ICERs of approximately ±10% versus the base case across all scenarios, demonstrate that the findings of this analysis are robust.
However, the current study has some limitations, which should be acknowledged. Although the strength of the NMA was enhanced by the use of patient-level data from phase 3 clinical trials, and the results of the NMA were consistent with other published NMAs performed in this populationCitation41, there was a degree of heterogeneity among the studies included in the NMA with respect to Eastern Cooperative Oncology Group scores, disease burden, previous local therapy, and metastatic disease being newly diagnosed. The lack of data on all potential treatment effect-modifying variables commonly prevents the use of population-adjusted methods to address the issue of heterogeneity. Also, there were relatively few events at data cut-off in the ARCHES study, which limited the OS data used in the modeling predictions. However, pooling of survival data from two clinical trials has improved the extrapolation of OS results, which otherwise would have been performed using relatively immature data. Furthermore, assumptions regarding treatment sequencing used in the modeling were validated solely on expert opinion, as no real-world data were available. It should be noted that the limited availability of data to guide treatment-sequencing decisions in prostate cancerCitation42 and in oncology in general remains an important issue. Finally, these results are derived from the data generated in specific clinical studies, namely ARCHES, ENZAMET, and TITAN. Therefore, the generalizability of these data to the wider population may be limited in terms of ethnicity and other demographic variables not controlled for in the respective studies. In ARCHES and TITAN, the majority of the population was White (approximately 80% and 68%), while Black or African-American participants represented <2.0%. The remaining participants were primarily Asian. Ethnicity was not reported in ENZAMET. Inclusion of a higher percentage of Black or African-American patients with prostate cancer who may experience a higher incidence of the disease or have a poorer prognosisCitation43,Citation44 may impact the overall cost-effectiveness of both treatments.
Conclusions
Enzalutamide was the most effective treatment option for mCSPC in the Canadian market, with the greatest LYs and QALYs, and incurred the most costs. Enzalutamide lies on the cost-effectiveness frontier at a sequential ICER of $92,868 per QALY compared to ADT alone, with apalutamide being extendedly dominated. These results provide important information for payers, clinicians, and patients. The findings suggest that the increased gains in LYs and QALYs may make enzalutamide the preferred option for treatment in the Canadian setting.
Transparency
Declaration of funding
The study was funded by Astellas Pharma Inc. and Pfizer Inc., the co-developers of enzalutamide. The sponsors had a role in the study design, data analysis and interpretation, and writing of the report. All authors had full access to all data and the corresponding author had final responsibility for the decision to submit for publication. The manuscript was written with editorial support from medical writers, funded by the sponsors. The authors developed the analysis plan and all stages of the manuscript in collaboration with Astellas Pharma Inc. and Pfizer Inc.
Declaration of financial/other relationships
FS has received grants from Astellas, AstraZeneca, Bayer, BMS, Janssen, Merck, Myovant, Pfizer, and Sanofi; consulting fees from Amgen, Astellas, AstraZeneca, Bayer, BMS, Janssen, Merck, Myovant, Pfizer, and Sanofi; and honoraria from Amgen, Astellas, AstraZeneca, Bayer, BMS, Janssen, Merck, Myovant, Pfizer, and Sanofi. AC is an employee of Astellas Pharma Inc. BH is an employee of Astellas Pharma Inc. SM is an employee of IQVIA, which received funding from Astellas to conduct the statistical analyses for this work under consultancy contract. AG is an employee of Astellas Pharma Inc. SN has received grant support from Astellas and honoraria from Astellas and Janssen. BS has no competing interests to declare.
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Author contributions
FS: Conceptualization, Data curation, Formal analysis, Investigation, Supervision, Writing – original draft. AC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. BH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. SM: Conceptualization, Formal analysis, Methodology, Visualization, Writing – review & editing. AG: Conceptualization, Methodology, Data interpretation, Writing – review & editing. SN: Methodology, Visualization, Writing – review & editing. BS: Conceptualization, Methodology, Supervision, Writing – review & editing.
Supplemental Material
Download PDF (405.3 KB)Acknowledgements
The development of the economic model was supported by Gittan Blezer from IQVIA Inc. Medical writing support was provided by Aitor Alvarez-Fernandez, PhD, and Paul Littlebury, PhD, from Bioscript and editorial assistance was provided by Stephanie Rippon, MBio, and Jane Beck, MA, from Complete HealthVizion, all funded by the study sponsors.
Data availability statement
Researchers may request access to anonymized participant-level data, trial-level data, and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
References
- Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian cancer statistics 2016. Toronto, ON: Canadian Cancer Society; 2016; [cited 2022 March 24]. Available from: www.cancer.ca/Canadian-Cancer-Statistics-2016-EN.pdf.
- Malone S, Shayegan B, Basappa NS, et al. Management algorithms for metastatic prostate cancer. Can Urol Assoc J. 2019;14(2):50–60.
- de Oliveira C, Bremner KE, Pataky R, et al. Trends in use and cost of initial cancer treatment in Ontario: a population-based descriptive study. CMAJ Open. 2013;1(4):E151–E158.
- So AI, Chi KN, Danielson B, et al. Canadian Urological Association-Canadian Urologic Oncology Group guideline on metastatic castration-naive and castration-sensitive prostate cancer. Can Urol Assoc J. 2020;14(2):17–23.
- Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737–746.
- Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36(11):1080–1087.
- Gravis G, Boher JM, Chen YH, et al. Burden of metastatic castrate naive prostate cancer patients, to identify men more likely to benefit from early docetaxel: further analyses of CHAARTED and GETUG-AFU15 studies. Eur Urol. 2018;73(6):847–855.
- Danielson B, Saad F, So A, et al. Management algorithms for prostate-specific antigen progression in prostate cancer: biochemical recurrence after definitive therapy and progression to non-metastatic castrate-resistant prostate cancer. Can Urol Assoc J. 2019;13(12):420–426.
- Appukkuttan S, Tangirala K, Babajanyan S, et al. A retrospective claims analysis of advanced prostate cancer costs and resource use. Pharmacoecon Open. 2020;4(3):439–447.
- Sullivan PW, Mulani PM, Fishman M, et al. Quality of life findings from a multicenter, multinational, observational study of patients with metastatic hormone-refractory prostate cancer. Qual Life Res. 2007;16(4):571–575.
- Schaeffer E, Srinivas S, Antonarakis ES, et al. Prostate cancer, version 3.2022, NCCN clinical practice guidelines in oncology: National Comprehensive Cancer Network. 2022; [cited 2022 February 23] Available from https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- Saad F, Canil C, Finelli A, et al. Controversial issues in the management of patients with advanced prostate cancer: results from a Canadian consensus forum. Can Urol Assoc J. 2020;14(4):E137–E149.
- Alberta Health Services. Advanced/metastatic prostate cancer. 2018; [April 2021]. Available from: https://www.albertahealthservices.ca/assets/info/hp/cancer/if-hp-cancer-guide-gu010-met-prostate.pdf.
- Rice MA, Malhotra SV, Stoyanova T. Second-generation antiandrogens: from discovery to standard of care in castration resistant prostate cancer. Front Oncol. 2019;9:801.
- Weiner AB, Nettey OS, Morgans AK. Management of metastatic hormone-sensitive prostate cancer (mHSPC): an evolving treatment paradigm. Curr Treat Options Oncol. 2019;20(9):69.
- Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37(32):2974–2986.
- Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121–131.
- Stenzl A, Dunshee C, De Giorgi U, et al. Effect of enzalutamide plus androgen deprivation therapy on health-related quality of life in patients with metastatic hormone-sensitive prostate cancer: an analysis of the ARCHES randomised, placebo-controlled, phase 3 study. Eur Urol. 2020;78(4):603–614.
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381(1):13–24.
- Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies: Canada, CADTH. 2017. Available from: https://www.cadth.ca/dv/guidelines-economic-evaluation-health-technologies-canada-4th-edition.
- Zhang PF, Xie D, Li Q. Cost-effectiveness analysis of cabazitaxel for metastatic castration resistant prostate cancer after docetaxel and androgen-signaling-targeted inhibitor resistance. BMC Cancer. 2021;21(1):35.
- Beca J, Majeed H, Chan KKW, et al. Cost-effectiveness of docetaxel in high-volume hormone-sensitive metastatic prostate cancer. Can Urol Assoc J. 2019;13(12):396–403.
- Rashid M, Ramesh M, Shamshavali K, et al. Efficacy and safety of non-steroidal anti-androgens in patients with metastatic prostate cancer: meta-analysis of randomized controlled trials. Rev Recent Clin Trials. 2020;15(1):34–47.
- Beer TM, Tombal B. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(18):1755–1756.
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005.
- de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–1154.
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–148.
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512.
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197.
- Skaltsa K, Longworth L, Ivanescu C, et al. Mapping the FACT-P to the preference-based EQ-5D questionnaire in metastatic castration-resistant prostate cancer. Value Health. 2014;17(2):238–244.
- Wang L, Paller C, Hong H, et al. Comparison of treatments for nonmetastatic castration-resistant prostate cancer: matching-adjusted indirect comparison and network meta-analysis. J Natl Cancer Inst. 2022;114(2):191–202.
- Schedule of Benefits for Laboratory Services: Ministry of Health, Ontario Health Insurance Plan Laboratories and Genetics Branch 2020. Available from: https://health.gov.on.ca/en/pro/programs/ohip/sob/lab/lab_mn2020.pdf.
- Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). G Ital Cardiol (Rome). 2017;18(7):547–612.
- Ontario Drug Benefit Formulary/Comparative Drug Index. 2021. Available from: https://www.formulary.health.gov.on.ca/formulary/.
- Hay JW, Smeeding J, Carroll NV, et al. Good research practices for measuring drug costs in cost effectiveness analyses: issues and recommendations: the ISPOR Drug Cost Task Force report-Part I. Value Health. 2010;13(1):3–7.
- Garrison LP, Jr., Mansley EC, Abbott TA, 3rd, et al. Good research practices for measuring drug costs in cost-effectiveness analyses: a societal perspective: the ISPOR Drug Cost Task Force report-Part II. Value Health. 2010;13(1):8–13.
- National Institute for Health and Care Excellence. Developing NICE guidelines: the manual. 2014. Available from: https://www.nice.org.uk/process/pmg20/resources/developing-nice-guidelines-the-manual-pdf-72286708700869.
- Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network Meta-analysis for health-care decision making: report of the ISPOR task force on indirect treatment comparisons good research practices: part 1. Value Health. 2011;14(4):417–428.
- Chen J, Ni Y, Sun G, et al. Comparison of current systemic combination therapies for metastatic hormone-sensitive prostate cancer and selection of candidates for optimal treatment: a systematic review and Bayesian network meta-analysis. Front Oncol. 2020;10:519388.
- Mori K, Mostafaei H, Sari Motlagh R, et al. Systemic therapies for metastatic hormone-sensitive prostate cancer: network meta-analysis. BJU Int. 2021;129(4):423–433.
- Sathianathen NJ, Koschel S, Thangasamy IA, et al. Indirect comparisons of efficacy between combination approaches in metastatic hormone-sensitive prostate cancer: a systematic review and network meta-analysis. Eur Urol. 2020;77(3):365–372.
- Komura K, Sweeney CJ, Inamoto T, et al. Current treatment strategies for advanced prostate cancer. Int J Urol. 2018;25(3):220–231.
- DeSantis CE, Siegel RL, Sauer AG, et al. Cancer statistics for African Americans, 2016: progress and opportunities in reducing racial disparities. CA Cancer J Clin. 2016;66(4):290–308.
- Siegel DA, O’Neil ME, Richards TB, et al. Prostate cancer incidence and survival, by stage and race/ethnicity – United States, 2001–2017. MMWR Morb Mortal Wkly Rep. 2020;69(41):1473–1480.