Abstract
Background
As the body of evidence on COVID-19 and post-vaccination outcomes continues to expand, this analysis sought to evaluate the public health impact of the Pfizer-BioNTech COVID-19 Vaccine, BNT162b2, during the first year of its rollout in the US.
Methods
A combined Markov decision tree model compared clinical and economic outcomes of the Pfizer-BioNTech COVID-19 Vaccine (BNT162b2) versus no vaccination in individuals aged ≥12 years. Age-stratified epidemiological, clinical, economic, and humanistic parameters were derived from existing data and published literature. Scenario analysis explored the impact of using lower and upper bounds of parameters on the results. The health benefits were estimated as the number of COVID-19 symptomatic cases, hospitalizations and deaths averted, and Quality Adjusted Life Years (QALYs) saved. The economic benefits were estimated as the amount of healthcare and societal cost savings associated with the vaccine-preventable health outcomes.
Results
It was estimated that, in 2021, the Pfizer-BioNTech COVID-19 Vaccine (BNT162b2) contributed to averting almost 9 million symptomatic cases, close to 700,000 hospitalizations, and over 110,000 deaths, resulting in an estimated $30.4 billion direct healthcare cost savings, $43.7 billion indirect cost savings related to productivity loss, as well as discounted gains of 1.1 million QALYs. Scenario analyses showed that these results were robust; the use of alternative plausible ranges of parameters did not change the interpretation of the findings.
Conclusions
The Pfizer-BioNTech COVID-19 Vaccine (BNT162b2) contributed to generate substantial public health impact and vaccine-preventable cost savings in the first year of its rollout in the US. The vaccine was estimated to prevent millions of COVID-19 symptomatic cases and thousands of hospitalizations and deaths, and these averted outcomes translated into cost-savings in the billions of US dollars and thousands of QALYs saved. As only direct impacts of vaccination were considered, these estimates may be conservative.
PLAIN LANGUAGE SUMMARY
Why was this study done?
Vaccination against COVID-19 is a crucial part of the public health response to the pandemic. The first vaccine against COVID-19 to be rolled out in the US was the Pfizer-BioNTech vaccine (also called Comirnaty or BNT162b2). In this study, we investigated how the BNT162b2 vaccine affected US public health and the economy in 2021, the first year of its use.
What did the researchers do and find?
We developed a mathematical model and populated it with published data on COVID-19 to estimate the benefits of receiving 2 BNT162b2 vaccine doses versus receiving no vaccination, in people 12 years and older.
Vaccination against COVID-19 is a crucial part of the public health response to the pandemic. The first vaccine against COVID-19 to be rolled out in the US was the Pfizer-BioNTech vaccine (also called Comirnaty or BNT162b2). In this study, we investigated how the BNT162b2 vaccine affected US public health and the economy in 2021, the first year of its use.
We estimated that the BNT162b2 vaccine helped prevent around 8.7 million symptomatic COVID-19 illnesses. We also estimated that the vaccine prevented almost 700,000 COVID-19–associated hospitalizations, and more than 110,000 deaths. We estimated that it saved around $30 billion on healthcare costs and $44 billion related to people being unable to work due to illness. Sensitivity and scenario analyses showed that, although changing the model parameters did affect the results, this did not alter the overall conclusion that the vaccine leads to favorable and substantial public health impact.
This study did not include estimates for children under 12 years old, or research the benefits of a booster. We cannot assume the results would be the same for other COVID-19 vaccines. This study also did not account for the Omicron variant, which did not emerge until late in 2021.
What do researchers think the results mean?
In the US, in 2021, the BNT162b2 COVID-19 vaccine contributed to preventing millions of people from getting symptomatic COVID-19 and saved billions of dollars. These results support recommendations for continued widespread vaccine use.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has claimed millions of lives globallyCitation1, with the United States (US) sharing a significant burden of more than nine hundred thousand deaths during the past two yearsCitation2. As a public health response to curb the pandemic, multiple mitigation strategies including mass vaccination have been implementedCitation3.
The clinical effectiveness of COVID-19 vaccines in preventing infections and severe disease has been established in randomized clinical trials (RCTs)Citation4–7, while a rich body of real-world evidence (RWE) complements RCTs resultsCitation8,Citation9. Leveraging national surveillance data, studies quantified the population-level impact of COVID-19 vaccination in the US and showed that vaccines are clinically effective in reducing symptomatic cases, deaths, and hospitalizationsCitation10–13. However, only a few studies assessed the economic impact of COVID-19 vaccination in the USCitation14–19. Kohli et al.Citation15, and Padula et al.Citation16, published in the early stages of the pandemic, assessed the cost-effectiveness of a hypothetical COVID-19 vaccine meeting the WHO and Food and Drug Administration (FDA) target product profiles. Li et al.Citation17, estimated the cost-effectiveness of a booster strategy of BNT162b2 in individuals aged ≥65 years. Although the three studies demonstrated cost-effectiveness versus no vaccination, they focused on the healthcare system perspective, which may arguably underestimate the full value of vaccination. COVID-19 causes productivity loss, with an estimated 10 sick days per infected personCitation20, and ∼5.4% reduction in the labor force participation in the USCitation21,Citation22. Analyses under a societal perspective are recommended for a more comprehensive assessment, incorporating impact on patients and society as a wholeCitation23,Citation24. Bartsch et al.Citation18, estimated that a non-specific COVID-19 vaccine can save billions of US dollars in productivity losses. Incorporating the broader macro-economic impact (e.g. GDP loss, deferred care), Kirson et al.Citation19, estimated that COVID-19 vaccines can generate trillions in savings.
More than one-year through the US vaccination program, extensive RCTs and RWE data are available on the clinical profile of the three vaccines (Pfizer-BioNTech; Moderna; Johnson & Johnson/Janssen) authorized by the FDACitation25–27. Surveillance data has been widely availableCitation28–30, and research on COVID-19 economic burdenCitation31–34 has emerged. These studies can be holistically combined to generate more accurate estimates of the societal value of vaccination.
The Pfizer-BioNTech COVID-19 Vaccine (BNT162b2) was the first vaccine available under Emergency Use Authorization (EUA) since December 2020, and became licensed as a two-dose primary series in individuals aged ≥16 years in August 2021Citation25,Citation35,Citation36. It was the only vaccine with FDA approval in 2021 and the only vaccine authorized for children; in the 12–15 years age group since May 2021, and in the 5–11 age group since October 2021Citation36. Based upon the Centers for Disease Control and Prevention (CDC) estimates, six out of ten (∼57%) individuals fully vaccinated in the US received Pfizer-BioNTech COVID-19 Vaccine (BNT162b2) in 2021Citation37. Representing the highest proportion of the vaccinated population, its use and outcomes have been heavily investigated in large, and high-quality studiesCitation37. This comprehensive body of evidence enables further research into its societal impact.
We employed quantitative methods to assess the public health and economic impact of two-dose BNT162b2 in the first year of its rollout in the US. The main analysis focused on the population aged ≥12 years, as the 5–11 age group was not eligible during most of the study period. The analyses focused exclusively on the two-dose primary series as intervention as a single booster dose was broadly recommended in individuals older than 12 in January 2022Citation38, with a time interval of at least 5 months after completion of dose 2Citation39.
Methods
Study design
A combined Markov and decision tree model with transition probabilities based on COVID-19 literature, was developed to estimate the one-year public health impact of Pfizer-BioNTech COVID-19 Vaccine (BNT162b2) compared to no vaccination. The base case analysis was conducted in the US target population aged ≥12 years, from a societal perspective. Age-stratified epidemiological, clinical, economic, and humanistic parameters were derived from published literature.
The expected public health impact was estimated as the number of COVID-19 deaths and symptomatic cases averted, with the latter differentiated among those requiring outpatient versus inpatient care. The expected economic impact was estimated as the amount of avoided healthcare and societal costs corresponding to the health outcomes averted. Productivity and QALY loss associated with early death was accounted for on a lifetime horizon.
The model was programmed in Microsoft Excel 2021. The manuscript was developed in alignment with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 guidelines (Supplementary Table S1) Citation40.
Model structure
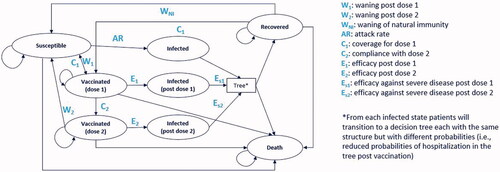
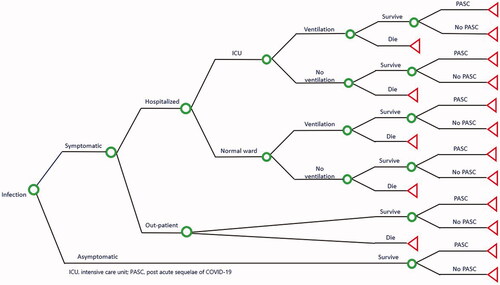
A Markov model () was constructed with eight mutually exclusive health states based on: (i) Susceptible-Infected-Recovered (SIR) structure which has been used in monitoring and predicting the spread of COVID-19Citation41, and (ii) Vaccinated states of partial (dose 1) or full vaccination (dose 2) depending on the vaccine coverage and breakthrough infections. The “infected states” (infected, infected post dose 1, infected post dose 2) of the Markov model were linked with a Decision tree () for transition probabilities of symptomatic and asymptomatic infections, and sequelae such as hospitalization, receiving intensive care (ICU) and/or invasive mechanical ventilation (IMV), and post-acute sequelae of COVID-19 (PASC).
Individuals entered the Markov model (; ) in the “susceptible” or “recovered” health states with weekly cycles for one full year. At each weekly cycle, individuals could transition to other health states or remain in the current state. From the susceptible state, individuals could become infected or receive the vaccine (dose 1 with partial vaccination followed by dose 2 of full vaccination). An age-dependent yearly attack rate in the susceptible population defined the share of the model cohort that would become infected with COVID-19 and vaccination was determined by age-dependent vaccine coverage (further explained in model parameters). From the vaccinated states, patients could experience breakthrough infections or become susceptible again due to waning of vaccine protection. The former was governed by the vaccine efficacy, which could vary by dose, age group and the circulating variants of COVID-19. Waning of the efficacy of the vaccine and of infection-induced immunity was captured through their corresponding duration of protection. In each cycle of the Markov model, a fraction of the cohort was moved from the “vaccinated” (1 or 2 doses) and “recovered” health states to the “susceptible” health state, representing the gradual decrease of protection level in the population due to waning.
Table 1. Model Parameters.
Once individuals got infected in “infected states” (infected, infected post dose 1, infected post dose 2), they moved to the decision tree (; ) for symptomatic or asymptomatic infection. We assumed that individuals with asymptomatic infection did not die or require any inpatient or outpatient treatment, but could experience PASC complications. The term PASC, also known as “long COVID”, is used to describe the post-acute sequelae and long-term symptoms that can be experienced from weeks to months by persons recovering from COVID-19Citation49. Symptomatic cases were moved further into the decision tree according to outcome probabilities informed by the individual’s health state specific efficacy against symptomatic disease and against hospitalization. The probability that infected individuals experienced symptoms was based on peer-reviewed literatureCitation42. Symptomatic cases were assumed to be managed in the outpatient or hospitalized setting, and incurred healthcare costs for clinical care including, respectively, visits, testing, medication, and hospitalization treatment. Hospitalized patients remained in the general ward or were admitted to the ICU and could receive IMV in either setting. Further, individuals that survived infection were then subject to a probability of developing PASC and incurred the costs of managing these symptoms. The PASC probabilities were sourced from published literatureCitation50,Citation51. We also assumed that individuals who got infected were immune to reinfection for an average of nine months after the infectionCitation44.
At the end of the decision tree, individuals returned to the Markov model component to either the “recovered” or “death” state, based on the outcome of the decision tree. Death following infection in the decision-tree was due to COVID-19, which reflected the case fatality rates (, Supplementary Table S9), while death from any other state was due to all other cause mortality. The “recovered state” represented individuals that had recovered from infection and had infection-induced immunity to disease.
Model parameters
The model included a multitude of inputs derived from published literature and supported by medical review. The main inputs are reported in , further details are included in the electronic Supplementary material (Supplementary Tables S2–14).
Population demographics
The study population was stratified into six age groups (12–17 years, 18–29 years, 30–49 years, 50–64 years, 65–74 years, and ≥75 years) to reflect age-dependent outcomes of COVID-19, as well as to facilitate age-dependent scenarios for vaccination coverage for dose 1, compliance with dose 2, and vaccine efficacy. Estimates of population sizes were adopted from the US Census Bureau population projections for 2019Citation52 (Supplementary Table S2).
Infection inputs
The model is based on an estimate of the proportions of susceptible and recovered individuals at the start of 2021, as well as on an estimate of the proportion of susceptible that would have been infected with SARS-CoV-2 over the year without vaccination.
The US infection- and vaccine-induced SARS-CoV-2 seroprevalence data from Jones et al.Citation29, and the Morbidity and Mortality Weekly Report (MMWR)Citation53 were used to define the proportions with infection-induced immunity at model start (, Supplementary S3). The case projections were informed by estimates of the risk of COVID-19 outcomes before any COVID-19 vaccines became available; specifically, the probability of infection in the susceptible population was calculated from age-dependent one-year attack rates extrapolated from Reese et al.Citation42 (Supplementary Table S4). Once the cases were distributed according to the demographic structure previously described, age-stratified data reported by the CDC during the vaccination eraCitation42 was used to assess face validity and inform scenario analyses.
Similarly, in the absence of age-, variant- and dose-stratified data on the infection-induced duration of protection, it was assumed that duration of protection from infection-induced immunity would last for nine months. These assumptions were based upon emerging data on infection-induced long-term protectionCitation29,Citation44,Citation45,Citation52,Citation53 and medical opinion, and were tested in scenario analyses.
The CoVariants – Global initiative on sharing all influenza data (GISAID) databaseCitation43 was used to calculate the COVID-19 variant distribution. The frequencies of COVID-19 original strain, Alpha, Beta, Gamma, and Delta during different months were extracted to estimate their respective proportions over the year (, Supplementary Table S6). The proportion of sequences for Beta was close to 0% hence this variant was not included due to its very low circulation in the USCitation43. Additionally, data from the CDC was used to assess face validityCitation37.
Vaccine inputs
Using the two-dose series of the Pfizer-BioNTech COVID-19 Vaccine (BNT162b2) as the intervention, vaccine-specific inputs such as coverage, compliance with the second dose, VE, and duration of protection, were integrated from various sources and based upon medical opinion. Consistent with current vaccine implementation efforts, we assumed individuals were eligible for vaccination regardless of prior infection.
For estimating the age group-specific vaccination coverage rates of dose 1 and dose 2, the demographic trends of individuals aged ≥12 years receiving COVID-19 vaccinations in the US reported by the CDC COVID-19 Data Tracker were usedCitation54, wherein, coverage by age was averaged over 2021 to estimate the 12-month representative rates, using the area under the curve (AUC) method (Supplementary Table S6). The age group-specific rates of vaccine coverage (defined as the percentage of eligible population receiving first dose of primary vaccination during model horizon) and vaccine compliance with second dose (defined as the percentage of population receiving second dose of primary vaccination during model horizon, among those receiving primary vaccination) were estimated from the CDC Data TrackerCitation54 (Supplementary Table S7).
Vaccine effectiveness (VE) of dose 1 and dose 2 against infection, symptomatic disease, and hospitalization was sourced from different RWE observational studies from the US and, whenever needed, other countries (See supplement section 2.6 and Supplementary Table S8 for further details). VE from RWE was chosen instead of vaccine efficacy from RCTs because RWE had the additional granularity required for the model projections (i.e. across study periods relevant to the multiple variants that were prevalent in 2021). Though the VE varied by COVID-19 variant and endpoint, it was assumed to be equal across age groups in the absence of combined age-, variant- and dose-stratified data (, Supplementary Table S8). Similarly, in the absence of age-, variant- and dose-stratified data on the vaccine duration of protection, it was conservatively assumed that the dose 1 and the dose 2 would provide protection for, respectively, 6 and 12 months until complete loss of efficacy. These assumptions were based upon emerging data on vaccine-induced and infection-induced long-term protectionCitation29,Citation44,Citation45,Citation52,Citation53, and medical opinion, which were also tested in scenario analyses using the lower and higher estimates.
Clinical inputs
As individuals from the infected states moved to the decision tree, probabilities of experiencing symptoms and sequelae were derived from published sources (, Supplementary Table S9). The probability of experiencing symptoms and the age-stratified probabilities of hospitalization among symptomatic individuals were derived from Reese et al.Citation42. A retrospective analysis of the US Premier Healthcare database (COVID-19 Special Release) by Di Fusco et al.Citation31, was used for the distribution of symptomatic cases by general ward or ICU and receipt of IMV, case fatality rates, and costs and length of stay for hospitalization. The mortality rates in the outpatient care setting were sourced from a retrospective analysis of the US Optum database (Supplementary Table S9)Citation33.
Healthcare resource utilization (HCRU) and cost inputs
We incorporated direct and indirect costs associated with the averted health outcomes (, Supplementary Tables S11–13). Direct medical costs included COVID-19 testing, disease management in inpatient (hospitalization) or outpatient (general physician [GP] visits, emergency room, and over-the-counter medication) setting. Hospitalization costs, stratified by general ward or ICU, and receipt of IMV, were sourced from Di Fusco et al.Citation31. The cost of managing PASC was also included as a one-off aggregated cost accrued at the time of infection, which included cost elements for number of GP visits, number of COVID-19 tests, the percentage of patients readmittedCitation48 and/or experiencing PASC. Due to uncertainty around the long-term outcomes, scenario analyses tested the lowest and highest reported re-admission rates (“Low”: 4%, “High”: 15%)Citation48.
Indirect costs included productivity loss due to short-term illness and premature death (Supplementary Table S13). Lost productivity costs due to illness were calculated based on the workforce participation rate, labor cost per week, and number of working days lost. The indirect cost associated with PASC based on working time lost for PASC patients was also included in the societal perspective. Long-term costs were discounted at a rate of 3% per year in accordance with economic evaluation guidelines from the Advisory Committee on Immunization Practices (ACIP)Citation24 and the Second Panel on Cost-Effectiveness in Health and MedicineCitation23. All costs were expressed as 2020 US dollars.
Health utilities
Quality-adjusted life years (QALYs) were estimated by applying utility decrements to the short and long-term symptomatic outcomes, and subtracting them from the general population age-dependent utility norms (Supplementary Table S14). In the absence of robust COVID-19 specific disutility weights, we used proxies from other infectious diseases such as Clostridium difficile infection and influenza, which have been used in previously published COVID-19 studiesCitation15,Citation17,Citation55. Long-term QALY loss associated with early death was included on a lifetime horizon and discounted at 3% annually. Further details on health utilities are available in the Supplementary section 2.9.
Analyses
The base case predicted the health outcomes of the Pfizer-BioNTech COVID-19 Vaccine (BNT162b2) in terms of the number of COVID-19 symptomatic cases, hospitalizations, and deaths averted over a year, alongside the corresponding direct healthcare and productivity costs saved, and QALYs gained, compared with no vaccination, from a societal perspective.
Two scenario analyses were performed to assess the impact of the parameter uncertainty and to evaluate the robustness of the base case findings. The scenarios used extreme but plausible ranges of parameters, which were informed by the lower and the upper bounds of confidence intervals (CIs) of the values used in the base case, or, in their absence, by medical opinion. The lower bounds were used for a “Low” scenario, and the upper bounds were used for a “High” scenario for all parameters except those related to the proportion of individuals with infection-induced immunity at model start, and the duration of protection from infection-induced immunity; for both the parameters, their upper bound values were used in the “Low” scenario. The parameters tested were those carrying higher uncertainty due to wider CIs, or limited evidence. These included the inputs determining the clinical course of COVID-19 in the absence of vaccination (i.e. attack rate) (Supplementary Table S4), the proportion of subjects with infection-induced immunity (Supplementary Table S3), the duration of infection-induced immunity (), the probability of symptoms (Supplementary Table S9), the hospitalization rate for new admissions (Supplementary Table S9) and re-admissions (), and the probability of outpatient deaths (Supplementary Table S9). The inputs related to the clinical profile of the vaccine were varied as well: the variant and dose-specific VE against infection, VE against symptomatic disease, VE against severe disease (Supplementary Table S8), as well as dose-specific duration of vaccine-induced protection ().
Rapid literature review efforts highlighted that a rich body of evidence exists on the burden of disease and the clinical effectiveness of the vaccine, and data on healthcare resource utilization (HCRU) and costs is quickly emerging. As additional evidence was reviewed, alternative plausible ranges for model inputs were derived and used in sensitivity analyses to identify the most sensitive parameters and to characterize the uncertainty. As such, extensive sensitivity analyses were conducted on over 15 model parameters to independently evaluate the impact of individual parameters on the baseline results, using values from alternative sources. One or multiple parameters within the same category were changed at a time using different sets of input values (, Supplementary Tables S2–12).
Table 4. Sensitivity analysis.
On an exploratory basis, the sensitivity analyses also examined the impact of the year-end rollout in children aged 5–11, building on the growing clinical and economic evidence in this age group (Supplementary Tables S2–14).
Implementation costs were also considered on an exploratory basis. This study focused on the assessment of the preventable costs associated with vaccine-preventable disease; it did not consider implementation costs, such as those related to infrastructure, supply, training and human resource management, outreach, or ancillary care management. These costs have been considered largely unknown, and not a primary driver for decision-making on the authorization of COVID-19 vaccines in the context of the pandemicCitation67. The cost of vaccine administration for the US payer system has however been pointed outCitation67, and adverse events have been reported in the RCTsCitation6,Citation68. Sensitivity analyses explored the effects of these two additional cost categories on the overall cost saving estimates (, Supplementary Table S12).
Finally, sensitivity analyses were run to assess the impact of alternative utility decrements and duration of symptoms input values on the QALY loss. Given that literature on COVID-19 specific utilities is scarce, arbitrary values (± 10%) informed the sensitivity analyses on utility decrements. A longer duration of symptoms was tested: 14 daysCitation15, versus 5 days used in the base case (Supplementary Table S14).
Results
Burden of disease in the absence of vaccination
shows the estimated burden of disease in the absence of COVID-19 vaccination in the base case, “Low” and “High” scenarios.
Table 2. Estimated burden of disease in the absence of COVID-19 vaccination.
It was estimated that, by the beginning of the vaccination campaign, about 33 million (“Low”: 31 million, “High”: 35 million) US individuals aged ≥12 and older had been infected with SARS CoV-2 and recovered, representing ∼12% of the eligible population. This approximation was based on the seroprevalence studiesCitation29,Citation53, and was conservative, although consistent with prior literature (11.9% by 30th October according to Sullivan et al.Citation69, and 14% by mid-November according to Angulo et al.Citation70).
Leveraging nationwide COVID-19 burden of disease data reported prior to the introduction of COVID-19 vaccinationCitation31, the base case and scenarios estimated that, of the eligible and susceptible population, 24.2% (“Low”: 17%, “High”: 31.8%) would have become infected with COVID-19 during 2021 in the absence of vaccination. These estimates were consistent with CDC burden of disease data for 2021 including the vaccination period (21.7% based uponCitation2) as well as seroprevalence dataCitation71.
Based upon a review of genome sequencing dataCitation43, it was calculated that about 22% of all infections were of the original strain, 17% were Alpha, ∼0% were Beta, 2% were Gamma, and almost 60% were Delta. This distribution was found to be consistent with additional literatureCitation56,Citation72. Using age-stratified probabilities of symptoms and hospitalization rates reported by Reese et al.Citation42, (), about 3.5 million (“Low”: 2.3 million, “High”: 5.6 million) hospitalizations were estimated to have occurred in 2021 in the absence of vaccination. This amount corresponds to a 5.8% (“Low”: 5.5%, “High”: 6.2%) overall symptomatic hospitalization rate across age groups, which aligns with prior literature (3.4% according to Angulo et al.Citation70) and is consistent with a total of over 4.4 million hospitalizations since the start of the pandemic, per the CDC Tracker (accessed on 2/17/2022)Citation2.
Using age-stratified mortality rates reported in real-world retrospective studiesCitation31,Citation33, we estimated that almost 500,000 (“Low”: 323,000, “High”: 810,000) deaths would have occurred in 2021 in the absence of vaccination. This estimate is consistent with a total of over 920,000 deaths since the start of the pandemic, per the CDC COVID Data Tracker (accessed on 2/17/2022)Citation2, and with published estimates of excess deaths among unvaccinatedCitation73.
Base case results
Based upon the clinical and economic inputs set for the base case (), it was estimated that, compared to no vaccination, the two-dose series of the Pfizer-BioNTech COVID-19 Vaccine (BNT162b2) prevented almost 8.7 million symptomatic cases, averted approximately 690,000 hospitalizations, and saved over 110,000 lives ().
Table 3. Total cumulative number of health outcomes averted by BNT162b2, and associated cost-savings.
Up to, respectively, 17.8%, 54.8% and 77.3% of the vaccine-prevented symptomatic cases, hospitalizations, and deaths were estimated to have occurred among individuals aged ≥65 years.
The total hospitalizations prevented were estimated to be composed mainly of stays in a normal ward without mechanical ventilation (501,191 [72.7%]), although the vaccine was also estimated to prevent more severe hospitalizations (32,104 [4.7%] stays in a normal ward with mechanical ventilation, 65,279 [9.5%] ICU stays without mechanical ventilation, and 91,223 [13.2%] ICU stays with mechanical ventilation). The reduction in COVID-19 burden led to an estimated total direct cost savings of $30.4 billion, driven by inpatient and PASC costs.
Overall, about 1.1 million discounted life-years (LY) and QALYs were gained. Around 85.5% of this QALY gain stemmed from avoiding COVID-19 mortality, while quality of life losses corresponding to morbidity from infection symptoms and long-term effects of mechanical ventilation had a moderate effect.
The observed reductions in COVID-19 symptomatic cases were also associated with productivity gains of $43.7 billion. Around 35.7% of this gain was related to the productivity loss due to early death and 64.3% to lost workdays during illness.
Scenario analyses
shows the outcomes averted in the two alternative scenarios (“Low”, and “High”). Using the most conservative estimates of burden of disease and clinical effectiveness, the “Low” scenario estimated that the Pfizer-BioNTech COVID-19 Vaccine (BNT162b2) prevented over 5 million symptomatic cases, averted over 260,000 hospitalizations, and saved over 45,000 lives.
The “High” scenario estimated that the Pfizer-BioNTech COVID-19 Vaccine (BNT162b2) prevented over 13.8 million symptomatic cases, averted over 1.2 million hospitalizations, and saved over 200,000 lives.
Sensitivity analyses
Sensitivity analyses results are presented in . The model was most sensitive to attack rates and hospitalization rates, whereas, probability of symptoms, clinical effectiveness, HCRU, and cost had moderate impacts on the outcomes of interest.
As expected, attack rates defined based upon the CDC reported cases in 2020 or 2021 without adjusting for under-reporting led to significantly lower burden averted. The CDC burden of disease estimates adjusted for under-reporting were in line with the seroprevalence data used in the base case and had only a marginal impact. As under-detection and under-reporting especially in the early phase of pandemic have been widely documentedCitation74, estimates of attack rates based on the reported cases are arguably not realistic and too conservative. As additional literature offered hospitalization rates across different time periods, age and risk-based populations, the hospitalization rates had a significant impact especially on the number of deaths and hospitalizations averted, which has led to a moderate to high effect on the direct costs and productivity loss. Alternative values for VE and vaccine-induced duration of protection moderately affected all the outcomes of interest, whereas, infection-induced duration of protection (i.e. +/– 3 months of base case at 9 months) had a marginal impact. The HCRU and costs had a moderate impact on direct care costs. Sensitivity analyses with 10% higher and lower utility decrement showed a marginal effect on the QALYs saved of ±0.2%. Increasing the duration of symptoms from 5 days (base case) to 14 daysCitation15 led to a marginal increase of 3.2% in the QALYs saved.
The inclusion of the year-end rollout in children 5–11 slightly increased the outcomes averted, however, to a very low extent, driven by low vaccine coverage in the earliest phase of rollout in November and December 2021, as well as lower burden of disease than adults. Finally, the inclusion of additional care management costs (adverse events management and vaccine administration costs) had a low to moderate impact on the direct costs; those costs were offset by the total cost savings associated with vaccine-preventable disease.
Discussion
The analyses show that the Pfizer BioNTech COVID-19 Vaccine (BNT162b2) has had a profound public health impact in the US in 2021, by preventing millions of symptomatic cases, thousands of hospitalizations, and deaths, which translated into significant cost-savings in the billions of US dollars, considering direct costs only (i.e. payer perspective), as well as a societal perspective incorporative of productivity losses for the affected individuals. Such findings are consistent with other analyses that stressed the relevance of the social burden of disease when analyzing the outcomes. This result was consistent in sensitivity and scenario analyses, and was highly impactful even excluding the potential indirect benefits of vaccination (linked to reduced transmission)Citation75, and the broader macroeconomic benefits associated with return to normalcy.
Relatively similar to prior modelsCitation15–17, the results were most sensitive to inputs related to burden of disease and clinical effectiveness of the vaccine. Scenario analyses exploring parameter uncertainty showed that the base case results were robust. The most conservative estimates, represented in the “Low” scenario, confirmed the same trends and direction of results of the base case. The meaning and direction of the results also remained unchanged after running sensitivity analyses using alternative sets of input values. To our knowledge, this is the first study bringing together and simultaneously analyzing multiple types of outcomes and data (epidemiological, clinical, economic, and humanistic) to assess the public health impact of the Pfizer BioNTech COVID-19 Vaccine (BNT162b2) in the whole US eligible population. The study was informed by targeted literature review efforts that retrieved a large amount of recent and US-based data.
The quantitative analyses were based on a relatively simple, transparent, and interpretable model framework that was tailored to the purpose of the research question. The exploration of parameter uncertainty was conducted via extensive sensitivity analyses, whose results strengthened the findings of the base case and highlighted that, using either highly conservative or less conservative assumptions, the Pfizer-BioNTech COVID-19 Vaccine (BNT162b2) generated substantial health benefits and cost-savings.
The results of this study should, however, be considered in the context of several assumptions and limitations.
While the vast majority of the model inputs were derived from published COVID-19 literature and national surveillance data, uncertainty existed in parameter estimates and was tested in sensitivity analyses. Other forms of uncertainty (i.e. structural and methodological) were not explicitly tested. The structures and mechanics used in the model were informed by medical opinion and were consistent with other published modelsCitation15–17, the existing data and understanding of the disease. Several simplifications and assumptions were made to minimize unnecessary complexity and deal with data limitations. Due to limited usable data on the spread of SARS-CoV-2 in the community and the effect of vaccination on transmission, our model simplified the epidemiology dynamics and time-dependent effects in a static Markov and decision tree model. Moreover, the model did not directly assess the transmission process and the indirect and herd immunity effects of vaccination, nor the potential therapeutic effects of vaccination in reducing the severity of cases and the impact of PASC, nor the potential macroeconomic savings that could have been achieved over the year through temporal elimination of levels of non-pharmaceutical interventions (NPIs) (e.g. restricted social interactions). Hence, this parsimonious model framework may have generated conservative estimates. These conservative estimates should, however, be considered in the context of a limited assessment of implementation costs, which was conducted on a small scale in a sensitivity analysis. Further studies assessing the implementation process and costs are warranted to improve the understanding of the full resource use associated with the COVID-19 vaccination program.
As this analysis used the Pfizer-BioNTech COVID-19 Vaccine (BNT162b2) as the intervention, results are not generalizable to other vaccines currently approved and authorized in the US, or in other countries. Similarly, the present study did not explicitly capture the effect of different existing or future interventions such as COVID-19 treatments, which may have a synergistic effect in further reducing disease severity.
Differently from existing studiesCitation15–17, this study included variant-specific and outcome-specific VE for dose 1 and dose 2 of the Pfizer-BioNTech COVID-19 Vaccine (BNT162b2) (Supplementary Table S7). VE estimates were partly informed by input data from other countries. Moreover, due to the absence of granular age-stratified data, VE estimates were assumed to be the same across the age groups, and no further stratification to risk groups was assessed. This is a limitation of the study, since RCTs and RWE show that VE varies across age and risk groupsCitation76.
The vast majority of the inputs used were COVID-19 specific, however, in the absence of robust COVID-19 specific disutility weights, we used proxies from other infectious diseases such as Clostridium difficile infection and influenza, which have been used in previously published COVID-19 studiesCitation15,Citation17,Citation55. While this may increase uncertainty in the results, sensitivity analysis demonstrated that utilities had limited impact on the overall results.
The findings of this study may not fully reflect the current or future disease trends (e.g. prevalence of Omicron variant), vaccine clinical profile, healthcare resource use and costs. Specifically, the current study excluded the impact of the highly contagious B.1.1.529 Omicron variant, which emerged in the US at the end of the analytic time horizon of this study, December 2021Citation77,Citation78. With Omicron becoming the dominant COVID variant in the US from end of DecemberCitation79, future analyses with an extended time horizon should incorporate its effects on the COVID-19 dynamics clinical, economic and humanistic perspective. Moreover, results are neither generalizable to indications nor to populations those were not specifically covered in this analysis; those including children younger than 12, specific high-risk populations such as immunocompromised who require additional vaccine doses for enhanced protectionCitation80, and general population ≥12 years who are eligible for booster doseCitation38.
As COVID-19 data continues to rapidly expand and evolve, future studies are warranted to assess the public health impact in those additional settings as well as in the longer-term.
Conclusions
This analysis shows that the Pfizer-BioNTech COVID-19 Vaccine (BNT162b2) generated substantial gains in health outcomes and cost savings in the US in 2021. It adds to the growing body of evidence demonstrating the societal benefit of the fast and extensive rollout of the vaccine in the US. It supports FDA and CDC recommendations for broad use of the vaccine, and highlights the opportunity to continue widespread uptake to prevent COVID-19 related disease and generate substantial benefits from a broad, patient-centric, societal perspective.
Transparency
Declaration of funding
This study was sponsored by Pfizer Inc.
Declaration of financial/other relationships
MDF, MMM, TLW, AC, and JY are employees of Pfizer and may hold stock or stock options. KM, KAD, SDB, and JR are employees of Evidera, which was a paid consultant to Pfizer in connection with the development of this study. SV is an employee of HealthEcon Consulting, Inc. and an external consultant for Pfizer who has received consulting fees from Pfizer in connection with the development of this study and manuscript.
A reviewer on this manuscript has disclosed that they receive a travel grant from Gilead Sciences Inc. All the peer reviewers on this manuscript have received an honorarium from JME for their review work.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author contributions
All authors contributed to the study conception, design, data acquisition, analysis, interpretation, and drafting and revising the manuscript. Editorial support was provided by SV of HealthEcon Consulting, Inc
Compliance with ethics guidelines
These analyses used data from previously conducted studies and did not include data from any new studies with human participants or animals performed by any of the authors, hence ethical approval was not required.
As the datasets used for this study are public no additional permission for use was required.
Supplemental Material
Download MS Word (163.8 KB)Acknowledgements
The authors acknowledge Moe Kyaw, Diana Mendes, and Jessica Atwell (Pfizer Inc employees) for specific contributions to this research project.
Availability of data and materials
Data generated or analyzed during this study are available upon request.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- World Health Organization. The true death toll of COVID-19: Estimating global excess mortality. 2022; [cited 2022 Feb 10]. Available from: https://www.who.int/data/stories/the-true-death-toll-of-covid-19-estimating-global-excess-mortality.
- Centers for Disease Control and Prevention (CDC): Estimated COVID-19 Burden 2021; [cited 2022 Feb 10]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/burden.html.
- National Strategy for the COVID-19 Response and Pandemic Preparedness. 2021.
- Korang SK, von Rohden E, Veroniki AA, et al. Vaccines to prevent COVID-19: a living systematic review with trial sequential analysis and network Meta-analysis of randomized clinical trials. PLoS One. 2022;17(1):e0260733.
- Sharif N, Alzahrani KJ, Ahmed SN, et al. Efficacy, immunogenicity and safety of COVID-19 vaccines: a systematic review and meta-analysis. Front Immunol. 2021;2021;12
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):605–2615.
- Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416.
- Kow CS, Hasan SS. Real-world effectiveness of BNT162b2 mRNA vaccine: a Meta-analysis of large observational studies. Inflammopharmacology. 2021;29(4):1075–1090.
- Zheng C, Shao W, Chen X, et al. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis. 2022;114:252–260.
- Moghadas SM, Vilches TN, Zhang K, et al. The impact of vaccination on coronavirus disease 2019 (COVID-19) outbreaks in the United States. Clin Infect Dis. 2021;73(12):2257–2264.
- Chen X, Huang H, Ju J, et al. Impact of vaccination on the COVID-19 pandemic in U.S. states. Sci Rep. 2022;12(1):1554.
- Samson LW, W T, EJ O, et al. Associations between county-level vaccination rates and COVID-19 outcomes among medicare beneficiaries. ASPE: Office Of The Assistant Secretary For Planning And Evaluation; 2021.
- Vilches TN, Moghadas SM, Sah P, et al. Estimating COVID-19 infections, hospitalizations, and deaths following the US vaccination campaigns during the pandemic. JAMA Netw Open. 2022;5(1):e2142725.
- Gupta S, Cantor J, Simon KI, et al. Vaccinations against COVID-19 may have averted up to 140,000 deaths in the United States. Health Aff. 2021;40(9):1465–1472.
- Kohli M, Maschio M, Becker D, et al. The potential public health and economic value of a hypothetical COVID-19 vaccine in the United States: use of cost-effectiveness modeling to inform vaccination prioritization. Vaccine. 2021;39(7):1157–1164.
- Padula WV, Malaviya S, Reid NM, et al. Economic value of vaccines to address the COVID-19 pandemic: a U.S. cost-effectiveness and budget impact analysis. J Med Econ. 2021;24(1):1060–1069.
- Li R, Liu H, Fairley CK, et al. Cost-effectiveness analysis of BNT162b2 COVID-19 booster vaccination in the United States. medRxiv. 2021. 2021.11.14.21266318.
- Bartsch SM, Wedlock PT, O'Shea KJ, et al. Lives and costs saved by expanding and expediting coronavirus disease 2019 vaccination. J Infect Dis. 2021;224(6):938–948.
- Kirson N, Swallow E, Lu J, et al. The societal economic value of COVID-19 vaccines in the United States. J Med Econ. 2022;25(1):119–128.
- Centers for Disease Control and Prevention (CDC). COVID-19: Ending Isolation and Precautions for People with COVID-19: Interim Guidance 2021; [cited 2022 Feb 10]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html.
- US Census Bureau. Pandemic Impact on Mortality and Economy Varies Across Age Groups and Geographies 2021; [cited 2022 Feb 10]. Available from: https://www.census.gov/library/stories/2021/03/initial-impact-covid-19-on-united-states-economy-more-widespread-than-on-mortality.html#:∼:text=The%20COVID%2D19%20pandemic%20has,also%20devastated%20the%20nation's%20economy.&text=Declines%20in%20the%20employment%2Dto,country%20due%20to%20the%20pandemic.
- Kochhar R, Bennett J. U.S. labor market inches back from the COVID-19 shock, but recovery is far from complete: Pew Research Center; 2021; [cited 2022 Feb 10]. Available from: https://www.pewresearch.org/fact-tank/2021/04/14/u-s-labor-market-inches-back-from-the-covid-19-shock-but-recovery-is-far-from-complete/.
- Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second panel on Cost-Effectiveness in health and medicine. Jama. 2016;316(10):1093–1103.
- Centers for Disease Control and Prevention (CDC). Guidance for Health Economics Studies Presented to the Advisory Committee on Immunization Practices (ACIP), 2019. Update 2019; [cited 2022 Feb 10]. Available from: https://www.cdc.gov/vaccines/acip/committee/downloads/Economics-Guidance-for-ACIP-2019.pdf.
- US FDA. Comirnaty and Pfizer-BioNTech COVID-19 Vaccine 2021; [cited 2022 Feb 10]. Available from: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine.
- US FDA. Spikevax and Moderna COVID-19 Vaccine. 2021; [cited 2022 Feb 10]. Available from: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/spikevax-and-moderna-covid-19-vaccine.
- US FDA. Janssen COVID-19 Vaccine 2021; [cited 2022 Feb 10]. Available from: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/janssen-covid-19-vaccine.
- COVID-19 SeroHub [Internet]. 2021. Available from: https://covid19serohub.nih.gov/.
- Jones JM, Stone M, Sulaeman H, et al. Estimated US infection- and Vaccine-Induced SARS-CoV-2 seroprevalence based on blood donations, july 2020-May 2021. Jama. 2021;326(14):1400–1409.
- Busch MP, Stone M. Serosurveillance for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) incidence using global blood donor populations. Clin Infect Dis. 2021;72(2):254–256.
- Di Fusco M, Shea KM, Lin J, et al. Health outcomes and economic burden of hospitalized COVID-19 patients in the United States. J Med Econ. 2021;24(1):308–317.
- Ohsfeldt RL, Choong CK-C, Mc Collam PL, et al. Inpatient hospital costs for COVID-19 patients in the United States. Adv Ther. 2021;38(11):5557–5595.
- Scott A, Chambers R, Reimbaeva M, et al. Real-world retrospective analysis of patient characteristics, healthcare resource utilization, costs, and treatment patterns among unvaccinated adults with COVID-19 diagnosed in outpatient settings in the United States. J Med Econ. 2022;2022:1–68.
- Chen J, Vullikanti A, Santos J, et al. Epidemiological and economic impact of COVID-19 in the US. Sci Rep. 2021;11(1):20451.
- FDA Approves First COVID-19 Vaccine [press release]. 2021.
- Pfizer-BioNTech COVID-19 Vaccine COMIRNATY® Receives Full U.S. FDA Approval for Individuals 16 Years and Older [press release]. 2021.
- Centers for Disease Control and Prevention (CDC) COVID Data Tracker: COVID-19 Vaccinations in the United States. 2021; [cited 2022 Feb 10]. Available from: https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total.
- Centers for Disease Control and Prevention (CDC). CDC Expands Booster Shot Eligibility and Strengthens Recommendations for 12-17 Year Olds. 2022; [cited 2022 Feb 10]. Available from: https://www.cdc.gov/media/releases/2022/s0105-Booster-Shot.html.
- Centers for Disease Control and Prevention (CDC) COVID-19 Vaccine Boosters. 2022; [cited 2022 Feb 10]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html.
- Husereau D, Drummond M, Augustovski F, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMJ. 2022;376:e067975.
- Guzmán Ruiz Y, Vecino-Ortiz AI, Guzman-Tordecilla N, et al. Cost-Effectiveness of the COVID-19 test, trace and isolate program in Colombia. Lancet Reg Health Am. 2022;6:100109.
- Reese H, Iuliano AD, Patel NN, et al. Estimated incidence of coronavirus disease 2019 (COVID-19) illness and Hospitalization-United States, February-September 2020. Clin Infect Dis. 2021;72(12):e1010–e7.
- CoVariants Database. 2022; [cited 2022 Feb 10]. Available from: https://covariants.org/.
- Centers for Disease Control and Prevention (CDC). Science Brief: SARS-CoV-2 Infection-induced and Vaccine-induced Immunity. 2021.
- Kim P, Gordon SM, Sheehan MM, et al. Duration of SARS-CoV-2 natural immunity and protection against the Delta variant: a retrospective cohort study. Clin Infect Dis. 2021;2021:ciab999.
- CDC COVID Data Tracker. Demographic Trends of People Receiving COVID-19 Vaccinations in the United States; [cited 2002 Feb 16]. Available from: https://covid.cdc.gov/covid-data-tracker/#datatracker-home.
- Medicare Administrative Contractor (MAC) COVID-19 Test Pricing January 25, 2021.
- Lavery AM, Preston LE, Ko JY, et al. Characteristics of hospitalized COVID-19 patients discharged and experiencing Same-Hospital Readmission - United States, March-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(45):1695–1699.
- Yomogida K, Zhu S, Rubino F, et al. Post-Acute sequelae of SARS-CoV-2 infection among adults aged ≥18 Years – Long beach, California, april 1-December 10, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(37):1274–1277.
- Menges D, Ballouz T, Anagnostopoulos A, et al. Burden of post-COVID-19 syndrome and implications for healthcare service planning: a population-based cohort study. PLoS One. 2021;16(7):e0254523.
- Asadi-Pooya AA, Nemati H, Shahisavandi M, et al. Long COVID in children and adolescents. World J Pediatr. 2021;17(5):495–499.
- US Census Bureau. 2019; [cited 2002 Feb 12]. Available from: www.census.gov.
- Hobbs CV, Drobeniuc J, Kittle T, et al. Estimated SARS-CoV-2 seroprevalence among persons aged <18 years — Mississippi, may–september 2020. MMWR Morb Mortal Wkly Rep. 2021;70(9):312–315.
- Centers for Disease Control and Prevention (CDC) COVID Data Tracker. 2022; [cited 2022 Feb 10]. Available from: https://covid.cdc.gov/covid-data-tracker/#datatracker-home.
- Campbell JD, Whittington MD, Rind DM, et al. Alternative Pricing Models for Remdesivir and Other Potential Treatments for COVID-19; Updated Report. Institute for Clinical and Economic Review November 10. 2020.
- Malden DE, Bruxvoort KJ, Tseng HF, et al. Distribution of SARS-CoV-2 variants in a large integrated health care System – California, March-July 2021. MMWR Morb Mortal Wkly Rep. 2021;70(40):1415–1419.
- Centers for Disease Control and Prevention (CDC) COVID-19 Pandemic Planning Scenarios - Scenario 5 2020; [cited 2022 Feb 16]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html.
- EPIC-HR CSR Section 5, Figure 4. Feb 16, 2022.
- Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of covid-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–520.
- Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385(21):1941–1950.
- Rosenthal N, Cao Z, Gundrum J, et al. Risk factors associated with in-Hospital mortality in a US national sample of patients with COVID-19. JAMA Netw Open. 2020;3(12):e2029058.
- Nasreen S, Chung H, He S, et al. Effectiveness of mRNA and ChAdOx1 COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe outcomes with variants of concern in Ontario. medRxiv. 2021. 2021.06.28.21259420.
- Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407–1416.
- CMS. Medicare Administrative Contractor (MAC) COVID-19 Test Pricing. 2021; [cited 2022 Feb 16]. Available from: https://www.cms.gov/files/document/mac-covid-19-test-pricing.pdf.
- Medicare COVID-19 Vaccine Shot Payment; [cited 2022 Feb 16]. Available from: https://www.cms.gov/medicare/covid-19/medicare-covid-19-vaccine-shot-payment.
- Biospace. UPDATED Comparing COVID-19 Vaccines: Timelines, Types and Prices 2021; [cited 2022 Feb 10]. Available from: https://www.biospace.com/article/comparing-covid-19-vaccines-pfizer-biontech-moderna-astrazeneca-oxford-j-and-j-russia-s-sputnik-v/. (accessed Apr 10, 2022)
- Advisory Committee on Immunization Practices (ACIP). COVID-19 ACIP Vaccine Recommendations; [cited 2022 Feb 10]. Available from: https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
- Thomas SJ, Moreira ED, Jr., Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761–1773.
- Sullivan PS, Siegler AJ, Shioda K, et al. Severe acute respiratory syndrome coronavirus 2 cumulative incidence, United States, August 2020–December 2020. Clin Infect Dis. 2022;74(7):1141–1150.
- Angulo FJ, Finelli L, Swerdlow DL. Estimation of US SARS-CoV-2 infections, symptomatic infections, hospitalizations, and deaths using seroprevalence surveys. JAMA Netw Open. 2021;4(1):e2033706.
- Stadlbauer D, Tan J, Jiang K, et al. Repeated cross-sectional sero-monitoring of SARS-CoV-2 in New York City. Nature. 2021;590(7844):146–150.
- MMWR. Morbidity and mortality weekly report, Vol. 71, January 21, 2022.71(3).
- Centers for Disease Control and Prevention (CDC): Excess Deaths Associated with COVID-19. 2022; [cited 2022 Feb 16]. Available from: https://www.cdc.gov/nchs/nvss/vsrr/covid19/excess_deaths.htm. (accessed Feb 16, 2022)
- Wu SL, Mertens AN, Crider YS, et al. Substantial underestimation of SARS-CoV-2 infection in the United States. Nat Commun. 2020;11(1):4507.
- Prunas O, Warren JL, Crawford FW, et al. Vaccination with BNT162b2 reduces transmission of SARS-CoV-2 to household contacts in Israel. medRxiv. 2021. https://doi.org/10.1101/2021.07.13.21260393
- Centers for Disease Control and Prevention (CDC). Science Brief: COVID-19 Vaccines and Vaccination 2021; [cited 2022 Feb 16]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/fully-vaccinated-people.html.
- Centers for Disease Control and Prevention (CDC). First Confirmed Case of Omicron Variant Detected in the United States [press release]. Dec 12021.
- Johnson AG, Amin AB, Ali AR, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of Delta and omicron variant emergence - 25 U.S. Jurisdictions, April 4–December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71(4):132–138.
- Statista. Statista. Omicron BA.2 Variant to Become Dominant in the U.S. 2022. [Available from: https://www.statista.com/chart/27076/coronavirus-variants-in-the-us/. (accessed Apr 12, 2022)
- US FDA. Coronavirus (COVID-19) Update: FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals [press release]. 2021.