Abstract
Aims
Renin-angiotensin-aldosterone system inhibitors (RAASi) therapy is commonly used to reduce the risk of death and to slow down disease progression in patients with chronic kidney disease (CKD), heart failure (HF) and hypertension. However, the cardio-renal benefits of RAASi therapy are also associated with an increased risk of hyperkalemia (HK), which may lead to dose reduction or discontinuation of therapy. Patiromer has demonstrated to reduce the risk of HK, which enables to maintain optimal doses of RAASi therapy. This study aimed to assess the cost-effectiveness of patiromer for the management of HK in CKD patients with and without HF in Spain.
Methods
A Markov model was developed to evaluate the costs and benefits of patiromer for the management of HK in patients with CKD stages 3–4 with and without HF treated with RAASi over a lifetime horizon. The main outcomes included total direct costs (€2021), quality-adjusted life-years (QALYs), life-years gained (LYG) and incremental cost-effectiveness ratio (ICER). Deterministic one-way and probabilistic sensitivity analyses were performed to assess the robustness of the results.
Results
Patiromer was more effective compared to no patiromer (5.76 vs 5.57 QALYs; 7.73 vs 7.50 LYG), and resulted in an incremental cost of €3,574, yielding an ICER of €19,092/QALY gained and of €15,236/LYG. Sensitivity analyses suggested that the results were robust to changes in most input parameters.
Conclusions
Patiromer is a cost-effective intervention in maintaining normokalemia and enabling optimal RAASi therapy in patients with CKD stages 3–4 with and without HF in Spain.
Introduction
Chronic kidney disease (CKD) is considered a public health problem and is one of the leading causes of mortality due to the aging of the populationCitation1. National guidelines recommend renin-angiotensin-aldosterone system inhibitors (RAASi), such as angiotensin-converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB), as the first and most important therapeutic step to prevent CKD progression and other pathologiesCitation2. RAASi therapy has demonstrated the reduction of blood pressure and proteinuria, delay glomerular filtration rate decline, and decreases the risks of kidney failure, cardiovascular morbidity and all-cause mortalityCitation3. However, its use is associated with an increased risk of hyperkalemia (HK)Citation3,Citation4. At the onset of HK, a major therapeutic dilemma arises from its benefit–risk balance that may lead to RAASi discontinuation or dose reduction and hinder the management of the underlying disease.
Over the past decades, ion exchange resins have been the most commonly used treatment to address HK, but they are associated with several drawbacks, such as safety-related contraindications due to serious gastrointestinal adverse events, risk of hypokalaemia, poor tolerability that can lead to low adherence to treatment, etc.Citation5–7. Recent Spanish real-world data reported that only 36.8% of the patients were adherent to the treatment in the first year and 17.5% in the third yearCitation7. Patiromer is a sodium-free, cation exchange polymer that is not absorbed and is able to bind free potassium in the lumen of the gastrointestinal tract, thereby reducing its absorptionCitation6. In the OPAL-HK study, patiromer demonstrated the reduction of serum potassium levels and prevent the recurrence of HK; and, consequently, patiromer allowed to maintain RAASi optimal dosesCitation8. Patiromer has significantly changed the management of CKD by offering a solution for the maintenance of normokalemia in patients treated with RAASi.
Patiromer has already been approved in the United States and in the European Union, and the National Institute for Health and Care Excellence (NICE) has recommended it for treating HK in patients with CKD with and without HFCitation9. In Spain, patiromer was commercialized in July 2019. Based on the indications of the Spanish health authorities, patiromer is currently reimbursed in patients with advanced CKD and class III–IV heart failure (HF) and mild to moderate HK (5.5–6.4 mmol/L), who are receiving RAASi and with failure or intolerance to ion exchange resins. The aim of this study is to evaluate the cost-effectiveness of patiromer in the management of HK in CKD patients with and without HF from the perspective of the Spanish National Health System (NHS).
Methods
Patient population
The target patient population in this analysis were adults (≥18 years old) with CKD stages 3–4 (≥15–60 mL/min/1.73m2) with and without HF (New York Heart Association [NYHA] functional class I, II, III or IV), serum potassium levels >5.5 mmol/L and treated with RAASi. According to the OPAL-HK trial, serum potassium levels from 5.5 to 6.5 mmol/L are defined as moderate to severe HKCitation8. Baseline patient demographic and clinical characteristics were obtained from the 107 patients included in the 8-week randomized withdrawal phase of the OPAL-HK trialCitation8,Citation10 ().
Table 1. Baseline patient demographic and clinical characteristics.
Intervention and comparator
The intervention evaluated was a daily treatment regimen of patiromer (8.4 or 16.8 g/day). The comparator was the standard-of-care (hereafter referred to as “No patiromer”), defined as the acute management for the correction of serum potassium and lifestyle interventions for the background maintenance of serum potassium (dietary intervention, modification of concomitant medications, etc.).
Model structure
A Markov model was designed to evaluate the cost-effectiveness of patiromer versus no patiromer for the control and maintenance of normokalemia in CKD patients. The model consisted of different health states, including acute HK, adverse events (AEs), major adverse cardiac events (MACE), hospitalizations, changes in RAASi use, mortality, and CKD and HF progression (). MACE, hospitalization and death were absorbing states. The model simulates the natural history of disease for patients with CKD stages 3–4 with and without HF, using standard approaches to classify disease severity and predict disease progression. For CKD patients, progression through CKD stages is followed until the onset of end-stage renal disease (ESRD) and the initiation of renal replacement therapy, including dialysis or renal transplant.
Figure 1. Markov model for HK in CKD patients with or without HF. The squares represent different health states that are mutually exclusive. CKD stages 3–4 and NHYA I–IV represent starting health states. Abbreviations. CKD, chronic kidney disease; HF, heart failure; HK, hyperkalemia; MACE, major adverse cardiac events; NYHA, New York Heart Association; RAASi, renin-angiotensin-aldosterone system inhibitors.
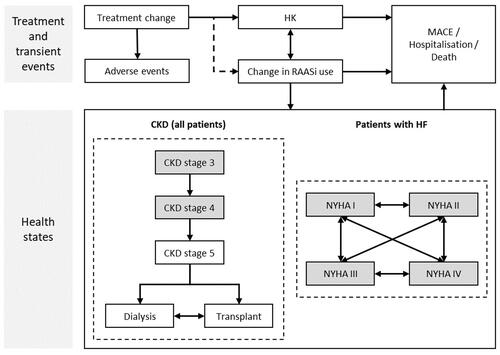
All patients who were initiated in the treatment arm were assumed to receive patiromer for at least 1 month and, after that, patients were stratified depending on the response to treatment. Responders continued to receive patiromer with its associated risk of events, while non-responders ceased treatment and incurred the risk of events as with no patiromer. Beyond the first month, patients receiving patiromer may discontinue at a constant monthly rate (10.3%), estimated from the OPAL-HK trial. The model also included an option to simulate re-treatment in patients who discontinue patiromer.
The analysis adopted a Spanish NHS perspective, taking only direct healthcare costs into account. A cycle length of 1 month and a lifetime horizon were considered. A 3% annual discount rate was applied for both costs and health effects, in accordance with the Spanish recommendations for the economic evaluation of drugsCitation11.
Clinical efficacy
Clinical parameters were mainly derived from the OPAL-HK studyCitation8. HK events and RAASi dose alteration rates were predicted over the first 3 months based on the OPAL-HK studyCitation8,Citation10. Subsequently, these rates were based on published literature and were adjusted for treatment-dependent relative risks to capture the effect of patiromer on reducing HK incidence and RAASi discontinuationCitation12,Citation13. HK incidence was modified according to RAASi therapy (Appendix A).
Other events not treatment-related included were MACE, hospitalizations and mortality. Baseline event rates were sourced from published literature and specific for CKD and HF. Annual MACE, hospitalization and mortality rate by stage (3–5) were included for CKD patientsCitation18, while annual MACE rate by RAASi dose and monthly probability of hospitalization by stage (NYHA I–IV) were considered for HF patientsCitation13,Citation20. Mortality was modeled using a multivariable Cox model for survival among HF patientsCitation22. MACE and mortality rates in CKD patientsCitation21 and hospitalization events in HF patientsCitation14 were adjusted by RAASi therapy and dose (Appendix A).
The progression through CKD stages was modeled according to health state transition probabilitiesCitation15,Citation16,Citation23. RAASi therapy was associated with delaying progression to ESRD and therefore the progression rates between CKD stages were adjusted for RAASi useCitation21. For ESRD patients, changes in modality of care (dialysis/transplant), the incidence of dialysis-related complications and mortality were includedCitation17. The progression of HF patients was modeled via transitions between NYHA classifications (I–IV)Citation19 (Appendix A).
Costs and resource use
A literature search was performed to identify sources for cost estimates. All cost parameter values were obtained from national databasesCitation24–29 and were updated to the year 2021 based on the Spanish Consumer Price Index ().
Table 2. Costs and utilities included.
The acquisition cost of patiromer treatment was determined as the weighted mean of 8.4 g (90%) and 16.8 g (10%) daily dose cost with its use in clinical practice, according to the opinion of Spanish clinical experts. The cost was derived from the unit price (retail price), applying the deduction described in the Royal Decree Law 8/2010 (7.5%)Citation30,Citation37. No cost was applied for no patiromer arm.
Direct medical costs of drugs (RAASi), CKD management, acute HK, MACE and hospitalization events were included in the analysis. Costs associated with ongoing RAASi therapy, including ACE inhibitors, ARB and/or mineralocorticoid receptor agonist (MRA), were estimated based on their use in the Spanish clinical practice. Two different costs were calculated by RAASi dose: optimal (maximum) and sub-optimal (sub-maximum). The sub-optimal dose cost was assumed as half of the optimal dose cost. In addition to this, management costs associated with RAASi dose change (discontinuation, down-titration, return to maximum) were applied in the month of change, including primary care and specialist visits, hospitalizations and urea and electrolytes tests. Annual CKD management costs were stratified by stage, including stages 3–5, dialysis and transplant maintenance. The one-off cost of dialysis access, dialysis complications and transplant procedure were also included. Costs of acute HK management were defined by serum potassium levels (>5.5 to ≤6 mmol/L; >6.0 mmol/L) and included healthcare resource use for outpatient and inpatient care, respectively. MACE and hospitalization costs were only applied in month of event ().
Utilities
The impact of CKD, HF and events on health-related quality of life was assessed using utilities and utility decrements and was expressed as quality-adjusted life-years (QALYs). For each treatment arm, QALYs were calculated by multiplying the length of time in life-years gained (LYG) that patients spent in the different health states, with the particular utility score. Data from international published literature were usedCitation31–35,Citation38, as there were no suitable studies for Spain. Baseline utility was age and sex-dependent and sourced from an international study that used EQ-5D questionnaire data from EuroQoLCitation39. Transient events such as HK, hospitalization and MACE were associated with a disutility applied additively in the cycle of incidence only ()Citation33–36. No treatment-related disutility was considered.
Sensitivity analysis
Deterministic one-way and probabilistic sensitivity analyses (PSA) were performed to assess the robustness of the model and to determine the impact of parameter uncertainty on the incremental cost-effectiveness ratio (ICER).
One-way scenario analyses were modeled, modifying discount rates, time horizon, patiromer discontinuation monthly rateCitation40, the proportion of patients with HF, and baseline ageCitation4.
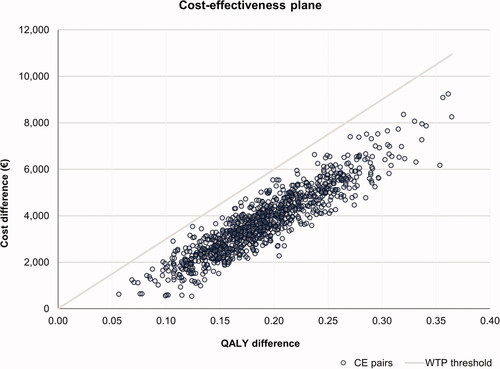
The PSA was conducted over 1,000 simulations, varying simultaneously all parameters included in the model. Cost inputs and acute HK event annual rate were modeled using a gamma distribution. Probabilities, utilities, and disutilities parameters were modeled using a beta distribution. The standard error (SE) was assumed to be 10% of the mean estimate when it was not available. The results were represented in a cost-effectiveness plane scatter diagram, which showed the uncertainty around the base-case result. A cost-effectiveness threshold of €30,000 per QALY gained was assumed.
Results
Base case
Patiromer was associated with greater QALYs gained per patient than no patiromer over a lifetime horizon (5.76 vs 5.57 QALYs, respectively). In addition, patiromer was associated with additional LYG compared with no patiromer (7.73 vs. 7.50 LYG, respectively) (). This increase in effectiveness is mainly due to the fact that patiromer delays the progression to ESRD in patients with and without HF, that is, patients treated with patiromer remain longer in less severe CKD stages that are associated with lower mortality and morbidity (lower MACE and hospitalization rates) compared to no patiromer.
Table 3. Base case and one-way scenario analyses results: patiromer vs. no patiromer (€2021).
Treatment with patiromer resulted in an incremental total lifetime average cost per patient of €3,574, compared to no patiromer (€119,642 vs. €116,068, respectively). The majority of the cost difference was due to higher drug acquisition costs associated with patiromer treatment (€1,527) and with the management of CKD (€1,546) thanks to the fact that patiromer delayed the progression from CKD to ESRD and patients remained longer in CKD (stages 3–5). On the other hand, patiromer was associated with significantly lower acute HK management costs than no patiromer (−€180), as well as lower MACE events (−€73). In addition, patiromer was associated with fewer management costs related to RAASi dose titration than no patiromer due to patiromer enabling maintaining RAASi therapy in optimal doses (−€98).
The ICER was estimated to be €19,092 per QALY gained, suggesting that patiromer was cost-effective compared with no patiromer using a willingness-to-pay (WTP) threshold of €30,000 per QALY gained (). The cost per LYG was estimated at €15,236.
Sensitivity analyses
The results of the one-way sensitivity analysis confirmed the robustness of the base case. The probability of patiromer treatment discontinuation was the most influential parameter (). Considering the patiromer discontinuation rate observed in the AMETHYST trial (2.5%)Citation40, the ICER would increase to €25,818/QALY; while if the discontinuation rate is higher than the reported in the OPAL-HK trial (15%), the ICER would decrease to €17,864/QALY. In general, the results of the scenario analyses suggested that patiromer was cost-effective compared with no patiromer since the ICERs were still below the WTP threshold. This occurred in all cases analyzed. Besides, patiromer was demonstrated to be a cost-effective treatment for the subgroup of patients with CKD and without HF (€19,561/QALY), and for patients with CKD and HF (€18,377/QALY).
The PSA showed that patiromer would be a cost-effective intervention in all simulations, assuming a WTP threshold of €30,000/QALY gained. The cost-effectiveness plane is presented in .
Discussion
Our analysis shows that the addition of patiromer to RAASi therapy is a cost-effective alternative for the treatment of HK in the Spanish setting. The model demonstrated that patiromer treatment compared with no patiromer resulted in improvements in survival and health-related quality of life (5.76 vs. 5.57 QALYs) and reductions in progressing to ESRD, acute HK and MACE events, which almost offset the acquisition cost of patiromer (+€3,574). Therefore, patiromer is a cost-effective option for patients with CKD stages 3–4 with and without HF, a larger patient group than current reimbursed visa conditions in Spain. Besides, patiromer is cost-effective for patients regardless of a prior treatment since the inclusion criteria of the OPAL-HK study did not consider that patients had to fail prior treatment such as ion exchange resins. Thus, patiromer is cost-effective as long as the patient has HK.
To our knowledge, this is the first study to assess the cost-effectiveness of patiromer from the Spanish NHS perspective. Thus, the results of this analysis can generate evidence from a regulatory and clinical point of view for the use of patiromer in actual clinical practice.
HK is associated with an increased risk of all-cause death and healthcare expenditureCitation41,Citation42. A recent study by Orly et al.Citation7 estimated the impact of HK on the use of RAASi and its associated economic impact on the Spanish NHS. Compared to mild HK, patients with severe HK incurred higher costs (€12,705 vs. €4,468) and had a higher RAASi therapy discontinuation rate (51.8% vs. 39.8%) in the short term. Thus, patients with severe HK remained at high serum potassium levels and represented high healthcare expenditures. Patients with HK have a significant disease burden due to higher hospitalization rates, the proportion of patients with dialysis initiation and recurrent events. This increases healthcare costs attributable to HK events. Patiromer offers a solution to CKD patients receiving RAASi thanks to declining serum potassium levels and, consequently, the recurrence of HKCitation8. Furthermore, patiromer enables the maintenance of normokalemia and optimal RAASi therapy that was associated with delaying the onset of ESRD, improving survival and reducing renal replacement therapy-related costsCitation3.
Other economic evaluations of patiromer have been published previously. A recent study by Widén et al.Citation43. assessed the cost-effectiveness of patiromer in patients with CKD stages 3–4 receiving RAASi from a Swedish perspective. At a WTP threshold of €52,804 per QALY, patiromer was cost-effective with higher effectiveness (0.14 QALYs) and an incremental cost per patient (€6,109) than the standard of care, yielding an ICER of €43,307 per QALY gained. A study by Bounthavong et al.Citation44. evaluated the cost-effectiveness of patiromer in combination with RAASi for patients with CKD from the US payer perspective. Patiromer combined with RAASi also resulted in an incremental QALY of 0.35 (6.32 vs. 5.97) and an additional cost of $18,600 ($265,400 vs. $246,800), compared to RAASi alone. The ICER was $53,400 per QALY gainedCitation44. Another study by Bounthavong et al.Citation45. evaluated the use of patiromer in combination with spironolactone and ACE inhibitor in patients with HK and class III–IV HF from the US payer perspective, compared with ACE inhibitor alone. Patiromer in combination with spironolactone and ACE-inhibitor resulted in greater QALYs (2.79 vs. 2.60) and costs ($28,000 vs. $18,200), giving an ICER of $52,700 per QALY gainedCitation45. Thus, patiromer was cost-effective at a threshold of $100,000 per QALY. These studies show that even though there might be some limitations in extrapolating our results to other settings because the costs could be different, the use of patiromer is cost-effective. Finally, an economic analysis by de Sequera et al.Citation46 estimated the impact of a good HK control with patiromer in patients with CKD and/or HF from a societal perspective in Spain. Patiromer reduced annual costs by 32% (€3,126.84 per patient with CKD; €3,466.27 per patient with HF) thanks to the delay of ESRD and the reduction in premature mortality, respectively. These savings would offset its pharmacological costCitation46. All these results suggest that the use of patiromer is value for money in patients with CKD with and without HF.
On the other hand, no other cost-effectiveness analyses on current treatment options were identified for patients with CKD and HK in Spain. Therefore, our study is the first published in this area.
Our analysis was associated with several limitations. First, clinical parameters included in the model were derived from the OPAL-HK study, but patients were followed up for 12 weeks in the trial. Beyond this period, acute HK event incidence and RAASi discontinuation rate were sourced from published literature. However, results from PSA indicate that patiromer is a potentially cost-effective alternative in most of the simulations. Further evidence is needed to confirm the long-term efficacy of patiromer. Second, another possible limitation is that most of the inputs were derived from international sources due to a lack of Spanish studies, except for costs. In Spain, there are higher rates of CKD progression and mortality, and renal replacement therapy initiation than in other European countriesCitation47. Third, the OPAL-HK study compared the efficacy and safety of patiromer versus no patiromer, but further research is needed to evaluate the efficacy and costs of patiromer compared with sodium zirconium cyclosilicate, a recent reimbursed treatment in Spain, although indirect comparisons have multiple limitations. Fourth, the incidence of HK or death was only specifically modeled based on potassium levels and different CKD and HF stages; and not according to other parameters such as gender and age. However, subgroup analysis of the OPAL-HK study showed that the efficacy of patiromer was higher than no patiromer in all subpopulationsCitation8. Finally, indirect costs were not included because the analysis adopted the NHS perspective. However, the population was on average over 60 years old, therefore the potential productivity losses may be low.
Conclusions
Patiromer was projected to be cost-effective in the treatment of HK in CKD patients with and without HF, receiving RAASi therapy, from the Spanish NHS perspective. Even though patiromer treatment was associated with additional costs, acute HK and MACE management costs were lower and survival and quality of life improved. Patiromer demonstrated to be a therapeutic option for maintaining normokalemia and enabling optimal RAASi therapy in CKD patients with and without HF, a larger patient group than current reimbursed visa conditions in Spain.
Transparency
Declaration of funding
This work was funded by Vifor Pharma Group.
Declaration of financial/other relationships
MV and ARA are employed by Vifor Pharma Spain and Switzerland, respectively. DN and EP are employed by PharmaLex Spain and received financial support from Vifor Pharma Spain for the development of this study. JGJ, AGF and PS received an honorarium from the sponsor and participated as independent consultants.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
MV, ARA, DN and EP contributed to the concept and design of the study, and to the analysis and interpretation of the data. All authors contributed to the critical revision of the manuscript for important intellectual content and read and approved the final version. All authors sufficiently contributed to this research according to ICMJE criteria to qualify as listed authors.
Acknowledgements
No assistance in the preparation of this article is declared by the authors.
References
- Wang H, Naghavi M, Allen C, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the global burden of disease study 2015. The Lancet. 2016;388(10053):640–1544.
- Ballarín J, García F, Ibeas J, et al. Guía de Práctica Clínica sobre la Detección y el Manejo de la Enfermedad Renal Crónica del Servicio Nacional de Salud [Clinical practice guideline on detection and management of the chronic kidney disease. Ministerio de Sanidad, Servicios Sociales e Igualdad. Instituto Aragonés de Ciencias de la Salud [Internet]. 2016 [cited 2021 August 17]. Available from: https://portal.guiasalud.es/wp-content/uploads/2018/12/GPC_559_ERC_IACS_compl.pdf.
- Evans M, Palaka E, Furuland H, et al. The value of maintaining normokalaemia and enabling RAASi therapy in chronic kidney disease. BMC Nephrol. 2019;20(1):31.
- Belmar L, Galabia ER, Bada da Silva J, et al. Epidemiología de la hiperpotasemia en la enfermedad renal crónica [epidemiology of hyperkalemia in chronic kidney disease]. Nefrología. 2019;39(3):277–286. Spanish.
- Sterns RH, Rojas M, Bernstein P, et al. Ion-Exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21(5):733–735.
- AEMPS (Spanish Agency for Medicines and Medical Devices). AEMPS online medicinal product information centre (CIMA). AEMPS [Internet]. 2021 [cited 2021 November 18]. Available from: https://cima.aemps.es/cima/publico/home.html.
- Olry de Labry A, Díaz Ó, Romero-Requena JM, et al. Hyperkalaemia management and related costs in chronic kidney disease patients with comorbidities in Spain. Clin Kidney J. 2021;14(11):2391–2400.
- Weir MR, Bakris GL, Bushinsky DA, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372(3):211–221.
- National Institute for Health and Care Excellence. Patiromer for treating hyperkalaemia: guidance (TA623). National Institute for Health and Care Excellence [Internet]. 2020 [cited 2020 December 9]. Available from: https://www.nice.org.uk/guidance/ta623
- VIFOR PHARMA. OPAL-HK CSR. Data on file. 2014.
- Ortega A, Marín R, Fraga MD. Guía de evaluación económica e impacto presupuestario en los informes de evaluación de medicamentos. Guía práctica asociada al programa MADRE v 4.0. [Guideline for economic evaluation and budget impact in drug evaluation reports. Practice guideline associated with the MADRE v 4.0. program]. Sociedad Española de Farmacia Hospitalaria. 2021 [cited 2021 July 1]. Available from: https://gruposdetrabajo.sefh.es/genesis/genesis/Documents/GUIA_EE_IP_GENESIS-SEFH_19_01_2017.pdf.
- Horne L, Ashfaq A, MacLachlan S, et al. Epidemiology and health outcomes associated with hyperkalemia in a primary care setting in England. BMC Nephrol. 2019;20(1):85.
- Linde C, Bakhai A, Furuland H, et al. Real‐world associations of renin–angiotensin–aldosterone system inhibitor dose, hyperkalemia, and adverse clinical outcomes in a cohort of patients with new‐onset chronic kidney disease or heart failure in the United Kingdom. JAHA. 2019;8(22):e012655.
- Flather MD, Yusuf S, Køber L, et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. The Lancet. 2000;355(9215):1575–1581.
- Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609–619.
- Robb M, Hendry R. Annual report on kidney transplantation. NHS Blood and Transplant [Internet]. 2020 [cited 2021 December 9]. Available from: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/20032/kidney-annual-report-2019-20-final.pdf.
- UK Renal Association. UK Renal Registry 22nd Annual Report. UK Renal Association [Internet]. 2018 [cited 2021 December 9]. Available from: https://ukkidney.org/about-us/who-we-are/uk-renal-registry.
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305.
- Yao G, Freemantle N, Calvert MJ, et al. The long-term cost-effectiveness of cardiac resynchronization therapy with or without an implantable cardioverter-defibrillator. Euro Heart J. 2006;28(1):42–51.
- Ford E, Adams J, Graves N. Development of an economic model to assess the cost-effectiveness of hawthorn extract as an adjunct treatment for heart failure in Australia. BMJ Open. 2012;2(5):e001094.
- Xie X, Liu Y, Perkovic V, et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis. 2016;67(5):728–741.
- Levy WC, Mozaffarian D, Linker DT, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113(11):1424–1433.
- Nuijten M, Andress DL, Marx SE, et al. Cost effectiveness of paricalcitol versus a non-selective vitamin D receptor activator for secondary hyperparathyroidism in the UK. Clin Drug Investig. 2010;30(8):545–557.
- Gisbert R, Brosa M. Base de Datos de Costes Sanitarios y Ratios Coste-Efectividad Españoles: eSalud [Database of Spanish health costs and cost-effectiveness ratios]. Oblikue Consulting S.L. [Internet]. 2021 [cited 2021 June 2]. Available from: http://esalud.oblikue.com.
- Lorenzo-Sellares V, Pedrosa MI, Santana-Expósito B, et al. Análisis de costes y perfil sociocultural del enfermo renal. Impacto de la modalidad de tratamiento [cost analysis and sociocultural profile of kidney patients. Impact of the treatment method]. Nefrología. 2014;34:458–468. Spanish.
- Caballero Alcalde C, Calleja Hernández MA, Escoda Ruiz L, et al. Libro blanco de la poliquistosis renal autosomica dominante (PQRAD) en España. Alianza frente a la PQRAD. 2nd Ed. 2016. Available from: https://www.easp.es.
- Darbà J, Marsà A. Chronic kidney disease in Spain: analysis of patient characteristics, incidence and direct medical costs (2011–2017). J Med Econ. 2020;23(12):1623–1629.
- Vargas M. Documento Marco sobre Enfermedad Renal Crónica dentro de la Estrategia de Abordaje a la Cronicidad en el SNS. Ministerio De Sanidad Servicios Sociales E Igualdad [Document on chronic kidney disease within the chronicity approach strategy in the NHS]. Ministerio de Sanidad, Servicios Sociales e Igualdad [Internet]. 2015 [cited 2021 may 28]. Available from: https://www.sanidad.gob.es/organizacion/sns/planCalidadSNS/pdf/Enfermedad_Renal_Cronica_2015.pdf.
- Ministerio de Sanidad. Conjunto Mınimo Basico de Datos (CMBD) [Minimum basic data set (MBDS)]. Ministerio de Sanidad [Internet]. 2019 [cited 2021 July 1]. Available from: https://www.sanidad.gob.es/estadEstudios/estadisticas/cmbdhome.htm.
- Consejo General de Colegios Oficiales de Farmacéuticos. Portal Farma. BotPLUS. Consejo General de Colegios Oficiales de Farmaceuticos [Internet]. 2021 [cited 2021 June 2]. Available from: https://botplusweb.portalfarma.com/botplus.aspx.
- Gorodetskaya I, Zenios S, Mcculloch CE, et al. Health-related quality of life and estimates of utility in chronic kidney disease. Kidney Int. 2005;68(6):2801–2808.
- Lee AJ, Morgan C, Conway P, et al. Characterisation and comparison of health-related quality of life for patients with renal failure. Curr Med Res Op. 2005;21(11):1777–1783.
- Göhler A, Geisler BP, Manne JM, et al. Utility estimates for decision–analytic modeling in chronic heart Failure-Health states based on New York heart association classes and number of rehospitalizations. Value in Health. 2009;12(1):185–187.
- Kent S, Briggs A, Eckermann S, et al. Are value of information methods ready for prime time? An application to alternative treatment strategies for NSTEMI patients. Int J Technol Assess Health Care. 2013;29(4):435–442.
- Sennfält K, Magnusson M, Carlsson P. Comparison of hemodialysis and peritoneal dialysis–a cost-utility analysis. Perit Dial Int. 2002;22(1):39–47.
- National Institute for Health and Care Excellence. Clinical guideline [CG125]: chronic kidney disease (stage 5): peritoneal dialysis. National Institute for Health and Care Excellence. [Internet]. 2021 [cited 2021 July 21]. Available from: https://www.nice.org.uk/guidance/cg125.
- Ministerio de Sanidad. Listado de medicamentos afectados por las deducciones del Real Decreto-Ley 8/2010 [List of drugs affected by the deductions of the Royal Decree-Law 8/2010]. Ministerio de Sanidad. 2021 [cited 2021 July 1]. Available from: https://www.mscbs.gob.es/profesionales/farmacia/pdf/DeduccionesJulio2021.pdf.
- National Institute for Health and Care Excellence. Clinical guideline [NG107]: renal replacement therapy and conservative management. National Institute for Health and Care Excellence [Internet]. 2021 [cited 2021 July 21]. Available from: https://www.nice.org.uk/guidance/ng107.
- Szende A, Janssen B, Cabases J, editors. Self-reported population health: an international perspective based on EQ-5D. Dordrecht (The Netherlands): Springer; 2014.
- Bakris GL, Pitt B, Weir MR, et al. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA. 2015;314(2):151.
- Jiménez-Marrero S, Cainzos-Achirica M, Monterde D, et al. Impact on clinical outcomes and health costs of deranged potassium levels in patients with chronic cardiovascular, metabolic, and renal conditions. Rev Esp Cardiol. 2021;74(4):312–320.
- Collins AJ, Pitt B, Reaven N, et al. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017;46(3):213–221.
- Widén J, Ivarsson M, Schalin L, et al. Cost-Effectiveness analysis of patiromer in combination with renin-angiotensin-aldosterone system inhibitors for chronic kidney disease in Sweden. PharmacoEconomics. 2020;38(7):747–764.
- Bounthavong M, Adamson R, Dolan C, et al. The cost-effectiveness analysis of patiromer and renin-angiotensin-aldosterone system inhibitors therapy in diabetic nephropathy patients with hyperkalemia. J Manag Care Spec Pharm. 2018;24:S86.
- Bounthavong M, Butler J, Dolan CM, et al. Cost-effectiveness analysis of patiromer and spironolactone therapy in heart failure patients with hyperkalemia. PharmacoEconomics. 2018;36(12):1463–1473.
- De Sequera P, Bover R, Ivanova Y, et al. Análisis económico del uso de patiromer en España [Economic analysis of the use of patiromer in Spain]. Málaga (Spain): 22 Congreso Nacional de Hospitales y Gestión Sanitaria; 2021. Spanish.
- Brück K, Jager KJ, Zoccali C, et al. Different rates of progression and mortality in patients with chronic kidney disease at outpatient nephrology clinics across Europe. Kidney Int. 2018;93(6):1432–1441.