Abstract
Objectives
Cost-effectiveness analysis (CEA) is useful to assess the value of health care interventions based on clinical effectiveness and costs. However, standard CEA methods make important assumptions that may significantly increase the incremental cost-effectiveness ratio (ICER) for lifelong treatments for rare, chronic diseases. We used the cost-effectiveness of elexacaftor/tezacaftor/ivacaftor and ivacaftor (ELX/TEZ/IVA) for the treatment of cystic fibrosis as a case study to explore how alternative assumptions for (1) discounting, (2) utility measures, (3) disease management costs, and (4) static drug pricing impact cost-effectiveness outcomes.
Materials and methods
Cost-effectiveness of ELX/TEZ/IVA was evaluated using base-case inputs and assumptions reflecting standard CEA methods and was then compared with cost-effectiveness estimates obtained with alternate assumptions: (1) applying a lower discount rate to health benefits (1.5%) than costs (3%); (2) including a treatment-specific utility increment; (3) excluding disease management costs incurred during the period of extended survival attributable to ELX/TEZ/IVA treatment; and (4) decreasing the price of ELX/TEZ/IVA following loss of exclusivity.
Results
Modifying assumptions for these four factors together reduced the ICER by 75% from the base case, with the largest reduction (45%) occurring when the price trajectory was modified to allow for generic entry. Differential discounting, use of a treatment-specific utility increment, and exclusion of additional disease management costs each individually reduced the ICER by 36%, 14%, and 10%, respectively, from the base case.
Conclusions
This study illustrates the impact that modifications to standard CEA methods may have on measures of cost-effectiveness for rare, chronic diseases.
Introduction
Dramatic advances in drug discovery in the last decade have led to increasingly innovative treatments for once untreatable conditions, including many rare, chronic diseasesCitation1. The emergence of these treatments, coupled with limited health care budgets, has increased the importance of formal assessment frameworks, such as cost-effectiveness analysis (CEA), in reimbursement decisionsCitation2. CEA can be a useful tool for guiding health policy and disease management decisions to rationally allocate limited health care dollars across treatments differing in cost and clinical effectivenessCitation3.
CEA is a well-established part of health technology assessment (HTA) processes in countries such as the United Kingdom, Australia, and Canada to aid decision-making by national health care systems and is increasing in use worldwideCitation2–4. While minor variations exist across HTA agencies, CEA is typically conducted using a standard set of methods and assumptions. Standard CEA methods, as used by many HTAs, were developed to evaluate traditional therapies for common conditions and many have not been tailored to address unique aspects of rare disease treatments, including small patient populations, or the innovation associated with novel therapies (e.g. gene therapies)Citation5–8. Treatments for rare diseases often do not meet conventional criteria for cost-effectiveness using standard CEA methodsCitation1,Citation9–11.
Several HTA agencies have recognized the limitations of applying a single HTA process and CEA methodology to all therapeutic advances and have created alternative pathways for therapies with specific attributes relating to disease epidemiology (e.g. rare/ultra-rare disease) and level of innovationCitation12–14. For example, in England, the National Institute for Health and Care Excellence (NICE) developed the Highly Specialised Technology appraisal process that allows a higher cost-effectiveness threshold for rare-disease therapies with unique attributesCitation12. The Australian Pharmaceutical Benefits Advisory Committee uses the Life Saving Drugs Program to evaluate the cost-effectiveness of therapies for rare and life-threatening diseases that demonstrate substantial life extensionCitation13. The current ultra-rare disease framework published by the Institute for Clinical and Economic Review in the United States applies the standard cost-effectiveness model approach with only minor modificationsCitation14. However, despite the existence of alternative pathways, these criteria are not uniformly applied to rare disease treatments, with the vast majority of therapies evaluated under traditional routesCitation13,Citation15.
To better understand how modifications to methods and assumptions in standard CEA evaluations may alter estimates of the cost-effectiveness of lifelong treatments for rare, chronic diseases, we analyzed a recently approved treatment for cystic fibrosis (CF) as a case study representing a rare, chronic disease with substantial clinical burden.
CEA overview
CEA is a standard economic tool used to compare the costs and clinical effectiveness of a novel medical intervention to current alternative treatment options (or the ‘standard of care’). Costs considered in a CEA include the estimated cost of treatment and any expenses from adopting the intervention as well as costs incurred to manage the underlying condition. The most common unit of effectiveness in CEA is the quality-adjusted life-year (QALY), which combines the length of life and health-related quality of life (QoL) into a single metric. QALYs provide a common measure that can be used to compare therapeutic benefits across various diseases, which often have different clinical outcome measuresCitation4. The primary result of the CEA is the incremental cost-effectiveness ratio (ICER), which is derived by dividing the difference in total costs by the difference in QALYs across the interventions being compared. During HTA evaluations, the resulting ICER is often compared to a pre-determined threshold to determine if the new therapy is an effective use of resources (‘cost-effective’). The thresholds are set by HTAs and vary by jurisdiction – the Institute for Clinical and Economic Review in the United States uses an ICER threshold of up to $150,000 per QALYCitation16, while NICE in the UK uses a threshold of £20,000–£30,000 per QALYCitation17. No standard methodology for setting ICER thresholds exists and there is often little supportive evidence on their derivation, which leads to large discrepancies in the thresholds used across different marketsCitation18. Further, many ICER thresholds have not been updated since their origination and therefore do not account for government budgetary changes, inflation, innovation and advancements in technology, or increased research and development costsCitation19.
Limitations of standard CEA methods
Rare, chronic health conditions generally require intensive treatment beginning at a young age and continuing over a patient’s lifetime, with the goal of slowing disease progression to prolong and improve the quality of a patient’s life. Several modeling assumptions included in standard CEA frameworks, including choice of discount rates, quantification of patient quality of life, ongoing attribution of costs for disease management when life is extended due to the new therapy, and assumptions about how drug pricings evolve over time, can have unintended influences on modeled outcomes.
Limitations of standard CEA methods: discounting cost and health benefits
Standard CEAs discount costs and health benefits that occur after the first year of the model to reflect their present value. While the discount rate used in CEA can vary, uniform rates are typically applied to costs and health benefits. The application of discounting within a CEA means costs and health benefits occurring earlier in the model are given more weight than those occurring laterCitation20. Consequently, when discounting is applied to interventions where benefits are accrued over several decades, health benefits are heavily discounted, which may substantially impact the CEA. Treatments for rare, chronic diseases are more likely to generate health benefits over longer periods of time due to the lifelong and often progressive nature of the disease.
Moreover, applying equal discount rates for costs and health benefits disproportionately impacts the latter because costs are incurred from the first day of treatment initiation when the impact of discounting is less; however, when disease progression is slowed, the majority of health benefits accrue later and are therefore more heavily discounted. A differential discounting approach in which health benefits are discounted at a lower rate than costs has been suggested for evaluating lifelong, life-extending therapiesCitation20,Citation21. This aligns with recommendations from multiple HTA and government agencies, including the published guidelines by the Dutch National Health Care InstituteCitation22 and the United Kingdom’s Treasury Green Book, which is the basis of the discount rate used by NICECitation23.
Limitations of standard CEA methods: inability of the QALY to capture all important aspects of treatment
The QALY may capture only a subset of benefits and hence not adequately reflect a treatment’s value. This limitation is driven by difficulties in comprehensively measuring QoL improvements and omission of, or inconsistent inclusion of, many important concepts such as the ability to work or attend school, hope for the future, equity considerations, reduced uncertainty, and opportunities for patients to benefit from future advances in medicineCitation4.
For measuring QoL improvements, HTA agencies often prefer widely used generic (i.e. not disease-specific) utility instruments (e.g. EQ-5D). However, generic measures may also lack sensitivity in the assessment of QoL for chronic diseases present from birth because these patients may adapt to their condition and therefore rate themselves highly on generic QoL scalesCitation17,Citation24. As a result, QoL gains associated with substantial clinical improvement and patient-reported benefits derived from new therapies for rare, chronic diseases may not be adequately captured.
Limitations of standard CEA methods: routine health care costs
In standard CEA, all costs associated with disease management (e.g. ongoing physician consultations), including those accrued during additional years of survival resulting from the treatment, contribute to the estimate of the total cost. In CEA models, a patient stops incurring costs once they die; if the patient continues to live, they continue accruing both treatment costs as well as disease management costs that are unrelated to the treatmentCitation20. Consequently, total costs in a CEA for therapies that extend survival for patients with chronic conditions can be quite high simply due to survival extension alone.
Limitations of standard CEA methods: drug prices over time
Drug prices in standard CEAs are assumed to be unchanged for the full duration of the model time horizonCitation20 even though this is inconsistent with real-world pricing patterns, which demonstrate significant price reductions when branded medications lose exclusivity and generic options emergeCitation25,Citation26. The assumption that pricing is static is likely to result in substantially overstated total costs of treatment in a CEA. This is particularly problematic for drugs taken regularly and for which model horizons span decadesCitation20 compared with products taken over shorter durations (e.g. acute treatments or oncology products for which overall survival is generally short).
Methods
To illustrate how cost-effectiveness is affected by modifications to these standard CEA methods, we modeled the cost-effectiveness of a novel, effective treatment for CF, a rare, chronic, and life-shortening disease.
Model overview
CF is a genetic disease that affects more than 30,000 people in the United States and more than 80,000 people worldwideCitation27. People with CF (pwCF) experience frequent respiratory infections, pulmonary exacerbations, gastrointestinal symptoms, and growth deficienciesCitation27. CF is characterized by progressive lung function decline and frequent hospitalizations, which result in early mortalityCitation27,Citation28. No cure exists for CF and the median age at death for pwCF in the United States in 2020 was 34.1 yearsCitation29.
Elexacaftor/tezacaftor/ivacaftor and ivacaftor (ELX/TEZ/IVA) was approved in the United States and Europe in 2019 and 2020, respectively, to treat pwCF aged ≥12 years with CF transmembrane conductance regulator (CFTR) gene mutationsCitation30,Citation31; the CFTR mutations which ELX/TEZ/IVA is approved to treat are present in up to 90% of pwCFCitation27. Multiple phase III trials of ELX/TEZ/IVA have demonstrated significant improvements in lung function, nutritional outcomes, and QoL measures, as well as reductions in pulmonary exacerbations, in pwCF treated with this regimenCitation32–35.
A person-level simulation model was used to estimate the lifetime clinical benefits and cost-effectiveness of ELX/TEZ/IVA in addition to best supportive care (ELX/TEZ/IVA + best supportive care [BSC]) compared with BSC alone for pwCF aged ≥12 years with F508del/minimal function (F/MF) genotypes. BSC for CF includes airway clearance therapy, bronchodilators, mucolytics, antibiotics, and nutritional managementCitation36,Citation37. The model was derived from a previously published model that was used to predict long-term clinical outcomes in pwCF treated with lumacaftor/ivacaftorCitation38 and was further validated using real-world data from a long-term safety study of pwCF treated with ivacaftor over a 5-year periodCitation39. Details regarding the model structure are provided in the Appendix. Model inputs related to disease progression, ELX/TEZ/IVA clinical efficacy, cost, and utility are presented in .
Table 1. Model inputs for disease progression, ELX/TEZ/IVA clinical efficacy, cost, and utility.
Scenarios to illustrate effect of standard CEA methods and alternative assumptions
We began by evaluating cost-effectiveness using base-case inputs and assumptions reflecting standard CEA methods. We compared this with cost-effectiveness estimates obtained when using alternate assumptions of uniform vs. differential discounting of health benefits and costs, limited vs. comprehensive QoL (utility) measures, inclusion vs. exclusion of ongoing disease management costs when life is extended, and static vs. dynamic drug pricing over time (described in ). Specifically, (1) a lower discount rate was applied to health benefits (1.5%) than costs (3%); (2) a treatment-specific utility increment was applied to account for patient-reported benefits observed in clinical studies beyond respiratory benefits included in the standard CEA modeling of CF; (3) disease management costs incurred during the period of extended survival attributable to ELX/TEZ/IVA treatment were excluded from the total cost estimate; and (4) the price of ELX/TEZ/IVA was assumed to decrease following loss of exclusivity. Model assumptions were evaluated individually and collectively to assess the impact of each assumption and any interdependence between them.
Table 2. Model assumptions varied in scenario analyses.
Results
Base-case analysis: standard HTA methods
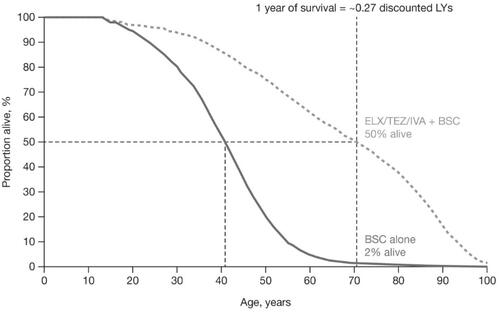
ELX/TEZ/IVA + BSC was projected to increase median survival in pwCF with F/MF genotypes by 29.7 years compared with treatment with BSC alone (70.4 vs. 40.8 years). The discount rate had a large effect on projected health benefits associated with ELX/TEZ/IVA, substantially reducing the overall incremental life-years (LY) and QALYs. Over the lifetime horizon, treatment with ELX/TEZ/IVA + BSC was associated with 25.0 additional undiscounted LYs, which decreased to 9.1 discounted LYs and 9.2 discounted QALYs when the traditional 3.0% discount rate was applied. At the time that half of the patients receiving ELX/TEZ/IVA + BSC were alive in the model (∼2% of patients receiving BSC were alive), each additional year of life contributed only about one-quarter (0.27) of a discounted LY ().
Figure 1. Projected impact of ELX/TEZ/IVA on survival. A comparison of the treatment-specific projected survival curves is presented. The incremental residual LYs (i.e. years since entering the model) is represented by the area between the two survival curves, whereas the incremental median predicted survival is represented by the distance between the two survival curves at the point where 50% of each cohort has died. Abbreviations. BSC, best supportive care; ELX/TEZ/IVA, elexacaftor/tezacaftor/ivacaftor and ivacaftor; LY, life-year.
The lifetime incremental cost associated with ELX/TEZ/IVA treatment was $4,416,000, comprising $5,642,000 in ELX/TEZ/IVA treatment costs, assuming static pricing for the lifetime treatment (on average, 42.5 years of treatment), and $1,226,000 in cost offsets from reduced disease severity. The base-case incremental cost-effectiveness ratio (ICER) was $482,000 per QALY gained over the lifetime horizon ().
Table 3. Pharmacoeconomic model results of base-case and scenario analysesa.
Scenario analyses: impact of modifying assumptions from standard HTA methods
The base-case ICER was highly sensitive to modeling assumptions (). Applying 1.5% discounting to health benefits increased the incremental QALYs provided by ELX/TEZ/IVA + BSC over BSC alone from 9.2 to 14.4, thereby reducing the ICER from $482,000 to $306,000. Allowing drug prices to be reduced after the loss of exclusivity decreased the incremental costs of ELX/TEZ/IVA from $4,416,000 to $2,435,000, thereby reducing the ICER by approximately 45% to $266,000. Excluding disease management costs incurred during the period of extended survival and including the treatment-specific utility increment reduced the base-case ICER by 10% and 14%, respectively. When applying all four assumptions simultaneously, ELX/TEZ/IVA + BSC treatment resulted in 16.4 additional discounted QALYs at an incremental lifetime cost of $2,007,000 compared with treatment with BSC alone, resulting in an ICER of $122,000 per QALY gained, a 75% reduction from the base case.
Discussion
Key findings of case study
This case study illustrated that the cost-effectiveness of ELX/TEZ/IVA is highly sensitive to the modeling assumptions used: uniform vs. differential discounting of health benefits and costs, limited vs. comprehensive QoL (utility) measures, inclusion vs. exclusion of ongoing disease management costs when life is extended, and static vs. dynamic drug pricing over time. Notably, three of these are basic modeling assumptions that are unrelated to the clinical benefits of the treatment; the application of utility scores is the only factor that is linked to the specific clinical benefits of treatments considered in this case study. When modifying these four standard assumptions by using plausible alternatives, ICERs for ELX/TEZ/IVA were reduced by 75% and fell under the threshold of ‘cost-effective’ as defined by the Institute for Clinical and Economic ReviewCitation10.
Our findings suggest that standard CEA approaches to discounting exert significant influence on ICERs for therapies that confer a substantial clinical benefit and extend life over long periods of time. In a similar example, Westra et al. investigated the impact of discounting approaches on the cost-effectiveness of vaccinating 12-year-old girls with the human papillomavirus 16/18 vaccineCitation40,Citation41. Girls are vaccinated at the age of 12 to prevent substantial morbidity and mortality associated with human papillomavirus and cervical cancer that could occur when they are much olderCitation40. The analysis by Westra et al. demonstrated that clinical benefits (i.e. QALYs) were reduced by approximately 80% when a flat 3% discount rate was applied compared with no discount rate, and ICERs increased proportionally (€7600 vs. €37,000)Citation40. Alternatively, discounting has little or no impact on the CEAs of therapies for which benefits are proximal to the start of the model, such as oncology interventions or the treatment of acute conditions like infections. A recent example evaluating the cost-effectiveness of nivolumab, a novel first-line treatment for metastatic renal cell carcinoma, reported that the projected life expectancy for patients treated with nivolumab plus ipilimumab was only 3.99 life years and that reducing the discount rate from 3% to 0% changed the ICERs by less than 10%Citation42.
There are many examples where utility scores do not comprehensively capture the impacts of disease or the benefits of treatment, particularly for rare, chronic diseases. In a CEA of voretigene neparvovec-rzyl treatment for RPE65-mediated retinal disease, a rare, genetic condition that leads to complete blindness in young adults and children, patients were projected to avoid blindness for an additional 10.6 years with treatmentCitation43. However, patients accrued only 1.3 additional QALYs with the treatment vs. the comparator, because the utilities used to represent QoL for patients were based on data from other eye conditionsCitation43. This modest QoL improvement does not reflect how patients and their families described the adverse effects of blindnessCitation43. The Institute for Clinical and Economic Review acknowledged that certain benefits, including self-confidence, mobility, and independence gained from treatment, may not be adequately captured in the QALY in their assessmentCitation43. This is similar to the QoL impacts experienced by patients treated with ELX/TEZ/IVA, who reported increases in energy levels and mood, reduced psychosocial burden (including depression and anxiety), ability to plan for the future, and hope for themselves and their community living with CFCitation44. Disease-specific patient-reported outcome measures in phase III clinical trials of ELX/TEZ/IVA similarly demonstrated a broad range of QoL benefitsCitation32,Citation33,Citation45,Citation46. However, utility values derived from generic instruments preferred in the standard CEA approach only capture respiratory improvements and fail to adequately quantify relevant aspects of QoL for pwCFCitation24,Citation47,Citation48. These cases illustrate the challenge of relying on standard CEA methods to comprehensively reflect all QoL benefits of novel therapies for chronic diseases.
Inclusion of ongoing disease management costs when life is extended by novel treatments reduces the cost-effectiveness of drugs in a way that is counter to the value most people would attribute to increased survival. In the most extreme cases, a life-extending treatment may not be considered cost-effective even when priced at $0. This was the case in the NICE evaluation of cinacalcet, a treatment for secondary hyperparathyroidism in patients with end-stage renal disease on maintenance dialysis. Because the treatment is projected to modestly extend life, and patients still require dialysis during this extended period, the additional costs associated with maintenance dialysis outweigh the additional QALYs gained. Because of this, the therapy would not be considered cost-effective even if the price were $0Citation49. This is an extreme but important example of how standard inclusion of disease management costs can produce an unrealistic valuation of life-extending products for chronic diseases. In our case study, omitting additional disease management costs during the period of additional survival attributable to ELX/TEZ/IVA reduced the ICER by 10%. Our case study for ELX/TEZ/IVA did not assume direct reductions in costs associated with standard of care treatment (e.g. reductions in cost per PEx episode or symptomatic therapies). This was likely a conservative assumption based on emerging evidenceCitation50,Citation51, and the inclusion of additional direct cost-offsets would reduce the impact of costs incurred during the additional years of survival.
Finally, changes in drug prices over time, such as those associated with the introduction of generic competition, are not included in current HTA methods. It is well accepted that drug prices decrease substantially after patent expiration, typically by 80%–90% in markets with large generic entriesCitation25. Therapies that treat people for decades will almost certainly be subject to these well-documented price decreases. In our case study, assuming a price reduction at the time of expected generic entry had the greatest effect on the base-case ICER, with a potential decrease of 45%. A Canadian CEA evaluating islet cell transplant in patients with type 1 diabetes demonstrated a similar impact; assuming generic pricing for the lifelong immunosuppression therapy required following transplant reduced the ICER of islet cell transplant by 25%Citation52. While the challenges of predicting pricing patterns over a long time horizon are important to acknowledge, standard HTA assumptions of static pricing can overestimate treatment costs over long time horizons, particularly for rare, chronic diseases.
Limitations
Our case study analyzed the cost-effectiveness of a treatment for a single disease, CF, and therefore might not reflect how modifications to standard HTA methods affect cost-effectiveness evaluations in other diseases. Moreover, our case study does not represent an exhaustive list of all factors that could influence the cost-effectiveness of treatments.
With respect to the economic analysis of ELX/TEZ/IVA presented, due to the lack of extensive long-term clinical data, results from clinical trials were extrapolated into long-term outcomes using a well-validated predictive modelCitation38,Citation39.
Implications for health care decision makers
The case study and additional examples discussed highlight how assessments of the value of a treatment based on standard HTA methods may be sensitive to alternative assumptions, particularly when evaluating new treatments for rare, chronic diseases. The consequences of leaving these alternatives unexplored include potential delays in patient access to important therapies for conditions with significant unmet needs. We highlighted relatively straightforward options in our scenario analyses that could address these limitations: differential discounting of health benefits and costs; comprehensive QoL (utility) measures that capture the full benefits to patients, their families, and caregivers; exclusion of ongoing disease management costs when life is extended; and dynamic drug pricing over time.
Because the alternative frameworks discussed herein would not have substantial impacts on ICERs estimated for common conditions, most of which do not require treatment from an early age and/or do not severely limit life expectancy, altering HTA frameworks to account for the important differences of rare, chronic diseases would not undermine existing systems. While some flexibility is built into most HTA systems, it is inconsistently applied and typically limited in scope. For example, most HTA systems formally or informally allow for higher ICER thresholds for rare disease treatments while retaining standard cost-effectiveness methods, but as demonstrated by our case study, the ICER threshold would need to be increased well beyond most current limits for rare diseases to address the limitations discussed.
Conclusions
This study highlights how standard CEA methods can impact the perceived value of novel interventions, even when the clinical benefits are profound, and how addressing these inherent limitations changes CEA outcomes. HTA frameworks should be sufficiently flexible to account for the limitations apparent when standard methods are applied to treatments for rare, chronic diseases, to more accurately and equitably reflect the full value such treatments provide, to continue to encourage the development of innovative treatments for these serious diseases, and to ensure appropriate access for patients.
Transparency
Declaration of funding
The study was funded by Vertex Pharmaceuticals Incorporated.
Declaration of financial/other relationships
J.L.R., A.L., and J.B. are employees of Vertex Pharmaceuticals Incorporated and may own stock or stock options in that company. P.G. has nothing to disclose. A.B.J. reports receiving consulting fees unrelated to this work from Pfizer, Hill-Rom Services, Bristol Myers Squibb, Novartis, Amgen, Eli Lilly, Vertex Pharmaceuticals Incorporated, AstraZeneca, Celgene, Tesaro, Sanofi US, Biogen, Precision Health Economics, and Analysis Group.
The peer reviewers on this manuscript have received an honorarium from JME for their review work.
One of the reviewers has disclosed that they have been on an advisory board for Vertex in their work on another genetic lung disease.
Author contributions
All authors participated in the concept and design of the study. Data were collected by J.L.R., A.L., and J.B. J.L.R., A.L., J.B., P.G., and A.B.J. participated in the analysis and interpretation of study data. All authors contributed to the critical revision of the manuscript for important intellectual content and gave final approval of the manuscript for publication.
Supplemental Material
Download MS Word (24.8 KB)Acknowledgements
Editorial coordination and support were provided by Morgan Deng, who is an employee of Vertex Pharmaceuticals Incorporated and may own stock or stock options in that company. Medical writing and editorial support were provided by Samantha Keller, Christopher Edwards, and Karen Kaluza Smith, of ArticulateScience, LLC, and was funded by Vertex Pharmaceuticals Incorporated. Editorial support was provided by Alice Newman, Lauren Smith, and Adam Paton, of Complete HealthVizion, funded by Vertex Pharmaceuticals Incorporated.
References
- Schuller Y, Hollak CE, Biegstraaten M. The quality of economic evaluations of ultra-orphan drugs in Europe – a systematic review. Orphanet J Rare Dis. 2015;10:783.
- Ciani O, Jommi C. The role of health technology assessment bodies in shaping drug development. Drug Des Devel Ther. 2014;8:2273–2281.
- Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103.
- Lakdawalla DN, Doshi JA, Garrison LPJ, et al. Defining elements of value in health care-a health economics approach: an ISPOR special task force report [3]. Value Health. 2018;21(2):131–139.
- National Institute for Health and Care Excellence. Developing NICE guidelines: the manual. Published October 2014. Last updated October 2020; 2020. Available from: https://www.nice.org.uk/process/pmg20/chapter/introduction.
- Ramsey SD, Willke RJ, Glick H, et al. Cost-effectiveness analysis alongside clinical trials II-An ISPOR good research practices task force report. Value Health. 2015;18(2):161–172.
- Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies: Canada. 4th ed. Ottawa: CADTH; 2017. Available from: https://www.cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf.
- The Pharmaceutical Benefits Advisory Committee. PBAC guidelines, section 3: economic evaluation; 2016. Available from: https://pbac.pbs.gov.au/section-3-economic-evaluation.html.
- Drummond MF. Challenges in the economic evaluation of orphan drugs. Eurohealth. 2008;14(2):16–17.
- Institute for Clinical and Economic Review. Assessing the effectiveness and value of drugs for rare conditions; 2017. Available from: https://icer.org/wp-content/uploads/2020/10/ICER_Assessing-the-Value-of-Drugs-for-Rare-Conditions_051017-1.pdf.
- Picavet E, Cassiman D, Simoens S. What is known about the cost-effectiveness of orphan drugs? Evidence from cost-utility analyses. J Clin Pharm Ther. 2015;40(3):304–307.
- National Institute for Health and Care Excellence. Interim process and methods of the Highly Specialised Technologies Programme updated to reflect 2017 changes; 2017. Available from: https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-highly-specialised-technologies-guidance/HST-interim-methods-process-guide-may-17.pdf.
- Taylor C, Jan S, Thompson K. Funding therapies for rare diseases: an ethical dilemma with a potential solution. Aust Health Rev. 2018;42(1):117–119.
- Institute for Clinical and Economic Review. Modifications to the ICER value assessment framework for treatments for ultra‐rare diseases; 2017. Available from: https://icer.org/wp-content/uploads/2020/10/ICER-Adaptations-of-Value-Framework-for-Rare-Diseases.pdf.
- Brown R, Ioannou P, Cadwell K. A review of six years of the NICE Highly Specialised Technology (HST) programme. Value Health. 2019;22:S860.
- Institute for Clinical and Economic Review. 2020-2023 Value Assessment Framework; 2020. Available from: https://icer.org/wp-content/uploads/2020/11/ICER_2020_2023_VAF_02032022.pdf.
- National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013; 2013. [cited 2019 September 19]. Available from: https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781.
- Cameron D, Ubels J, Norstrom F. On what basis are medical cost-effectiveness thresholds set? Clashing opinions and an absence of data: a systematic review. Glob Health Action. 2018;11(1):1447828.
- Nanavaty M, Kaura S, Mwamburi M, et al. The use of incremental cost-effectiveness ratio thresholds in health technology assessment decisions; 2015. Available from: https://www.hmpgloballearningnetwork.com/site/jcp/article/use-incremental-cost-effectiveness-ratio-thresholds-health-technology-assessment-decisions.
- Cahill JR. Traditional cost-effectiveness formulas and precision medicines. Health Affairs. 2020 [cited Blog summarizing the limitations of cost-effectiveness analyses of treatments for rare, chronic diseases. p.]. Available from: 10.1377/forefront.20200527.292431
- Paulden M, O'Mahony JF, McCabe C. Discounting the recommendations of the second panel on cost-effectiveness in health and medicine. Pharmacoeconomics. 2017;35(1):5–13.
- Zorginstituut Nederland. Guideline for economic evaluations in healthcare; 2016. Available from: https://english.zorginstituutnederland.nl/publications/reports/2016/06/16/guideline-for-economic-evaluations-in-healthcare.
- HM Treasury. The green book: central government guidance on appraisal and evaluation. London; 2018.
- Solem CT, Vera-Llonch M, Liu S, et al. Impact of pulmonary exacerbations and lung function on generic health-related quality of life in patients with cystic fibrosis. Health Qual Life Outcomes. 2016;14:63.
- IMS Institute for Healthcare Informatics. Price declines after branded medicines lost exclusivity in the U.S. 2016. [cited 2019 September 17]. Available from: https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/price-declines-after-branded-medicines-lose-exclusivity-in-the-us.pdf.
- Vondeling GT, Cao Q, Postma MJ, et al. The impact of patent expiry on drug prices: a systematic literature review. Appl Health Econ Health Policy. 2018;16(5):653–660.
- Bell SC, Mall MA, Gutierrez H, et al. The future of cystic fibrosis care: a global perspective. Lancet Respir Med. 2020;8(1):65–124.
- Mall MA, Mayer-Hamblett N, Rowe SM. Cystic fibrosis: emergence of highly effective targeted therapeutics and potential clinical implications. Am J Respir Crit Care Med. 2020;201(10):1193–1208.
- Cystic Fibrosis Foundation. Cystic fibrosis foundation patient registry 2020 annual data report. Bethesda, MD: Cystic Fibrosis Foundation; 2021.
- Vertex Pharmaceuticals Incorporated. Trikafta® (elexacaftor/tezacaftor/ivacaftor) [package insert]. Boston, MA: Vertex Pharmaceuticals Incorporated; 2021.
- Vertex Pharmaceuticals (Ireland) Limited. Kaftrio® (elexacaftor/tezacaftor/ivacaftor) [summary of product characteristics]. Dublin, Ireland: Vertex Pharmaceuticals (Ireland) Limited; 2021.
- Middleton PG, Mall MA, Dřevínek P, et al. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381(19):1809–1819.
- Heijerman HGM, McKone EF, Downey DG, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019;394(10212):1940–1948.
- Barry PJ, Mall MA, Álvarez A, et al. Triple therapy for cystic fibrosis Phe508del-gating and -residual function genotypes. N Engl J Med. 2021;385(9):815–825.
- Daines CL, Tullis E, Costa S, et al. editors. Long-term safety and efficacy of elexacaftor/tezacaftor/ivacaftor in people with cystic fibrosis and at least one F508del allele: 96-week interim results from an open-label extension study. 35th Annual North American Cystic Fibrosis Conference (virtual); 2021.
- Cystic Fibrosis Foundation. Cystic fibrosis foundation patient registry 2015 annual data report. Bethesda, MD: Cystic Fibrosis Foundation; 2016.
- Mogayzel PJ Jr, Naureckas ET, Robinson KA, et al. Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2013;187(7):680–689.
- Rubin JL, O'Callaghan L, Pelligra C, et al. Modeling long-term health outcomes of patients with cystic fibrosis homozygous for F508del-CFTR treated with lumacaftor/ivacaftor. Ther Adv Respir Dis. 2019;13:1753466618820186.
- McGarry L, Lopez A, Chandler C, et al. Validation of modeled 5-year survival outcomes among patients with Cystic Fibrosis (CF) treated with the CF Transmembrane Conductance Regulator Modulator (CFTRm) ivacaftor using US CF Foundation Patient Registry (USCFFPR) data. Value Health. 2020;23(suppl 1):S8.
- Westra TA, Parouty M, Brouwer WB, et al. On discounting of health gains from human papillomavirus vaccination: effects of different approaches. Value Health. 2012;15(3):562–567.
- Rogoza RM, Westra TA, Ferko N, et al. Cost-effectiveness of prophylactic vaccination against human papillomavirus 16/18 for the prevention of cervical cancer: adaptation of an existing cohort model to the situation in The Netherlands. Vaccine. 2009;27(35):4776–4783.
- Wan X, Zhang Y, Tan C, et al. First-line nivolumab plus ipilimumab vs sunitinib for metastatic renal cell carcinoma: a cost-effectiveness analysis. JAMA Oncol. 2019;5(4):491–496.
- Banken R, Rind D, Cramer G, et al. Voretigene neparvovec for biallelic RPE65-mediated retinal disease: effectiveness and value; 2018. Available from: https://icer.org/wp-content/uploads/2020/10/MWCEPAC_VORETIGENE_FINAL_EVIDENCE_REPORT_02142018.pdf.
- Tice JA, Kuntz KM, Wherry K, et al. Modulator treatments for cystic fibrosis: effectiveness and value; final evidence report and meeting summary; 2020. Available from: https://icer.org/wp-content/uploads/2020/08/ICER_CF_Final_Report_092320.pdf.
- Fajac I, Van Brunt K, Daines C, et al. Impact of elexacaftor/tezacaftor/ivacaftor triple combination therapy on health-related quality of life in people with cystic fibrosis heterozygous for F508del and a minimal function mutation: results from a phase 3 clinical study. J Cyst Fibros. 2020;19(suppl 2):S118–S119.
- Majoor C, Van Brunt K, Daines C, et al. Impact of elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) triple combination therapy on Health-Related Quality of Life (HRQoL) in People with Cystic Fibrosis (pwCF) homozygous for F508del (F/F): results from a phase 3 clinical study. J Cyst Fibros. 2020;19(suppl 2):S32.
- Acaster S, Mukuria C, Rowen D, et al. Development of the cystic fibrosis questionnaire-revised preference based scoring algorithm. Pediatr Pulmonol. 2019;758:S443.
- Whiting P, Al M, Burgers L, et al. Ivacaftor for the treatment of patients with cystic fibrosis and the G551D mutation: a systematic review and cost-effectiveness analysis. Health Technology Assessment. 2014;18(18):106.
- Davis S. Assessing technologies that are not cost-effective at a zero price. London: National Institute for Health and Care Excellence (NICE): NICE Decision Support Unit Methods Development; 2014.
- Toporek A, Merlo CA, West NE. Use of chronic maintenance therapies for cystic fibrosis in patients on elexacaftor, tezacaftor, ivacaftor at a single center. Am J Respir Crit Care Med. 2021;203:A2024.
- Brown C, Powers C, Teibel H, et al. editors. Patients on elexacaftor/tezacaftor/ivacaftor decrease chronic daily therapy within two months of initiation. North America Cystic Fibrosis Conference (virtual); 2020.
- Wallner K, Shapiro AM, Senior PA, et al. Cost effectiveness and value of information analyses of islet cell transplantation in the management of 'unstable' type 1 diabetes mellitus. BMC Endocr Disord. 2016;16:17.
- Konstan MW, Morgan WJ, Butler SM, et al. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr. 2007;151(2):134–139.e1.
- Konstan MW, Wagener JS, VanDevanter DR, et al. Risk factors for rate of decline in FEV1 in adults with cystic fibrosis. J Cyst Fibros. 2012;11(5):405–411.
- Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: epidemiology and pathogenesis. Thorax. 2007;62(4):360–367.
- Truven Health Analytics. Red Book Online 2020. [cited 2019 January 3]. Available from: http://www.micromedexsolutions.com/micromedex2/librarian.
- Hu X, Sawicki G, Bonafede M, et al. An early view of characteristics and treatment patterns of patients initiated on tezacaftor/ivacaftor in the United States: an administrative claims data analysis. Manag Care Spec Pharm. 2019;25(suppl):S71.
- Vertex Pharmaceuticals Incorporated. Trikafta® (elexacaftor/tezacaftor/ivacaftor) [package insert]. Boston MA: Vertex Pharmaceuticals Incorporated; 2019.
- Centers for Medicare and Medicaid Services. Episode-of-Care Payment 2019. Available from: https://www.cmshospitalchartbook.com/visualization/2019/episode-care-payment.
- McGarry L, Lopez A, Booth J, et al. Application of the CFQ-R-8D to estimate utility benefit of elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) in people with cystic fibrosis (CF). Value Health. 2020;23(suppl 2):S731–S732.
