Abstract
Aims
To compare the efficacy of tezepelumab with other approved biologics via indirect treatment comparisons (ITCs) in patients aged ≥ 12 years with severe uncontrolled asthma.
Materials and methods
Data from randomized controlled trials (RCTs) identified from a systematic literature review were synthesized using two different ITC approaches: network meta-analysis (NMA) and simulated treatment comparison (STC). Outcomes of interest were annualized asthma exacerbation rate (AAER) and AAER for exacerbations leading to hospitalization or emergency room visit. To address potential heterogeneity between study populations, various subgroup analyses were performed for the NMA (based on blood eosinophil count, fractional exhaled nitric oxide level, and presence of allergic asthma), and for the STC, models were adjusted for potential treatment effect modifiers. Sensitivity analyses were performed to assess the impact of study design (exclusion of non-placebo-controlled studies and non-phase 3 or 4 studies). Results were reported as rate ratios (RRs) with 95% credible/confidence intervals and ranking statistics were computed for the NMAs.
Results
Sixteen RCTs were included in at least one of the ITCs. All biologics (tezepelumab, dupilumab, benralizumab, mepolizumab, reslizumab, and omalizumab) had similar efficacy, with no statistically significant RRs for either exacerbation outcome; however, tezepelumab was favorably associated with numerically lower AAERs and was ranked first in the network for both types of exacerbation outcome. This trend was consistent in the subgroup and sensitivity analyses. As with the primary NMA, the STC results did not demonstrate any significant differences between biologics, but point estimates were favorable towards tezepelumab.
Limitations
Heterogeneity between trials was observed among eligibility criteria and clinically important patient characteristics; however, the impact on findings is expected to be low, based on consistency across analyses.
Conclusions
Findings from both ITCs (NMA and STC) support the use of tezepelumab in a broad patient population of severe uncontrolled asthma of any phenotype.
Introduction
Severe asthma accounts for approximately 5–10% of all asthma casesCitation1. Despite treatment with guideline-recommended standard of care therapy, which may include medium- to high-dose inhaled corticosteroids and long-acting β2-agonists, or a short course of oral corticosteroids (e.g. prednisolone), many patients have uncontrolled disease, calling for other treatment optionsCitation1,Citation2. Prior to December 2021, there were five monoclonal antibodies (biologics) approved by both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of severe, uncontrolled asthma: omalizumab (anti-immunoglobulin [Ig] E); mepolizumab and reslizumab (anti-interleukin [IL]-5); benralizumab (anti-IL-5 receptor α); and dupilumab (anti-IL-4 receptor α)Citation3–7. These biologics have demonstrated annualized asthma exacerbation rate (AAER) reductions of 48–59% versus placebo in pivotal clinical trialsCitation8–12. However, their treatment effects are mainly limited to eosinophilic and allergic asthma. They have demonstrated limited efficacy in patients with blood eosinophil counts (Eos) of less than 150 cells/µLCitation8,Citation9,Citation11,Citation12, and efficacy has been inconsistent in patients with Eos of 150–300 cells/µLCitation8,Citation9,Citation11,Citation12. Even in patients with eosinophilic asthma, for which many of these biologics are indicated, efficacy can vary across individuals. Thus, there is a need for additional biologic treatments that have a high efficacy across the whole spectrum of severe, uncontrolled asthma.
In December 2021, tezepelumab was approved by the FDA for the treatment of severe asthma, and is the only biologic approved for severe asthma with no phenotype (e.g. eosinophilic or allergic) or biomarker limitation within its approved labelCitation13,Citation14. Tezepelumab is a human immunoglobulin G2 (IgG2) monoclonal antibody that targets thymic stromal lymphopoietin (TSLP), preventing its interaction with the heterodimeric TSLP receptorCitation15, and impacting both type 2 and non-type 2 inflammatory pathways, including allergic inflammation, eosinophilic inflammation, and airway hyperresponsivenessCitation16,Citation17. TSLP is an epithelial cell-derived cytokine produced in response to proinflammatory stimuli (such as viruses, allergens, and irritants) and is a key factor in the regulation of type 2 immunity through its activity on dendritic cells, T- and B-cells, and innate immune cellsCitation18–20. TSLP may regulate allergic responses more broadly and through more diverse pathways than biologic therapies that target immunoglobulin E (IgE) or type 2 effector cytokines, such as IL-4, IL-5, or IL-13Citation17.
The efficacy and safety of tezepelumab for the treatment of severe, uncontrolled asthma has been demonstrated in the PATHWAY and NAVIGATOR trials, which were both randomized and placebo-controlled. In the PATHWAY trial (phase 2, NCT02054130), tezepelumab 210 mg every 4 weeks was associated with a significant, 71% reduction in AAER versus placeboCitation15. In NAVIGATOR (phase 3, NCT03347279), the same regimen of tezepelumab led to reductions in AAER, regardless of baseline Eos, fractional exhaled nitric oxide (FeNO) level, or allergic status. In the overall population, tezepelumab was associated with a 56% reduction in AAER, and in patients with an Eos less than 300 cells/µL (which accounted for 58% of the study population), tezepelumab was associated with a 41% reduction versus placeboCitation21,Citation22; both reductions were statistically significant. These findings suggest that tezepelumab is effective in a broader population of patients with severe, uncontrolled asthma than any one of the currently approved biologics, and, in addition, tezepelumab is effective in patients without an eosinophilic phenotype. However, the comparative efficacy of tezepelumab versus any of the five previously approved biologics has not been evaluated in randomized controlled trials (RCTs), in part because other biologics are only approved in subsets of severe, uncontrolled asthma.
In the absence of head-to-head clinical trial data, indirect treatment comparisons (ITCs), such as network meta-analyses (NMAs) and simulated treatment comparisons (STCs), can be used to compare the efficacy of treatmentsCitation23,Citation24. These evidence synthesis methods differ from conventional pairwise meta-analyses in that conventional meta-analyses are used to compare two treatments that have been compared directly in individual trials; in ITCs, multiple treatments can be compared even if they have never been included in the same trialCitation23–25. In NMAs, all relevant trials are analyzed in one network, with comparisons made between all treatment arms represented. This method is intuitive to follow, but does not fully account for potential heterogeneity in key study characteristics such as eligibility criteria and outcome definitionsCitation26. STCs have more complex methods, and have the advantage of allowing adjustment for patient characteristics when comparing outcomesCitation27. These valuable statistical methods have evolved over time to address the lack of head-to-head data and they are often required to inform reimbursement decisionsCitation23,Citation24,Citation28–32.
ITCs have been performed previously for the biologics approved prior to tezepelumabCitation33–43, and one non-industry sponsored ITC has been published that includes tezepelumabCitation44. This ITC was an NMA that included tezepelumab and three comparators (dupilumab, mepolizumab, and benralizumab), but omitted omalizumab and reslizumabCitation44. No ITC has yet included all available biologics for severe asthma. Further, all of the previous ITCs used one type of statistical method only. Different types of ITC have advantages and disadvantages, as mentioned above; thus, it is valuable to explore several different methods to assess the robustness of the overall ITC findings.
The aim of the current study was to use two types of ITC method – NMA and STC – to compare the efficacy of tezepelumab with five other approved biologics, in terms of reducing exacerbations in patients with severe, uncontrolled asthma.
Methods
Systematic review of the literature
A systematic literature review (SLR) was performed in October 2020 to identify all relevant data for inclusion in the ITCs. Three electronic databases (MEDLINE, Embase, and Cochrane Central Register of Controlled Trials) were searched (Supplementary Table S1). In addition, supplementary searches captured data from gray literature (Supplementary Appendix 1). Abstracts of identified publications were screened by two independent reviewers, against pre-defined eligibility criteria (). In summary, eligibility criteria captured patients aged 12 years or older with asthma that is uncontrolled despite adherence to Global Initiative for Asthma (GINA) step 4 or 5 treatmentCitation45, and captured RCT data for tezepelumab (210 mg every 4 weeks) and approved doses of anti-IgE, anti-IL-5 pathway, and anti-IL-4/IL-13 biologics. The data extracted from eligible studies included details on study and patient population characteristics, treatment design, and efficacy and safety endpoint definitions and values.
Table 1. Eligibility criteria for identification of relevant studies.
Quality assessments of the included RCTs were conducted using the National Institute for Health and Care Excellence (NICE) quality appraisal checklist for quantitative intervention studiesCitation46.
The SLR was performed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions and reported in alignment with the Preferred Reporting Items for Systematic Literature Reviews and Meta-Analyses (PRISMA) guidelinesCitation47,Citation48.
ITCs
Overview
Two types of ITC were performed – NMA and STC – to assess the robustness of treatment comparisons using fundamentally different approaches. NMAs synthesize data from RCTs and allow multiple pairwise comparisons across the interventions studied, yielding relative treatment effects for each comparison. This is possible because each RCT is connected to one or more other RCTs via a common comparator (e.g. placebo), forming a network. The NMA assumes all populations in the network are exchangeable/comparable. The impact of heterogeneity can be investigated through subgroup analyses or via meta-regression, although the ability to explore heterogeneity may be limited in NMAs conducted using aggregate trial data, provided published information is available. In contrast, STCs involve the use of individual patient data (IPD) from one trial to simulate efficacy estimates within a comparator trial population. This method permits adjustment for multiple factors that are associated with heterogeneity and may affect the treatment comparison (i.e. potential treatment effect modifiers). Such adjustment reduces the likelihood of confounding in ITC estimates.
Outcomes
The two outcomes selected for analysis were (1) AAER overall, capturing any exacerbation requiring treatment and/or healthcare utilization, and (2) AAER for exacerbations leading to hospitalization or emergency room visit. These outcomes were selected as they are commonly used as primary outcomes in RCTs for severe asthma.
ITC feasibility assessment
The ITC feasibility assessment was conducted to inform selection of studies that were the most comparable, and to confirm the most appropriate method of ITC to be used (Supplementary Table S2). Examination of study characteristics showed that most studies reported an annualized exacerbation rate, or could be converted as such, and definitions of exacerbation were similar across most RCTs, typically being defined as deterioration of asthma that required use of systemic corticosteroids or unscheduled hospital visits. Several exceptions were observed for omalizumab trials, where an exacerbation could also be defined as a doubling of the individual’s baseline inhaled beclometasone dipropionate (BDP) doseCitation49, worsening of symptoms leading to work absenteeismCitation50, or worsening of symptoms demonstrated by specific changes in peak expiratory flow (PEF) or forced expiratory volume in 1 s (FEV1)Citation49,Citation51,Citation52. Studies that only reported the number or proportion of patients with exacerbations were excluded from further consideration. The time point of exacerbation rate assessment varied from week 12 to week 52 across studies.
NMA
Bayesian NMAs were performed based on standard methods described in the NICE Decision Support Unit (DSU) Technical Support Document (TSD) 2Citation31. The primary analysis included the intention-to-treat populations of all relevant RCTs, regardless of phase, type of control arm, and whether blinding was used. Rates of exacerbations were modeled using a Poisson likelihood and a log link, in which the number of person-years at risk was used. Vague or flat priors, such as N (0, 1002), were assigned for basic parameters. Fixed-effects and random-effects models were both considered for the NMAs and the best model fit (fixed- vs random-effects) was selected based on deviance information criterion (DIC) values, total residual deviance, and random-effects standard deviation. Where model fit was similar, both models are presented. Rate ratios (RRs) and 95% credible intervals (CrIs) were calculated for every pairwise comparison in the network. Surface under the cumulative ranking curve (SUCRA) values were estimated as an additional measure to reflect ranking of each treatment in the networkCitation53. SUCRA values are expressed as a percentage (0–100%) and represent the relative probability of an intervention being among the best options in the network; the higher the value, the higher the ranking of the treatment in the network. Consistency could not be assessed because none of the biologics in the network were directly connected. NMAs were performed using R statistical software (version 3.5.3) with R package “rjags” (version 4–8) to interface with Just Another Gibbs Sampler (JAGS) software (version 4.3.0) for both model specification and Bayesian estimation.
Subgroup and sensitivity analyses for NMA
To explore the potential impact of differences in patient characteristics across studies on findings observed in the primary analysis, and to assess comparative efficacy in clinically relevant subpopulations, subgroup analyses were performed for the NMA. Subgroups were formed based on three sets of variables and cut-offs for which there was sufficient comparative data: (1) Eos (≥ and < 300 cells/µL, ≥ and < 150 cells/µL); (2) FeNO level (≥ 25 ppb and ≥ 50 ppb); and (3) the presence of allergic asthma (defined as a positive result to a perennial aeroallergen test and/or high IgE meeting eligibilty for anti-IgE therapy). Further subgroups, such as patients with both high Eos count and high FeNO, were explored but were not possible, owing to a lack of reported data for comparator biologics. In the subgroup analyses, only the AAER overall outcome was analyzed because there were insufficient data to analyze AAER leading to hospitalization or emergency room visit in subgroups.
Two sensitivity analyses were performed for the NMA. One analysis was restricted to blinded and placebo-controlled trials only while the second included only phase 3 and 4 studies.
STC
Anchored STC analyses were performed for tezepelumab versus each comparator, using the methods outlined in the NICE DSU TSD 18Citation32. In each anchored STC, a regression model was applied to IPD from the tezepelumab trial (NAVIGATOR [2020]) and the fitted model was used to simulate the effect of tezepelumab in the population studied in the comparator trial. For each comparator and outcome, one study identified in the SLR was chosen to be the comparator trial, based on its comparability with NAVIGATOR in terms of patient characteristics, study design, and availability of data. For both STCs, a list of potential treatment effect modifiers was selected, based on clinician input, as well as on modifiers used in previous published ITCsCitation41,Citation42. These included: demographics and clinical characteristics (age, sex, body mass index, disease duration; number of exacerbations in the past 12 months, lung function, FEV1 [% predicted]); biomarkers (Eos count, FeNO, total IgE); and treatment-related characteristics (oral corticosteroid [OCS] users, OCS dose at entry, inhaled corticosteroids [ICS] dose at entry). These potential treatment effect modifiers were included as covariates in the regression model, incorporating interaction terms between treatment and clinically relevant treatment effect modifiers. The statistical performance of each regression model was based on model convergence and model fit statistics (Akaike information criterion [AIC]). AAER overall and AAER leading to hospitalizations/emergency room visits were modeled using negative binomial regression, in which the number of person-years at risk was used; this approach is aligned with a previously conducted ITC that assessed exacerbationsCitation35. RRs and 95% confidence intervals (CIs) were calculated for tezepelumab versus comparators. The primary analyses of the anchor trial and the comparator trials used fully adjusted models that included all potential treatment modifiers available in the studies. No sensitivity or subgroup analyses were performed for the STC.
Results
Systematic review of the literature
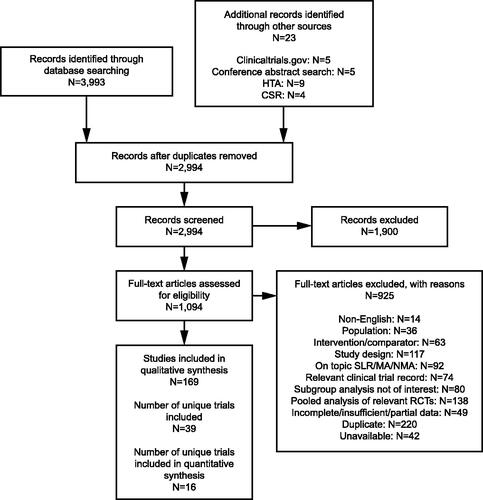
After screening 2,994 records from the electronic database and supplementary searches, 1,900 articles were excluded at different stages of the SLR. The final number of relevant unique studies was 39 (). The NAVIGATOR and SOURCE studies were additional trials to those identified through the SLR. Study characteristics and quality assessment findings of each study are presented in Supplementary Table S3. Twenty-six studies that reported the exacerbation outcomes of interest were included in the ITC feasibility assessment (Supplementary Table S2). After examining the findings, three studies were excluded from the NMA of both outcomes (AAER overall and AAER leading to hospitalization/emergency room visit) owing to comparability issues (Supplementary Table S4). These studies were also excluded as candidates for the STC.
Figure 1. PRISMA flow diagram. NAVIGATOR62 and SOURCECitation65 were additional trials to those identified through the systematic literature search. Abbreviations: CSR, clinical study report; HTA, Health Technology Assessment; MA, meta-analysis; NMA, network meta-analysis; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial; SLR, systematic literature review.
ITCs
NMA
Primary analysis
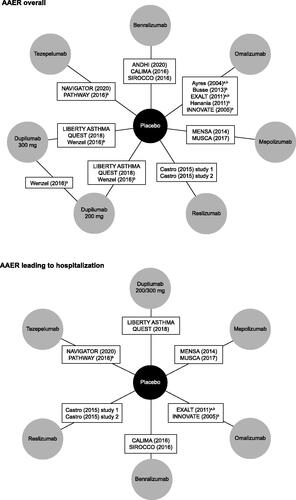
The NMA for AAER overall was informed by a network of eight treatment arms and 16 studies (). Dupilumab was represented by two treatment arms, depending on dose: 200 mg and 300 mg. The NMA for AAER leading to hospitalization/emergency room visit was informed by a network of seven treatment arms and 11 studies (). In this network, only pooled data for the 200 mg and 300 mg doses of dupilumab were available. Individual study data for the clinical outcomes are presented in Supplementary Tables S5 and S6.
Figure 2. Network diagram for primary NMAs of AAER overall and AAER leading to hospitalization/emergency room visit. aThese studies were excluded from the sensitivity analysis that restricted inclusion to blinded and placebo-controlled trials. bThese studies were excluded from the sensitivity analysis that restricted inclusion to phase 3 and 4 studies. In this sensitivity analysis, studies were excluded if they were not phase 3 or 4 studies, or if their source article did not report the study phase. Castro et al.Citation8 reported data for two different studies (NCT01287039 and NCT01285323), so contributes two treatment arms. LIBERTY ASTHMA QUEST reported data for two different treatment arms, based on dupilumab dose. Abbreviations: AAER, annualized asthma exacerbation rate; NMA, network meta-analysis.
For the primary NMA, random-effects models had the best fit for both outcomes and so are reported here. There were no statistically significant differences between any of the biologics in the network. However, tezepelumab was associated with a numerically lower AAER overall than all other treatment arms in the network. The strongest treatment effect observed among the biologics was that of tezepelumab versus omalizumab, where the RR (95% CrI) was 0.60 (0.35–1.01) (). Tezepelumab was ranked first in the network, with a SUCRA value of 84% (Supplementary Table S7).
Table 2. Primary NMA: rate ratios for AAER overall, reported as cross-tabulation of all treatment arms in the network.
For AAER leading to hospitalization/emergency room visit, the primary NMA showed again that there were no statistically significant differences between any of the biologics. Numerically, findings were favorable towards tezepelumab: tezepelumab had a lower exacerbation rate than all other treatment arms in the network () and was ranked first in the network, with a SUCRA value of 95% (Supplementary Table S7).
Table 3. Primary NMA: rate ratios for AAER leading to hospitalization/emergency room visit, reported as cross-tabulation of all treatment arms in the network.
Subgroup analyses for NMA
Studies included in the subgroup NMAs are listed in Supplementary Table S8. For the subgroup analysis of Eos higher or lower than 300 cells/µL, random-effects models are reported; for both the subgroups, there were no significant differences between any of the biologics ( and ). However, in both cases, tezepelumab was associated with a numerically lower AAER than almost all other biologics in the network (number of biologics included varied depending on availability of subgroups). The one exception was a favorable RR for dupilumab 300 mg versus tezepelumab for the Eos greater than or equal to 300 cells/µL subgroup ().
Table 4. Subgroup NMA for patients with eosinophil count ≥ 300 cells/µL: rate ratios for AAER overall, reported as cross-tabulation of all treatment arms in the network.
Table 5. Subgroup NMA for patients with eosinophil count <300 cells/µL: rate ratios for AAER overall, reported as cross-tabulation of all treatment arms in the network.
In the remaining subgroups, a random-effects model was the best fit for allergic asthma, while fixed-effects models were the best fit for Eos higher or lower than 150 cells/µL and for both FeNO subgroups. As with the primary analysis, all other subgroup findings demonstrated a consistent numerical benefit for tezepelumab versus comparators in terms of AAER overall (Supplementary Tables S9–13).
Sensitivity analyses for NMA
In the sensitivity analysis restricted to blinded and placebo-controlled trials, Ayres (2004)Citation50 and EXALT (2011) were excludedCitation52. In the sensitivity analysis of phase 3 and 4 studies only, two phase 2 trials (PATHWAY [2016]Citation15 and Wenzel [2016]Citation54) and five studies that did not report phase (Busse [2013]Citation49, EXALT [2011]Citation52, Hanania [2011]Citation55, INNOVATE [2005]Citation51, and Ayres [2004]Citation50) were excluded. Both sensitivity analyses for the NMA aligned with the primary results (Supplementary Tables S14–17).
STC
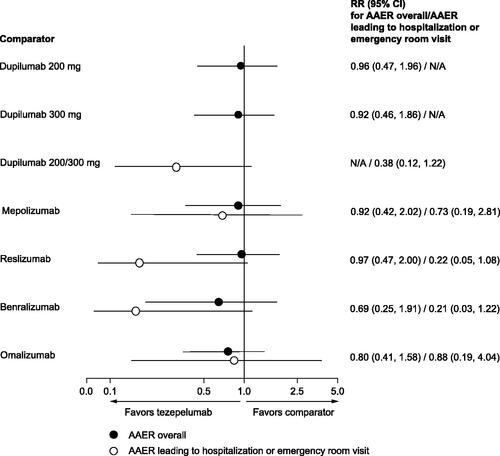
The comparator trials selected for the STC are illustrated in . Results from the STCs showed that there was a consistent numerical benefit for tezepelumab, across all comparisons and both outcomes, which did not reach statistical significance (). Baseline characteristics for study populations used in the STCs are provided in Supplementary Tables S18 and 19, and the potential treatment effect modifiers included in each analysis are provided in Supplementary Table S20.
Figure 3. Anchor and comparator trials used for the STC. Treatment effect of tezepelumab was simulated in the study population of each of the comparator trials. aCALIMA and SIROCCO were both suitable as comparator studies and had the same inclusion criteria; thus, these studies were pooled. bHanania et al.Citation55 was used for AAER overall because this study did not report results for AAER leading to hospitalization/emergency room visit. EXALT was used for AAER leading to hospitalization/emergency room visit. Abbreviations: AAER, annualized asthma exacerbation rate; STC, simulated treatment comparison.
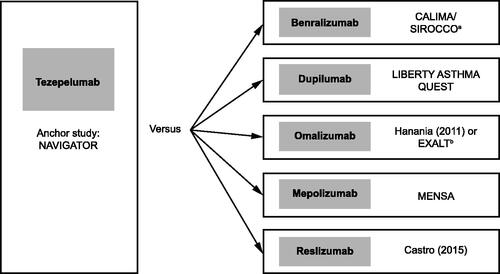
Figure 4. Findings from the STC, rate ratios for AAER, tezepelumab versus comparators. RR reflects the effect of tezepelumab in the comparator study population. Treatment effect modifiers included in the models of each comparator and outcome can be found in Supplementary Table S20. Abbreviations: AAER, annualized asthma exacerbation rate; CI, confidence interval; N/A, not applicable; RR, rate ratio; STC, simulated treatment comparison.
Discussion
Findings from two types of ITC (NMA and STC) showed there were no statistically significant differences between treatments, but tezepelumab was favorably and consistently associated with numerically lower rates of exacerbation than the other approved biologics in a population with overall severe, uncontrolled asthma, and in several subgroups defined by Eos, FeNO, and an allergic asthma subtype. Although there was a lack of statistical significance, the favorable trend for tezepelumab was consistent across both ITC methods used, and in sensitivity analyses. For both outcomes, ranking statistics of the NMA showed that tezepelumab had the highest probability of being the treatment in the network with the highest efficacy.
The findings of these analyses have a strong clinical relevance in the management of severe, uncontrolled asthma for three key reasons. First, only treatment arms with FDA- or EMA-licensed dosages were included, and so represent dose regimens used in the real world. Second, the outcomes selected for the analyses, which were based on exacerbation rate, are key drivers of healthcare resource utilization in severe, uncontrolled asthmaCitation56; therefore, using the biologic with the highest efficacy in this setting, as identified by the results reported here, could result in substantial savings to the healthcare system. Finally, among the subgroups analyzed were patients with severe, uncontrolled asthma without an eosinophilic phenotype. For this group in particular, there is currently an unmet need in terms of approved treatments, which are primarily indicated for eosinophilic asthma, or in the case of the EU indication for dupilumab, asthma with type 2 inflammationCitation3–5,Citation7.
This study has various strengths that support its findings. All aspects of the study are reported in the current article according to good practice reporting guidelines for NMAsCitation57. The study was informed by an SLR that was fully compliant with the 2020 PRISMA guidelinesCitation48, and includes trials that were assessed as high quality, according to the NICE quality appraisal checklist. Well-established methods were used, as outlined by the NICE DSU TSDsCitation31,Citation32. These included two different ITC approaches (NMA and STC), making the current study the first ITC of biologics for severe, uncontrolled asthma based on more than one type of widely accepted method. Thus, our findings are a valuable complement to similar published ITCs in severe asthma that only used one type of methodCitation33–44. In the current ITC, results were consistent between methods, which supports the robustness of the findings. Heterogeneity between trials was thoroughly assessed to determine the potential to conduct NMAs and to identify appropriate methods to address the observed differences in patient populations and study designs. As such, key subgroup analyses were performed for the NMAs, using Eos and FeNO count cut-offs and an allergic asthma subtype, and for the STCs, potential treatment effect modifiers were identified based on guidance from clinical experts. Sensitivity analyses were also performed to explore the impact of differences in study design.
There are several limitations to be aware of when interpreting the results of this study. Some heterogeneity was observed in eligibility criteria and clinically important patient characteristics across included trials. For example, differences were observed in Eos, the number of exacerbations in the past 12 months, and OCS use. To minimize the impact of this heterogeneity, these variables were explored as potential treatment effect modifiers in the STCs. Further, to account for differing time points across studies, we used the number of person-years at risk rather than the number of patients at risk. One key difference between studies was the target populations for each biologic. The reslizumab, mepolizumab, and benralizumab RCTs included patients with eosinophilic asthmaCitation8,Citation9,Citation58–61, while the omalizumab studies included patients with allergic asthmaCitation49–52,Citation55. The tezepelumab and dupilumab studies did not have such restrictionsCitation12,Citation15,Citation54,Citation62. We aimed to minimize the impact of these differences by exploring separate allergic asthma and Eos subgroups in the NMA, and by adjusting for baseline Eos and IgE in the STC. Some bias may remain in the results owing to unmeasured confounders, or owing to differences in exacerbation definition that could not be addressed, such as in the omalizumab trials. However, the consistency of the results across multiple types of analyses suggests that this bias is not substantial. Finally, the study may be limited by the age of the SLR, which was performed in 2020. However, we have captured all studies that were included in a similar, published SLR that was performed in mid-2021Citation44, and based on ad hoc targeted searches, we are not aware of any new RCTs that would have been eligible for inclusion. It should be noted that the analyses reported here do not provide any data on safety outcomes or on other clinically relevant efficacy outcomes, such as patient-reported outcomes (asthma control questionnaire score and health-related quality-of-life), forced expiratory volume in 1 s, and OCS use. Research that compares biologics in terms of these outcomes would be valuable in further informing treatment decisions.
Results in the current ITCs align with previous ITC results in terms of ranking of biologics, which supports the robustness of the current ITC. The current ITC and the other tezepelumab ITC by Ando et al.Citation44 are similar in their eligibility criteria and primary outcome used but there are also several key differences. The first key difference is that the current ITC used two methods, NMA and STC, while the Ando et al. study only used NMA. The added value of two types of ITC has been discussed above and, as already stated, we used well-established robust methods outlined by the NICE DSU TSDsCitation31,Citation32. There is some uncertainty regarding the methodology used in the Ando et al. ITC and the robustness of their approach because model fit statistics, such as standard deviation or DIC, and prior distributions are not reported. The second key difference is that the current ITC includes five treatments plus placebo, while Ando et al. included three treatments plus placebo, leading to a difference in the size of networks evaluated (16 vs 8 studies, respectively). The current ITC provides comparative evidence for omalizumab and reslizumab, which were omitted in the analysis by Ando et al. Despite being the earliest approved biologics, omalizumab and reslizumab remain relevant comparators in severe asthma, and were included in recommendations published by the European Academy of Allergy & Clinical Immunology in 2020Citation63. In terms of findings, the current ITC and Ando et al.’s ITC both showed that tezepelumab was ranked highest for efficacyCitation44. Results were also aligned in terms of findings in specific subgroups, with both analyses showing that results favored tezepelumab over dupilumab and benralizumab in patients with Eos less than 300 cells/µL, and favored tezepelumab over dupilumab in patients with FeNO greater or equal to 25 or 50 ppbCitation44. These consistencies support the robustness of the overall findings, in clinically relevant subgroups for which there is an unmet treatment need. However, the CrIs were wide for the subgroup analyses, owing to small sample sizes and limited data availability across studies. Thus, more evidence would be valuable in confirming these associations. Wide intervals also led to some cases where the statistical significance of the results of the current ITC did not always align with the individual RCT data that informed them. For example, the ITC subgroup results showed that the difference in AAER was not significantly different between tezepelumab and placebo in patients with Eos less than 300 cells/µL, even though the NAVIGATOR trial results did show a significant difference. The reason for this is that the use of random-effects models in the ITC, which was found to be the best model fit for this subgroup (vs a fixed-effects model), imparted statistical uncertainty to the estimates (i.e. wider 95% CrIs). This is a common occurrence in random-effects meta-analyses, making statistical significance difficult to achieve, even if it was demonstrated in the source resultsCitation64.
Conclusions
In conclusion, our findings support the use of tezepelumab in patients with severe, uncontrolled asthma, irrespective of phenotype or baseline biomarker level, which is consistent with its broad approval indication in the US. The efficacy findings across various subgroups may be observed because TSLP, the target of tezepelumab, is positioned higher up the inflammatory cascade than other targets, which may lead to broader effects on airway inflammation and structural airway changes.
Transparency
Declaration of funding
This study was funded by AstraZeneca. AstraZeneca is responsible for initiation of the study and decision to publish.
Declaration of financial/other interests
AMG has attended advisory boards for GlaxoSmithKline, Novartis, AstraZeneca, Teva, and Sanofi; has received speaker fees from Novartis, AstraZeneca, Sanofi, and Teva; has participated in research for which his host institution has been remunerated with AstraZeneca; has attended international conferences sponsored by Teva; and has consultancy agreements with AstraZeneca and Sanofi. JS and SS are employees of EVERSANA, which received funding from AstraZeneca for performing the systematic literature review and statistical analyses that informed this study. WE, JR, NM, and AQ are employees of AstraZeneca. PR and JPLA are employees of Amgen Inc.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (327.6 KB)Acknowledgements
Joanna Bielecki (EVERSANA) designed and executed the systematic literature search. Hannah Guiang, Teresa Kangappaden, Catarina Aniceto da Silva, and Stacey Priest (EVERSANA) contributed to study selection, data extraction, and ITC feasibility assessment. Lisa Law (ORCID iD: https://orcid.org/0000-0002-9837-6609) of Oxford PharmaGenesis, UK, provided medical writing support, which was funded by AstraZeneca, in accordance with Good Publication Practice 3 (GPP3).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43, 679–373.
- Chen S, Golam S, Myers J, et al. Systematic literature review of the clinical, humanistic, and economic burden associated with asthma uncontrolled by GINA steps 4 or 5 treatment. Curr Med Res Opin. 2018;34(12):2075–2088.
- European Medicines Agency. Fasenra: EPAR. Product information. 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/fasenra
- European Medicines Agency. Dupixent: EPAR. Product information. 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/dupixent
- European Medicines Agency. Nucala: EPAR. Product information. 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/nucala
- European Medicines Agency. Xolair: EPAR. Product information. 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/xolair
- European Medicines Agency. Cinqaero: EPAR. Product information. 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/cinqaero
- Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3(5):355–366.
- Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2115–2127.
- Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108(2):184–190.
- Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659.
- Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496.
- US Food & Drug Administration. TEZSPIRE™ (tezepelumab-ekko) injection, for subcutaneous use Initial U.S. Approval: 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761224s000lbl.pdf
- US Food & Drug Administration. FDA approves maintenance treatment for severe asthma. 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-maintenance-treatment-severe-asthma
- Corren J, Parnes JR, Wang L, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377(10):936–946.
- Diver S, Khalfaoui L, Emson C, et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (Cascade): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2021;9(11):1299–1312.
- Gauvreau GM, Sehmi R, Ambrose CS, et al. Thymic stromal lymphopoietin: its role and potential as a therapeutic target in asthma. Expert Opin Ther Targets. 2020;24(8):777–792.
- Allakhverdi Z, Comeau MR, Jessup HK, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204(2):253–258.
- Liu S, Verma M, Michalec L, et al. Steroid resistance of airway type 2 innate lymphoid cells from patients with severe asthma: the role of thymic stromal lymphopoietin. J Allergy Clin Immunol. 2018;141(1):257–268.e6.
- Soumelis V, Reche PA, Kanzler H, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3(7):673–680.
- ClinicalTrials.gov. Study to evaluate tezepelumab in adults and adolescents with severe uncontrolled asthma (NAVIGATOR). 2021. https://clinicaltrials.gov/ct2/show/NCT03347279
- Menzies-Gow A, Corren J, Bourdin A, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. 2021;384(19):1800–1809.
- Hoaglin DC, Hawkins N, Jansen JP, et al. Conducting indirect-treatment-comparison and network-Meta-analysis studies: report of the ISPOR task force on indirect treatment comparisons good research practices: part 2. Value Health. 2011;14(4):429–437.
- Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network Meta-analysis for health-care decision making: report of the ISPOR task force on indirect treatment comparisons good research practices: part 1. Value Health. 2011;14(4):417–428.
- Rouse B, Chaimani A, Li T. Network Meta-analysis: an introduction for clinicians. Intern Emerg Med. 2017;12(1):103–111.
- Higgins JP, Whitehead A. Borrowing strength from external trials in a Meta-analysis. Statist Med. 1996;15(24):2733–2749.
- Ishak KJ, Proskorovsky I, Benedict A. Simulation and matching-based approaches for indirect comparison of treatments. Pharmacoeconomics. 2015;33(6):537–549.
- CADTH. Guidelines for the economic evaluation of health technologies: Canada. 2022. https://www.cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf
- Eunethta. Methodological guideline for REA of pharmaceuticals: direct and indirect comparison. 2022. https://www.eunethta.eu/methodological-guideline-for-rea-of-pharmaceuticals-direct-and-indirect-comparison/
- Laws A, Tao R, Wang S, et al. A comparison of national guidelines for network Meta-analysis. Value Health. 2019;22(10):1178–1186.
- NICE Decision Support Unit (DSU). NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. 2021. https://www.ncbi.nlm.nih.gov/books/NBK310366/pdf/Bookshelf_NBK310366.pdf
- NICE Decision Support Unit (DSU). NICE DSU technical support document 18: methods for population-adjusted indirect comparisons in submissions to NICE. 2021. http://nicedsu.org.uk/wp-content/uploads/2017/05/Population-adjustment-TSD-FINAL.pdf
- Busse W, Chupp G, Nagase H, et al. Anti-IL-5 treatments in patients with severe asthma by blood eosinophil thresholds: indirect treatment comparison. J Allergy Clin Immunol. 2019;143(1):190–200 e120.
- Cabon Y, Molinari N, Marin G, et al. Comparison of anti-interleukin-5 therapies in patients with severe asthma: global and indirect Meta-analyses of randomized placebo-controlled trials. Clin Exp Allergy. 2017;47(1):129–138.
- Casale TB, Pacou M, Mesana L, et al. Reslizumab compared with benralizumab in patients with eosinophilic asthma: a systematic literature review and network Meta-analysis. J Allergy Clin Immunol Pract. 2019;7(1):122–130 e121.
- Bateman E, Khan A, Xu Y, et al. Pairwise indirect treatment comparison of dupilumab versus other biologics in patients with uncontrolled persistent asthma. Respir. Med. 2020; Pre-proof, doi:
- Cockle SM, Stynes G, Gunsoy NB, et al. Comparative effectiveness of mepolizumab and omalizumab in severe asthma: an indirect treatment comparison. Respir Med. 2017;123:140–148.
- Nachef Z, Krishnan A, Mashtare T, et al. Omalizumab versus mepolizumab as add-on therapy in asthma patients not well controlled on at least an inhaled corticosteroid: a network meta-analysis. J Asthma. 2018;55(1):89–100.
- NICE. Reslizumab for treating severe eosinophilic asthma. 2021. https://www.nice.org.uk/guidance/ta479
- NICE. Mepolizumab for treating severe eosinophilic asthma [ID3705] Committee Papers. 2020.
- Bourdin A, Husereau D, Molinari N, et al. Matching-adjusted indirect comparison of benralizumab versus interleukin-5 inhibitors for the treatment of severe asthma: a systematic review. Eur Respir J. 2018;52(5):1801393.
- Bourdin A, Husereau D, Molinari N, et al. Matching-adjusted comparison of oral corticosteroid reduction in asthma: systematic review of biologics. Clin Exp Allergy. 2020;50(4):442–452.
- International clinical trials registry platform. JPRN-UMIN000044672. 2022. https://trialsearch.who.int/?TrialID=JPRN-UMIN000044672
- Ando K, Fukuda Y, Tanaka A, et al. Comparative efficacy and safety of tezepelumab and other biologics in patients with inadequately controlled asthma according to thresholds of type 2 inflammatory biomarkers: a systematic review and network meta-analysis. Cells. 2022;11(5):819.
- Global Initiative for Asthma. 2021 GINA Report, Global Strategy for Asthma Management and Prevention. 2022. https://ginasthma.org/gina-reports/
- National Institute for Health and Care Excellence (NICE). Appendix F Quality appraisal checklist - quantitative intervention studies. 2021. https://www.nice.org.uk/process/pmg4/chapter/appendix-f-quality-appraisal-checklist-quantitative-intervention-studies
- Cochrane. Cochrane handbook for systematic reviews of interventions. 2022. https://training.cochrane.org/handbook
- PRISMA. PRISMA statement. 2022. http://www.prisma-statement.org/PRISMAStatement/PRISMAStatement
- Busse W, Spector S, Rosen K, et al. High eosinophil count: a potential biomarker for assessing successful omalizumab treatment effects. J Allergy Clin Immunol. 2013;132(2):485–486.e11.
- Ayres JG, Higgins B, Chilvers ER, et al. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with poorly controlled (moderate-to-severe) allergic asthma. Allergy. 2004;59(7):701–708.
- Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309–316.
- Bousquet J, Siergiejko Z, Świebocka E, et al. Persistency of response to omalizumab therapy in severe allergic (IgE-mediated) asthma. Allergy. 2011;66(5):671–678.
- Daly CH, Neupane B, Beyene J, et al. Empirical evaluation of SUCRA-based treatment ranks in network Meta-analysis: quantifying robustness using cohen's kappa. BMJ Open. 2019;9(9):e024625.
- Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388(10039):31–44.
- Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011;154(9):573–582.
- Chastek B, Korrer S, Nagar SP, et al. Economic burden of illness among patients with severe asthma in a managed care setting. JMCP. 2016;22(7):848–861.
- PRISMA. PRISMA for Network Meta-Analyses (PRISMA-NMA). 2021. http://www.prisma-statement.org/Extensions/NetworkMetaAnalysis
- Harrison TW, Chanez P, Menzella F, et al. Onset of effect and impact on health-related quality of life, exacerbation rate, lung function, and nasal polyposis symptoms for patients with severe eosinophilic asthma treated with benralizumab (ANDHI): a randomised, controlled, phase 3b trial. The Lancet Respiratory Medicine. 2021;9(3):260–274.
- FitzGerald JM, Bleecker ER, Nair P, CALIMA study investigators, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–2141.
- Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207.
- Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5(5):390–400.
- AstraZeneca/Amgen. Data on file. Clinical study report. A multicentre, randomised, double-blind, placebo controlled, parallel group, phase 3 study to evaluate the efficacy and safety of tezepelumab in adults and adolescents with severe uncontrolled asthma (NAVIGATOR). 2020.
- Agache I, Beltran J, Akdis C, et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI guidelines - recommendations on the use of biologicals in severe asthma. Allergy. 2020;75(5):1023–1042.
- Borenstein M, Hedges LV, Higgins JPT, et al. An introduction to meta-analysis; John Wiley & Sons, Ltd: 2009.
- AstraZeneca/Amgen. Data on file. Clinical study report. A multicentre, randomized, double-blind, placebo controlled, phase 3 study to evaluate the efficacy and safety of tezepelumab in reducing oral corticosteroid use in adults with oral corticosteroid dependent asthma (SOURCE). 2021.
