Abstract
Objectives
In advanced cancers, healthcare resource utilization (HCRU) and costs usually increase until death. However, few studies have measured HCRU over time in patients treated with immunotherapies. The objective was to describe the evolution of HCRU and costs over four years for patients with advanced non-small cell lung cancer (aNSCLC) initiating nivolumab.
Materials and methods
Based on the French hospital reimbursement database, all aNSCLC patients initiating nivolumab in the 2nd line or later in 2015 or 2016 were followed until 2019. HCRU (including hospitalizations and hospital visits) and costs (payer perspective) were described annually after nivolumab initiation. Trends in HCRU were analyzed with the Mann-Kendall test. As most patients did not reach the four-year follow-up, cost-analysis was performed without adjustment throughout, without adjustment in uncensored cases only or with adjustment using for all patients using the Bang&Tsiatis method.
Results
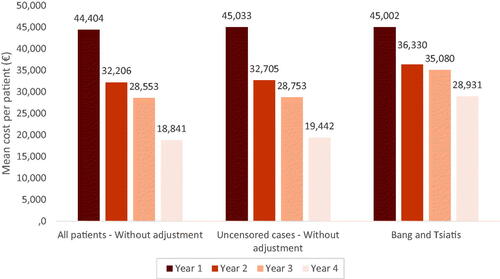
10,452 patients initiating nivolumab were evaluated. The percentage of patients hospitalized or with hospital visits decreased (p < .001) over the four-year follow-up with the exception of consultations. The number of hospital visits per patient decreased from 23.3 in Y1 to 13.2 in Y4 without adjustment and 18.3 with adjustment (p < .001). The overall hospitalization duration per patient (days) decreased from 36.0 (Y1) to 14.9 (Y4-unadjusted) and 20.5 (Y4-adjusted) (p < .001). Annual per capita costs also decreased. The method without adjustment provided the lowest cost over time (€44,404 (Y1), €32,206 (Y2); €28,552 (Y3); €18,841(Y4)) while the Bang&Tsiatis method presented the highest cost (€45,002 (Y1), €36,330 (Y2); €35,080 (Y3); €28,931 (Y4)).
Conclusion
HCRU and costs for NSCLC patients treated with nivolumab decreased over time. Cost estimates are dependent on the statistical method used to take into account uncertainty, but costs decreased over time whatever the method used.
1. Introduction
Lung cancer is one of the most frequently diagnosed cancers in Europe, with 470,000 new cases documented in 2018, and is the most frequent cause of death from cancerCitation1. Around 50,000 new patients are diagnosed with lung cancer in France every year, of whom 85% present with non-small cell lung cancer (NSCLC). A majority of patients are diagnosed with advanced or metastatic disease and thus have a poor prognosisCitation2,Citation3.
Since 2015, immune checkpoint inhibitors (ICI), such as nivolumab, atezolizumab and pembrolizumab, have become the standard of care in advanced disease, originally in second-line after platinum-based chemotherapy and, more recently, also in first-line as monotherapy or in combination with chemotherapy. These treatments have demonstrated a long-term survival benefit in second-line therapy with a survival rate of 13.4% for nivolumab at five years compared to 2.6% for docetaxelCitation4.
Cancer is the second most expensive disease in France after psychiatric disorders, corresponding to 16.3 billion euros in 2018, representing 11.5% of total health expenditure annually. Lung cancer is the second most expensive cancer after breast cancer, accounting for 1.8 billion euros of expenditure in 2018Citation5. The management cost ranged between €20 and €27,000 per patient with advanced disease in 2004, before the introduction of immunotherapiesCitation6.
To date, most economic studies of aNSCLC have focused on cytotoxic chemotherapies and have reported healthcare resource utilization (HCRU) data on the number and duration of hospitalizations, as well as the annual cost of hospitalization per patient. These outcomes have been evaluated by cancer stage, treatment line or countryCitation7,Citation8. Data also suggests that, for patients treated with cytotoxic chemotherapy, costs usually increase over treatment lines until deathCitation9. However, there is little information on how costs evolve over time as the nature and intensity of care change across disease management, especially for patients treated with ICIs. With respect to ICIs, only short-term data (i.e. from 1 to 4 years) on HCRU is available, comparing the three licensed ICIs (pembrolizumab, nivolumab and atezolizumab) with each other or with cytotoxic chemotherapy and did not explore how costs varied over the yearsCitation10,Citation11. However, the long-term evolution of costs in patients treated with ICIs is expected to differ from patients receiving chemotherapy, since more patients achieve a sustained partial or complete responseCitation12. In addition, the tolerability profile differs between ICIs and chemotherapy (both in terms of the nature and the severity of adverse events), as does the impact on quality of lifeCitation13. Consequently, HCRU and costs for patients treated with ICIs such as nivolumab may decrease over time.
The French National Health Data System (SNDS, Système National des Données de Santé) collates individual patient health and reimbursement data for more than 99% of the French population, covering both hospital and community care and both the public and private sectors. The SNDS uses a unique and anonymous patient identifier so that individual patients can be followed over their lifetime. Given its exhaustive coverage, the extensive range of HCRU data it contains, and its information quality, this database has been widely used for the epidemiological, burden of disease, or economic studiesCitation14.
The objective of this study was to describe how HCRU and costs evolve over the four years following the initiation of nivolumab as a second-line treatment for aNSCLC. A secondary objective was to compare different methods of cost adjustment in the context of censored data.
2. Methods
The study was performed using data from the UNIVOC cohort, a large cohort of 10,452 patients with NSCLC extracted from the SNDS databaseCitation15–17.
2.1. Data sources
Data were retrospectively extracted from the SNDS, in particular the French Hospital Medical Information database (Programme de Médicalisation des Systèmes d'Information – PMSI). The PMSI database covers the MCO sector (medicine, surgery and obstetrics facilities), HAD (home care), SSR (follow-up care and rehabilitation), ACE (outpatient hospital visits), and FICHCOMP (innovative and expensive drugs cost separately from the Disease-related group tariff or available through early access programs). It covers all overnight, day hospitalizations or visits in public and private French hospitalsCitation14. Each hospital stay results in a production of a standard discharge summary (“Résumé de Sortie Standardisée” RSS) following each visit or stay. The RSS is then anonymized to become the RSA (“Résumé de Sortie Anonyme”). The RSA contains information on patient characteristics (gender, age, residence code), the main diagnosis that led to hospital admission, the nature of the treatment and work-up (examinations) carried out, comorbidities and on complications. Diagnoses are coded using the International Classification of Diseases, 10th revision (ICD-10)Citation18 either as primary- (PD: the condition for which the patient was hospitalized), related- (RD: any underlying condition which may have been related to the PD), or significant associated-diagnoses (SAD: comorbidities or complications which may affect the course or cost of hospitalization). For each DRG, the hospital receives a fixed payment according to a national tariff, which is intended to cover all hospital expenses. The type and volume of health care resources used, as well as the cost per patient, can be collected over time, by linking all the hospital visits and stays based on the unique patient identifier.
2.2. Identification of patients
The study design and study population of UNIVOC have been described in previous publicationsCitation15–17 and are briefly described below. UNIVOC is a retrospective observational cohort of all French aNSCLC patients initiating nivolumab in second or further lines during the early access program of the drug (Jan-2015 – Dec-2016). Patients and data from UNIVOC were identified and collected from the PMSI database. Patients were included in the UNIVOC cohort when they had a hospital stay mentioning lung cancer (ICD-10 code: C34*) in 2015 or 2016. All the patients were followed until 31 December 2019 or death, if it occurred before.
2.3. Health care resources used and cost data collection
Regarding healthcare resource utilization (HCRU), the number of times nivolumab was administered was documented from the FICHCOMP/FICHCOMP ATU files. Hospital stays were split into categories, namely inpatient stays with at least an overnight spent in the hospital, home care organized by the hospital (hospitalisation à domicile; HAD), one-day stays (ODS), emergency room visits (ERV) and hospital consultations. Inpatient stays concerned all stays with at least one night spent in hospital, including intensive/resuscitation care, palliative care, hospitalizations for lung cancer, other hospitalizations (for any causes) and rehabilitation care.
Costs are presented according to the French social security perspective. Hospital costs were valued using the national tariffs for each year considered, and were expressed in 2020 Euros. Tariffs included nursing care, treatments, standard drugs, food and accommodation, and investment costs, as well as the supplements for the intensive care unit. For public hospitals, tariffs also covered medical and technical procedures. Expensive drugs, extracted from the FICHCOMP ATU (early access program period), were costed using the first publicly listed price. When the purchased price presented was superior to the tariffs, the price was limited to the tariff price. Costs of expensive drugs after reimbursement were extracted from the FICHCOMP. For private hospitals, costs were estimated using the official DRG tariffs for private hospitals to which physician’s fees were added (as they are not included in DRG tariffs and are reimbursed on a fee-for-service basis). Costs are presented as the mean cost per patient per year of follow-up.
2.4. Study outcomes
For each year of follow-up (1st–4th year), the percentage of patients requiring at least one visit or hospitalization was estimated. The total number of hospital visits per patient and per year (PPPY) was calculated, as the sum of all hospital visits or stays whatever the duration of the stay.
Regarding the duration of hospitalization, as their respective duration differs among the different categories of visits, the mean time spent at hospital PPPY was calculated. Data were available in the PMSI for inpatient and home care categories, whereas hypotheses were made for the categories without a night spent at the hospital (i.e. ODS, ERV, hospital consultations), based on experts' opinions (CC, JBA). We considered that a patient spent half a day at the hospital for ODS and ERV, and two hours for hospital consultations. The mean cost per patient and per year was calculated and reported by category of expenses and globally, valued in 2020 Euros.
2.5. Statistical analysis
Data presentation is principally descriptive: categorical data are expressed as proportions, whereas continuous data are expressed as means and standard deviations (SD). Time to treatment discontinuation (TTD), overall survival (OS) and follow-up duration from nivolumab initiation was estimated using Kaplan-Meier survival curves. Patients who survived were considered censored at the last follow-up date (31st December 2019).
The percentage of patients making at least one visit or at least one hospitalization stay, the mean number of visits and the time spent at the hospital were described. Costs are presented as the mean cost per patient and per year of follow-up. Trends over time in HCRU and costs were analyzed using the Mann-Kendall test applied to quarterly data. Mann-Kendall test is a non-parametric test for identifying trends in time series data.
As some patients did not reach four years of follow-up (i.e. patients initiating nivolumab in 2016), HCRU and costs are censored for some patients during their last year of follow-up. The cost analysis was then performed using three different methods for censored cost data. For the base case analysis, mean annual costs per patient were determined using available cost data for all patients without adjustment. Two sensitivity analyses were performed, the first without adjustment in uncensored cases only and the second applying the Bang and Tsiatis method for all patientsCitation19. The Bang and Tsiatis method allows us to consider the censor in the estimation of the cost and to avoid biased estimation because costs occurring after censoring are unknown. This method is based on inverse probability weighting, to evaluate the probability of a patient being censored at event time. A brief description of each cost estimation is presented in Supplementary Materials 1. For HCRU, no adjustment methods were identified in the literature. To adjust HCRU for censoring, we used two UNIVOC data extractions with different follow-ups (up until the end of follow-up in 2018 and 2019). Consequently, in the first extraction, data were complete for the first two years and censored for the third year. In the second extraction, data were complete for the first three years and censored for the fourth year. We compared the estimate of HCRU for the third year between the two extractions to assess the impact of censoring. The difference in HCRU (expressed as a percentage) between the two extractions was then applied to the fourth-year data to control for censoring. Statistical analyses were performed with R software (version 4.0.4).
2.6. Ethics
The study was conducted in accordance with the International Society for Pharmacoepidemiology (ISPE) Guidelines for Good Pharmacoepidemiology Practices (GPP) and applicable regulatory requirements. Since this was a retrospective study of an anonymized database and had no influence on patient care, ethics committee approval was not required. The study was performed according to the MR006 guideline of the French data protection agency (Commission Nationale de l’Informatique et des Libertés; CNIL) with respect to the confidentiality of individual patient data.
3. Results
3.1. Study population
Overall, 10,452 patients with an advanced NSCLC who initiated treatment with nivolumab between 1st January 2015 and 31st December 2016 were identified. The mean age at inclusion was 64 years and 71% were men. Most patients had non-squamous aNSCLC (55.5%).
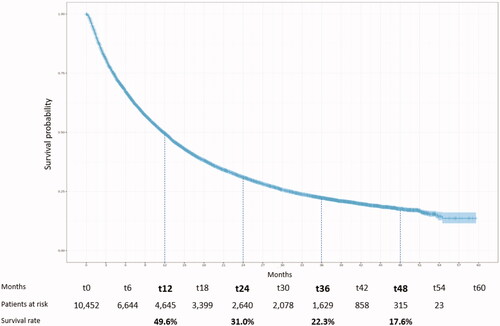
The median follow-up duration was 9.9 months [Q1 = 3.5; Q3 = 24.2]. The median TTD with nivolumab was 2.8 months [Q1 = 1.4; Q3 = 6.6]. The median OS was 11.8 months [Q1 = 4.2; Q3 = 31.1] (). Survival rates at 1, 2, 3 and 4 years were 49.6%, 31.0%, 22.3% and 17.6% respectively.
3.2. Healthcare resource utilization
3.2.1. Percentage of living patients with at least one hospital visit or one hospitalization
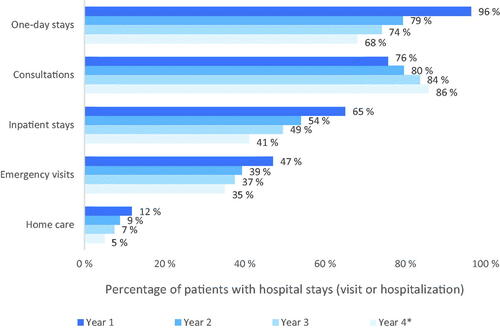
The most frequently documented type of visit was ODS and hospital consultations, with 96% of patients making an ODS and 76% attending a consultation during the first year of treatment. Over the follow-up period, the percentage of patients making an ODS declined (96% in Year 1 to 68% in Year 4 (p < .0001)), whereas the percentage of patients consulting increased (76% in Year 1 to 86% in Year 4 (p < .0001)). From the second year onwards, consultations became the most frequent category of hospital stay. In Year 1, 47% of patients made an ERV, and this proportion then decreased over time (). Inpatient stays concerned 65% of patients during Year 1 and then decreased over time. Home care only concerned 12% of patients in Year 1 and 5% in Year 4.
3.2.2. Number of hospital visits per patient
A patient with aNSCLC initiating nivolumab made on average of 23.3 hospital visits during the first year of treatment. This number then decreased over time, to 21.3 visits in Year 2 and 19.7 visits in Year 3. For Year 4, the retrieved and projected mean numbers of hospital visits per patient were 13.2 and 18.3 respectively. This decrease in the number of visits was statistically significant over time, with and without adjustment (p < .0001) ().
Figure 3. Mean hospital visits for all causes and per category per patient. *Year 4 has been adjusted. Of the 18.3 visits per patient, 13.2 represents the number of hospital visits per patient (without adjustment), while 5.1 is the number of additional visits after adjustment. **Emergency visits represent: 3% year 1, 3% year 2, 3% year 3 and 3% year 4. ***Home care visits represent: 1% year 1, 1% year 2, 1% year 3, 1% year 4.
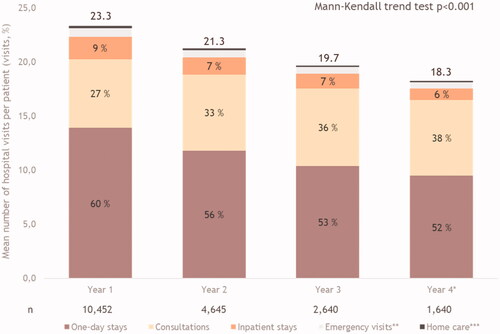
The number of visits to PPPY tended to decrease over the follow-up for all categories except for consultations. ODS represented more than half of the hospital visits throughout the follow-up period. Patients went to ODS mainly for treatment administration (86% in Year 1, 85% in Year 2, 83% in Year 3 and 82% of the visits). Consultations accounted for the second highest number of visits to PPPY. The number of emergency visits and home care visits PPPY was <1 throughout the four years of follow-up ().
3.2.3. Time spent at hospital per patient
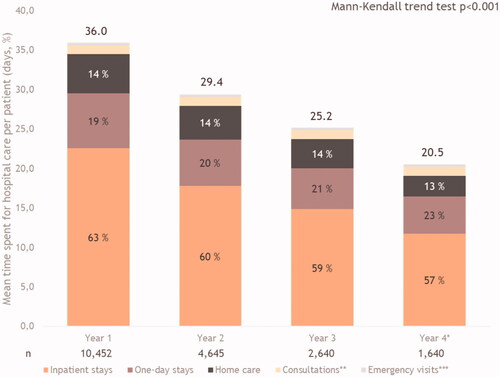
The time spent at the hospital was 36.0 days during the first year following nivolumab initiation, and significantly decreased thereafter to attain 20.5 days in Year 4 (p < .0001) (). It was mostly explained by the inpatient stays, which contributed 63% to 57% of the time spent in the hospital from Y1 to Y4, even if this proportion decreased over time (p < .001). The second highest contribution to time spent in the hospital came from ODS, which also decreased over the follow-up period. Home care, consultations and ERV represented <5 days PPPY throughout the four-year follow-up period ().
Figure 4. Mean time spent at the hospital for all causes and per category per patient. *Year 4 has been adjusted. Of 20.5 hospital days per patient, 14.9 represents the number of hospital visits per patient (without adjustment), while 5.6 is the number of additional visits after adjustment. **Consultations represent: 3% year 1, 4% year 2, 5% year 3, 6% year 4. ***Emergency visits represent: 1% year 1, 1% year 2, 1% year 3 and 1% year 4.
3.3. Cost analysis
3.3.1. Main analysis
The mean cost per patient was estimated to be €44,404 in Year 1 and €18,841 in Year 4, representing a significant decrease over time (p < .001). In all years, drugs paid as a surcharge on top of the DRG (i.e. expensive drugs, namely anti-PD1, anti-PDL1, bevacizumab or pemetrexed) were the largest cost component, representing at least 55% of the overall cost per patient. MCO hospitalization and ODS were the two categories with the next highest costs ().
Table 1. Description of costs (€) per patient per category.
3.4. Comparison of adjustment methods
For all the methods for cost adjustment assessed, a significant decrease in mean cost PPPY was observed across the follow-up period. The method without adjustment on all patients showed the lowest cost over the four-year follow-up period. All methods estimated a similar cost for Year 1. From Year 2 onwards, the methods without adjustment on all patients and on uncensored cases provided similar estimates. In contrast, the Bang and Tsiatis method yielded a smaller reduction in the estimated mean cost PPPY over time, from €45,002 in Year 1 to €28,931 in Year 4 ().
4. Discussion
This is the first study exploring HCRU and associated cost evolution in patients with aNSCLC treated with nivolumab in France over time. This study includes all patients initiating nivolumab for NSCLC between 2015 and 2016. Time on treatment is close to the ones observed in pivotal trials. Indeed, in pivotal trials, the median number of doses was 6 in CheckMate 057 and 8 in CheckMate 017. Nivolumab was administrated every two weeks corresponding to 2.8 and 3.7 months respectively on treatment. Considering the higher proportion of patients with non-squamous NSCLC compared to squamous NSCLC in our study, the time on treatment is close to the ones in the pivotal trials.
In this real-world data analysis, we observed a decrease in overall HCRU related to all-cause hospitalization over time, including the percentage of patients requiring at least one hospital stay, the mean number of hospital visits PPPY and the time spent at the hospital PPPY. In particular, we observed a decrease in all study outcomes over time for inpatient stays and ODS. The only category increasing over the follow-up was consultations, perhaps explained by a switch from hospitalization to consultations. Patients are less often hospitalized and stay less time at the hospital. These results suggest less intensive management over time with less HCRU.
Few studies have explored HCRU in aNSCLC management and most of these have concerned chemotherapies. A study in the NetherlandsCitation20 evaluated HCRU in 28 patients treated with chemotherapy in second line. The mean number of hospital visits and hospitalizations PPPY was higher than in our study. Moreover, the length of hospitalization was 36.6 days, while in our study the maximum length of hospital stays (including hospital visits or hospitalizations) was 36.0 days. Another study conducted in 8 European countries estimated that patients with advanced/metastatic NSCLC spent between 15.6 and 17.5 days in hospital per year or until death. Moreover, they observed that patients spent on average in France and Germany 5 days more in hospitalization than in other countriesCitation7. These results are not comparable with the overall results of our study since we included duration for all categories of hospitalization but seem close to MCO hospitalization results. The close or lower HCRU finding is similar to an American study exploring HCRU and cost evolution following the introduction of ICIs which found lower mean hospital stays and ERV with the introduction of these treatmentsCitation11. Thus, in addition to a level of HCRU close to or even lower than chemotherapy, the HCRU of patients treated with ICIs decrease.
In our study, we explored three methods to estimate the mean cost of PPPY. The mean cost per patient tended to decrease over the follow-up period with all these methods, showing the robustness of our results even with cost censored data. Cost estimations were similar between all methods for the first year. The mean cost per patient during the first year ranged from €44,404 to €45,002. After the first year, cost estimations were different between methods. The method without adjustment on all patients presented the lowest cost per patient while the Bang and Tsiatis method estimated the highest cost per patient. The mean cost per patient during the last year of follow-up ranged from €18,841 to €28,931. Whatever the method tested, our results indicated higher costs than those reported previously for the two years after treatment initiationCitation21. For example, the French National insurance published a reportCitation5 showing that expenses attributable to lung cancer have grown over the years, with the arrival of new treatments and the improved prognosis of the disease. They estimated that the mean expenditure per patient, independently of the stage, in 2017 was around €20,000. These results are close to what was estimated in an Italian cohort of patients diagnosed in 2017Citation22. However, according to the study by Tanguy-Melac et al., expenses are higher for patients with metastatic cancer and tend to increase until deathCitation9. Higher expenses in our study can be explained by the fact that our data are restricted to patients starting nivolumab in second-line treatment and over setting of advanced disease. However, we presented different findings for evolution until death, since the mean cost per patient seems to decrease over time.
Setting aside expensive treatments (funded in addition to the hospital stay) from the hospital stays, we observe this category was the most important cost driver throughout the follow-up period as reported in previous studies even before the introduction of ICIsCitation23,Citation24. After treatment, the two major cost drivers were MCO hospitalizations and ODS. MCO hospitalizations concern all hospitalizations for medicine, surgery and obstetrics. In this case, we assume most MCO hospitalizations are for NSCLC management (adverse events, symptoms, or exams), comorbidities management or palliative/intensive care. Even with less than 10 visits per patient per year, these stays can be expensive since patient stays at least one night at the hospital. The third cost driver concerns ODS. This category is a driver since it represents more than half of visits per year with a cost for administration or patient management. These cost items tended to decrease over time, which is consistent with a decrease in the of hospital visits observed in an Italian cohort, in which some patients were treated with immunotherapyCitation22. As in our study, the lowest cost category was ERV, although the Italian study reported higher absolute costs for these. The lower costs in our study might be explained by the use of nivolumab since Korytowsky et al. found that hospital costs excluding treatments have decreased since the introduction of ICIsCitation11. These results emphasize the importance of exploring each cost category and its evolution over time.
The limitations of this study are principally related to the PMSI database. For example, we were not able to explore outpatient (outside the hospital) HCRU and costs in this analysis or indirect costs. A previous study observed that indirect costs are almost as high as direct costs and should be considered in cancer costing studiesCitation25. Unfortunately, the reasons for stopping treatment are not available in the PMSI which decreases the interest to explore differences in HCRU and costs evolution according to management and the possible treatments received after nivolumab over time. Moreover, due to the large number of patients included in this study and the possible variety of treatment typologies between patients, this analysis would require machine learning techniques. Also, the estimates of the unit time spent at the hospital for ODS, ERV and consultations were obtained from expert opinion rather than from actual data. For statistical analysis, no specific method for HCRU adjustment on censoring but we were able to adjust thanks to the previous extraction of this cohort. Finally, we did not estimate the savings compared to non-ICIs treated patients.
However, thanks to the PMSI, we identified all patients initiating nivolumab for NSCLC during the early access program in France. We followed the HCRU and costs evolution of more than 10,000 patients treated with the only ICI available in 2015–2016. This study allowed a clear illustration of aNSCLC patients treated with the nivolumab hospital pathway. Finally, this is the first study informing on long-term HCRU and costs of patients treated with ICIs in France.
In conclusion, this large study indicates that HCRU and associated costs tend to decrease over time in patients with aNSCLC treated with immunotherapy. Further studies would be beneficial to determine whether long-survivor patients achieve a level of expenditure close to the general population with the same characteristics.
Transparency
Declaration of funding
Funding for the study was provided by Bristol Myers Squibb.
Declarations of financial/other interest
VG, FEC, AFG and DR are employees of BMS, purveyor of immunotherapies used in different cancers. CC reports consultancy fees from Astra Zeneca, Boehringer Ingelheim, MSD, Pierre Fabre Oncology, Lilly, Roche, BMS and Novartis. BJ and RJ are employees of HEVA. JBA was supported by grants from Fondation pour la Recherche Médicale (FRM). IB has no conflicting interests.
A reviewer on this manuscript has disclosed that they are an employee of Merck & Co Inc. The other peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
All authors have made substantial contributions. VG, FEC, AFG, DR, CC and JBA participated from the conception or design to the data interpretation. RJ and BJ participated in the data acquisition and data analysis. VG, FEC and IB participated in the data interpretation and the writing of the manuscript. All authors have approved the present manuscript.
Previous presentation
ISPOR-EU 2021, November 30-December 3.
Supplemental Material
Download MS Word (18.3 KB)Acknowledgement
The authors are grateful to Adam Doble (SARL Foxymed, Paris) for medical writing support.
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:691–387.
- Haute Autorité de Santé. Available from: https://www.has-sante.fr/upload/docs/application/pdf/2013-10/guide_k_bronchopulmonaires_finalweb__091013.pdf.
- Chouaïd C, Debieuvre D, Durand-Zaleski I, et al. Survival inequalities in patients with lung cancer in France: a nationwide cohort study (the TERRITOIRE study). PLoS One. 2017;12(8):e0182798.
- Borghaei H, Gettinger S, Vokes EE, et al. Five-Year outcomes from the randomized, phase III trials CheckMate 017 and 057: Nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol. 2021;39(7):723–733.
- Caisse Nationale d’Assurance Maladie. Available from: https://assurance-maladie.ameli.fr/sites/default/files/rapport-charges-et-produits-2020.pdf.
- Chouaïd C, Molinier L, Combescure C, et al. Economics of the clinical management of lung cancer in France: an analysis using a markov model. Br J Cancer. 2004;90(2):397–402.
- Vergnenègre A, Carrato A, Thomas M, et al. Real-world healthcare resource utilization in a european non-small cell lung cancer population: the EPICLIN-Lung study. Curr Med Res Opin. 2014;30(3):463–470.
- Lee DH, Isobe H, Wirtz H, et al. Health care resource use among patients with advanced non-small cell lung cancer: the PIvOTAL retrospective observational study. BMC Health Serv Res. 2018;18(1):147.
- Tanguy-Melac A, Denis P, Pestel L, et al. Intensity of care, expenditure, place and cause of death people with lung cancer in the year before their death: a french population based study. Bull Cancer. 2020;107(3):308–321.
- Huang Y, Diaby V. Pcn142 healthcare resource utilization analysis on immune checkpoint inhibitors in patients with metastatic lung cancer using claims database. Value in Health. 2020;23:S48.
- Korytowsky B, Radtchenko J, Nwokeji ED, et al. Understanding total cost of care in advanced non-small cell lung cancer pre- and postapproval of immuno-oncology therapies. Am J Manag Care. 2018;24(20):439–43S. 447
- Antonia SJ, Borghaei H, Ramalingam SS, et al. Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis. Lancet Oncol. 2019;20(10):1395–1408.
- Reck M, Brahmer J, Bennett B, et al. Evaluation of health-related quality of life and symptoms in patients with advanced non-squamous non-small cell lung cancer treated with nivolumab or docetaxel in CheckMate 057. Eur J Cancer. 2018;102:23–30.
- Bezin J, Duong M, Lassalle R, et al. The national healthcare system claims databases in France, SNIIRAM and EGB: powerful tools for pharmacoepidemiology. Pharmacoepidemiol Drug Saf. 2017;26(8):954–962.
- Giaj Levra M, Cotté FE, Corre R, et al. Immunotherapy rechallenge after nivolumab treatment in advanced non-small cell lung cancer in the real-world setting: a national data base analysis. Lung Cancer. 2020;140:99–106.
- Assié JB, Corre R, Levra MG, et al. Nivolumab treatment in advanced non-small cell lung cancer: real-world long-term outcomes within overall and special populations (the UNIVOC study). Ther Adv Med Oncol. 2020;12:1758835920967237.
- Chouaïd C, Grumberg V, Batisse A, et al. Machine learning-based analysis of treatment sequences typology in advanced non-small-cell lung cancer long-term survivors treated with nivolumab. JCO Clin Cancer Inform. 2022;6:e2100108.
- World Health Organization. ICD-10: international statistical classification of diseases and related health problems: tenth revision. 2nd ed. World Health Organization. 2004. https://apps.who.int/iris/handle/10665/42980
- Heejung B, Tsiatis A, et al. Estimating medical costs with censored data. Biometrika. 2000;87(2):329–343.
- Pompen M, Gok M, Novák A, et al. Direct costs associated with the disease management of patients with unresectable advanced non-small-cell lung cancer in The Netherlands. Lung Cancer. 2009;64(1):110–116.
- Buja A, Rivera M, De Polo A, et al. Estimated direct costs of non-small cell lung cancer by stage at diagnosis and disease management phase: a whole-disease model. Thorac Cancer. 2021;12(1):13–20.
- Buja A, Pasello G, De Luca G, et al. Non-small-cell lung cancer: real-world cost consequence analysis. JCO Oncol Pract. 2021;17(8):e1085–e1093.
- Gadby F, Descourt R, Robinet G, et al. Evolution of the costs and management of lung cancer between 2004 and 2014. Rev Mal Respir. 2020;37(1):1–7.
- Verleger K, Penrod JR, Manley Daumont M, et al. Costs and cost drivers associated with non-small-cell lung cancer patients who received two or more lines of therapy in Europe. Clinicoecon Outcomes Res. 2020;12:23–33.
- Bugge C, Saether EM, Brustugun OT, et al. Societal cost of cancer in Norway -results of taking a broader cost perspective. Health Policy. 2021;125(8):1100–1107.