Abstract
Aims
The objective of this study is to estimate the cost-effectiveness of KTE-X19 versus standard of care (SoC) in the treatment of patients with relapsed/refractory (R/R) mantle cell lymphoma (MCL) post-Bruton tyrosine kinase inhibitor (BTKi) treatment from a UK healthcare perspective.
Materials and Methods
A three-state partitioned survival model (pre-progression, post-progression and death) with a cycle length of one month was used to extrapolate progression-free and overall survival over a lifetime horizon. Population inputs along with KTE-X19 (brexucabtagene autoleucel) efficacy and safety data were derived from the single-arm trial ZUMA-2 (NCT02601313). The composition of SoC was informed by a literature-based meta-analysis, SoC efficacy data were obtained from the SCHOLAR-2 real-world study. Survival was modelled using standard parametric curves for SoC and a mixture-cure methodology for KTE-X19. It was assumed that patients whose disease had not progressed after five years experienced long-term remission. Costs, resource use and utility, and adverse event disutility inputs were obtained from published literature and publicly available data sources. An annual discount rate of 3.5% was applied to costs and health outcomes. Modelled outcomes for KTE-X19 and SoC included expected life years (LY), quality-adjusted life years (QALY) and total costs. Deterministic and probabilistic sensitivity analyses and scenario analyses were performed.
Results
Estimated median survival was 5.96 years for KTE-X19 and 1.38 for SoC. Discounted LYs, QALYs and lifetime costs were 8.27, 5.99 and £385,765 for KTE-X19 versus 1.98, 1.48 and £79,742 for SoC, respectively. The KTE-X19 versus SoC cost per QALY was £67,713 and the cost per LY was £48,645. Influential scenario analyses use alternative KTE-X19 survival curves and discount rates, and shorter time horizons.
Conclusion
Considering the survival and quality of life benefits compared to SoC, KTE-X19 for R/R MCL appears as a cost-effective treatment in the real-world UK setting.
Introduction
As a rare form of non-Hodgkin lymphoma (NHL), mantle cell lymphoma (MCL) originates from the accumulation of malignant B-cells in the mantle zone of lymph nodes. In the United Kingdom (UK), approximately 560 people are diagnosed with MCL annually, representing approximately 5% of all people diagnosed with NHLCitation1. With a median age at diagnosis of 72.9 and a male-to-female ratio of 2.6:1, MCL most often affects older menCitation1.
Current treatment options for MCL in the UK are defined by the British Society for Haematology and National Institute for Health and Care Excellence (NICE) which have established a clear treatment pathway for patients receiving first and second-line treatment for MCLCitation2,Citation3. In the first line, patients are recommended to receive a high-dose cytarabine regimen followed when possible by an autologous stem cell transplant (auto-SCT) with or without rituximab maintenanceCitation2,Citation4. Patients who are not eligible for auto-SCT will instead receive immunochemotherapy with or without rituximabCitation2,Citation3. Patients who are refractory or relapse are eligible to receive treatment with Bruton tyrosine kinase inhibitor (BTKi) ibrutinibCitation3,Citation4. Current treatment options for higher subsequent relapses are not well established. The use of alternative immunochemotherapy to that adopted in the first line, but also treatment with venetoclax and lenalidomide, commonly results in inferior response and rapid progressionCitation4–7.
With current treatment, MCL is generally incurableCitation8, and patients typically relapse, obtaining a worse prognosis with each subsequent treatment lineCitation8–11. A recent UK real-world analysis of patients receiving ibrutinib at first relapse, for instance, estimated median survival of 23.9 months post-BKTi initiationCitation12. However, the prognosis for patients who have relapsed or are refractory to ibrutinib is extremely poor, with the median second line and subsequent survival across treatments in the UK being estimated at 9.6 and 7.5 months, respectivelyCitation9,Citation13.
KTE-X19 (brexucabtagene autoleucel), a chimeric antigen receptor (CAR) T-cell therapy directed against CD19, is proposed as a treatment option for relapsed/refractory (R/R) MCL post two or more lines of systemic therapy including a BKTi. The efficacy and safety of KTE-X19 have been studied in phase 2, multicenter, single-arm open-label study (ZUMA-2, NCT02601313) in patients with R/R MCL and ≤5 prior therapies, including a BTKiCitation14,Citation15. Patients underwent leukapheresis and conditioning chemotherapy followed by a single infusion of KTE-X19. At a median follow-up of 17.5 months, KTE-X19 was associated with durable responses, with an objective response rate of 92% in the safety populationCitation16. In a more recent data set with a median follow-up of 25.5 months, KTE-X19 continued to be associated with durable responses with an objective response of 91% and a complete response rate of 68% in the safety population (Supplementary Table 6). In this recent dataset, the median duration of response was 24.8 months, progression-free survival (PFS) was 25.3 months and median overall survival (OS) had not yet been reached (Supplementary Table 6).
This paper investigates the comparative effectiveness and cost-effectiveness of KTE-X19 versus standard of care (SoC) containing cytotoxic chemotherapy, proteasome inhibitors, immunomodulatory drugs and Bcl-2 protein inhibitors for the treatment of patients with R/R MCL in the post-BTKi setting in England. While the analyses were conducted from the perspective of the National Health Service (NHS), personal and social services perspective, the modelled results may allow generalization to other settings.
Materials and methods
Overview
Based on the health-economic model submitted to the National Institute for Health and Care Excellence (NICE) in the UKCitation17, a partitioned survival model was built to reflect the clinical pathway, health outcomes and costs of patients with R/R MCL post-BTKi (such as ibrutinib), driven by their PFS and OS. Partitioned survival models have previously been used for the modelling of MCL and other oncology indications, as rather than defining transition probabilities, the model utilizes parametric survival models for OS and PFS to model health state membershipCitation18. The modelled population reflected the ZUMA-2 safety population in terms of patient characteristics at baseline (see Supplementary Table 1 for patient characteristics). The intervention KTE-X19 was compared to SoC, which contained multiple agents reflecting clinical practice (see ). In contrast to the model submitted to NICE, SoC efficacy was informed by the real-world study SCHOLAR-2, which was conducted in several European countriesCitation19 (see Supplementary Table 1 for patient characteristics) rather than by a literature-based meta-analysis. SoC composition in the SCHOLAR-2 study was not initially reportedCitation19, therefore, the SoC composition was informed by a literature-based meta-analysis of SoC used for the treatment of R/R MCL post BTKiCitation20, which agreed with the composition observed in the latest analysis of SCHOLAR-2 (see Supplementary Table 2). In the model, the SoC basket comparator contained cytotoxic chemotherapy (bendamustine, cytarabine and doxorubicin-hydrochloride), proteasome inhibitors (bortezomib), immunomodulatory drugs (lenalidomide, rituximab) and Bcl-2 protein inhibitors (venetoclax)Citation20. Although allogeneic stem cell transplantation was used as a consolidation strategy for patients with chemosensitive diseaseCitation3, it did not contribute to SoC as only a fraction of patients would be eligible due to its substantial toxicity and feasibility limitations including inadequate response to conditioning chemotherapy prior to consolidation with stem cell transplantation, inability to find a suitable stem cell donor and graft vs host diseaseCitation3,Citation21. KTE-X19 efficacy was informed by data analysis of the ZUMA-2 trial done at a median of 25.5 months of follow-up (Supplementary Figure 1), thus making use of longer follow-up data of the ZUMA-2 trial compared to the model reviewed by NICE. Mixture-cure modelling (MCM) methodology provided survival curves for KTE-X19 and standard parametric models provided survival curves for SoC to inform a three-state partitioned survival model. In line with UK guidelines, the cost-effectiveness model took a lifetime horizon and applied a 3.5% discount annually to both costs (2020 UK pound sterling, £) and health outcomesCitation22. The cycle length used was one month and a half-cycle correction was applied. The primary outcomes of the model were the incremental cost-effectiveness and cost-utility ratios (ICER and ICUR) of KTE-X19 versus SoC, calculated based on the cost per life year (LY) and cost per quality-adjusted life year (QALY), respectively.
Table 1. Model input data and sources.
Model structure
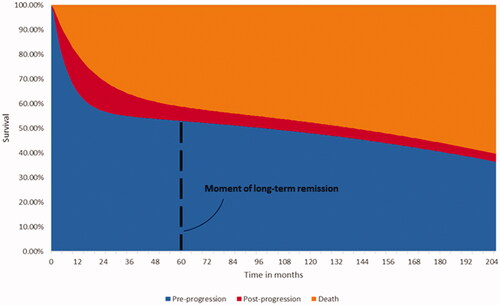
A partitioned survival model with health states for ‘pre-progression’, ‘post-progression’ and ‘death’ was developed. The model structure is presented in and further described in Simons, MaloneCitation23 where it was used in R/R MCL patients. Based on the 25.5 months ZUMA-2 trial data on PFS and OS (Supplementary Figure 1), the model estimated the transition of KTE-X19 patients through the health states by using MCM.
Survival
KTE-X19
Data from the 25.5 months follow-up of the safety population (n = 68) in the ZUMA-2 trial were used to model the efficacy of KTE-X19 (brexucabtagene autoleucel, Supplementary Figure 1), this population excluded patients who received conditioning chemotherapy but did not receive KTE-X19. MCM was fitted based on maximum likelihood and selected using the goodness of fit criteria including the Akaike information criterion (AIC) Citation24. The standard parametric models fitted demonstrated similar goodness of fit with the data. The MCM methodology was chosen to reflect the response dichotomy seen with the use of CAR T therapies, with a proportion of patients in the clinical trial setting appearing to show a deep and durable response with follow-up of 5-yearsCitation23,Citation25–29. Such models had been used in previous CAR T publications and aligned with recently published data suggesting that a proportion of R/R MCL patients may experience long-term remission after treatment with KTE-X19Citation16,Citation23,Citation25,Citation27,Citation28. The MCM methodology modelled survival conditional on this cure assumption by deriving a proportion of patients that are cured and at risk for dying only from background mortality (further described in Simons, MaloneCitation23) Survival in the non-cured proportion of patients who remain at risk of disease progression and death from R/R MCL was modelled by fitting standard parametric survival curvesCitation30,Citation31. The estimated percentages of patients experiencing long-term remission, as well as estimated median PFS and OS values, were consistent across the models. The exponential MCM was selected to extrapolate PFS and OS over the model’s lifetime horizon as it minimized AIC indicating the best fit with the trial data, and required the least number of parameters (see Supplementary Figure 1 and Supplementary Figure 2). To further reflect the presence of these latent groups, the model assumed that patients in the ‘pre-progression’ health state experience long-term remission after 5 years, which affected their utilities and healthcare resource use. This assumption has been applied in previous CAR T publications in other indicationsCitation28,Citation29. In a scenario analysis, the sex- and age-matched background mortality used in the OS curve was increased by a factor of 1.09 as described by Maurer et al.Citation32, to account for the possibility that prior treatment with chemotherapy and other treatments (such as SCT) may negatively impact survival in patients in R/R MCL post-BTKi long-term remission.
Standard of care
Survival outcomes with SoC were estimated based on data from a real-world study in patients with R/R MCL (SCHOLAR-2, N = 59) in France, the UK, Germany, Italy, Spain, Sweden and DenmarkCitation19. SCHOLAR-2 was a retrospective chart review study designed to collect data on the outcomes of adult patients with R/R MCL who received active treatment in the routine care setting after failing or being intolerant to BTKi therapyCitation19. The primary objective of SCHOLAR-2 was to estimate OS among real-world patients in the post-BTKi setting. OS was measured from the cohort index date to the date of death from any cause; patients who were known to be alive or had been lost to follow-up were censored at the last date they were known to be alive.
Standard parametric models, adjusting for background mortality, were fitted to the OS data. All curves except for the gamma curve converged. The exponential curve was chosen for the extrapolation of OS over time based on the shape of the distribution which fitted well with the entire trial data, and considering the goodness of fit statistics indicated the exponential curve provided the second-best fit after the Weibull curve which showed less good fit in the tail of the curve (see Supplementary Figure 3). PFS data was not collected in SCHOLAR-2, so to estimate PFS with SoC, a hazard ratio adjustment was applied to the OS survival estimate. This ratio of 0.727 was informed based on the OS and PFS results of a previously conducted literature-based meta-analysis in R/R MCLCitation20. The ratio was assumed to be constant over time (see Supplementary Figure 4).
A propensity score-matched analysis was conducted to reduce differences in patient characteristics between the ZUMA-2 and SCHOLAR-2 studiesCitation19. SCHOLAR-2 patients were matched to the ZUMA-2 safety cohort by means of a logistic regression model predicting treatment allocation. The model included patient gender, age, number of prior treatments, prior auto-SCT, prior BTKi duration and response to BTKi, and stage four R/R MCL as covariates, with the choice of covariates being determined based on clinical relevance, availability, and statistical considerations such as the overlap of distributions of propensity scores and sufficient variabilityCitation19 (see Supplementary Table 1 for matched patient characteristics). This reduced the effective sample size to 40.1 and resulted in only minor changes to the OS curve as the matched OS curve shifted slightly downwards during the first 20 months, aligned with the unmatched curve between 20 and 32 months of follow-up and then shifted slightly downwards again during the last months of follow-up (see Supplementary Figure 5). This implies that the outcomes of the matched SCHOLAR-2 population were similar or somewhat inferior to those of the unmatched SCHOLAR-2 population. Likewise, the hazard ratio between SCHOLAR-2 and ZUMA-2 before matching (0.37, confidence interval [CI] 0.20; 0.66) was higher compared to the hazard ratio after matching (0.33, CI 0.18; 0.59). As a conservative approach, the unmatched SCHOLAR-2 data was used in the economic modelCitation19.
Cost and resource use
The model considered costs of treatment with KTE-X19, costs of SoC treatment, adverse event (AE) costs, and costs related to disease management and end of life.
KTE-X19 and related costs and resource use
Costs of treatment with KTE-X19 included drug acquisition cost based on the manufacturer’s list price of £316,118 for a single infusion of 2 × 106 anti-CD19 CAR T cells/kgCitation14, along with leukapheresis, conditioning and bridging therapy and hospitalization costs, leading to total costs per KTE-X19 administration of £341,003. Conditioning chemotherapy was given prior to the administration of KTE-X19 to induce lymphodepletion and consisted of fludarabine (30 mg/m2 intravenous [IV]) and cyclophosphamide (500 mg/m2 IV)Citation14. Conditioning chemotherapy was assumed to occur in the inpatient setting. Based on ZUMA-2 trial data, hospitalization lasted 21.2 days (see Supplementary Table 3) and consisted of 22.7% of days in the intensive care unit (ICU), and 77.3% of days in non-ICU careCitation25. A proportion of patients received bridging therapy for disease control during CAR T manufacturing, the proportion of patients was obtained from the ZUMA-2 trial (36.8%)Citation14. Costs of bridging therapy with a cycle of rituximab, bendamustine and cytarabine between leukapheresis and KTE-X19 infusion were included to reflect the most common clinical practice in the UKCitation17.
A retreatment cost item was included to account for retreatment with conditioning chemotherapy in 7.4% of patients (see Supplementary Table 3), it was assumed that the second round of leukapheresis was not costed. All model input values and sources relating to costs and resource use with KTE-X19 treatment are summarized in .
Standard of care and related costs and resource use
The treatment regimens included in SoC are outlined in based on the definition of SoC in the literatureCitation20, which had been validated by UK clinical experts. The literature-based definition of SoC aligned with the treatments used in the SCHOLAR-2 study, which are presented in Supplementary Table 2. Costs of SoC consisted of acquisition costs based on British National Formulary (BNF, May 2021) costsCitation33. Administration cost applied to IV treatments and was informed by 2018–2019 NHS reference costs for IV administration of simple parenteral chemotherapy at first attendanceCitation34. Drug dosage and treatment duration for each treatment were taken from the respective guidance, except for venetoclax, for which there was no indicated posology for the treatment of MCL, whereby the average maximum dose in Eyre, FollowsCitation5 was used. Dose intensity of 100% with no vial sharing was generally assumed, a dose intensity of 70% was explored in a scenario analysisCitation35. All model input values and sources relating to costs and resource use with SoC treatment are summarized in .
Disease management costs and resource use
Evidence on health state-related health care resource utilization was not collected in the ZUMA-2 trial and was assumed to be equal to the health state-related health care resource utilization reported in the NICE technology appraisal for ibrutinib for R/R MCL [TA502] Citation36. Evidence used in the appraisal stemmed from a survey amongst active practicing hematologists and oncologists in the NHS and was validated by UK hematologic experts. outlines the cost in the pre-progression and post-progression health states. Health state-related costs were assumed independent of treatment and applied until the patient died or reached long-term remission. Long-term remission was assumed to commence after 60 months in pre-progression. The resource use of patients in long-term remission was assumed to include two doctor’s visits per year at a unit price of £171.46 per visit, see Supplementary Table 3 for details.
Adverse events
AEs of grade 3 or 4 with an incidence of ≥5% based on the ZUMA-2 trial were modelled for KTE-X19 (see Supplementary Table 4, additional details on AEs in Supplementary Table 5). Additionally, cytokine release syndrome (CRS) of grade ≥2 (61.8% of patients) was modelled by assuming treatment with tocilizumab, CRS-related hospitalization in the ICU was assumed to be included in the ICU care component of KTE-X19 administration. AEs were costed in line with NHS reference costsCitation34, hypogammaglobulinemia (20.6% of patients) was assumed to be treated with 0.5 g/kg of IV immunoglobulin every 4 weeks for 12 months, and BNF prices were appliedCitation33. Although AEs occurred with the individual SoC treatment components, limited safety data were identified from the literature and consequently, no AEs were modelled for SoC as a conservative assumption.
End of life costs
Costs relating to intensified care towards the end of life are applied to all patients irrespective of their cause of death and prior treatment. A fixed cost of £16,539 was applied reflective of 37 days of palliative care at a cost of £447 per day (see )Citation37,Citation38.
Utilities
In absence of utility values for R/R MCL patients post BTKi, health state utilities from the ibrutinib NICE submission in R/R MCL overall are used and applied irrespective of treatment. The utility of patients experiencing long-term remission was assumed to be equal to an age-matched UK general population and was informed by published estimates (see )Citation39. Pre-progression and post-progression utilities were adjusted not to exceed the utility of age- and gender-matched UK population. QALYs were derived from the multiplication of each health state’s utility and the time spent in the health stateCitation36.
Utility decrements were used to reflect the impact of AEs following the treatment with KTE-X19. These were calculated based on the incidence of the AE, the applicable utility decrement, and the duration of the AE. Decrements and duration were informed by patient-level data of the ZUMA-1 trial used in the NICE assessment of axicabtagene ciloleucel for the treatment of diffuse large B-cell lymphoma and primary mediastinal B-cell lymphoma after 2 or more systemic therapiesCitation25. The incidence of the AEs was informed by the safety population of the ZUMA-2 trial (see Supplementary Figure). In case no disutility was identified for an AE, the highest disutility of the remaining AEs (excluding CRS) was used as a proxy. Decrements were applied during the first model cycle, and in parallel to KTE-X19 administration.
Outcomes
The outcomes of treatment with KTE-X19 vs. SoC in terms of OS expected LYs, QALYs and total costs were calculated and presented alongside incremental costs and effects, and the ICER and ICUR. All presented results are discounted and considered a lifetime horizon.
Uncertainty analyses
In addition to deterministic results, results of probabilistic sensitivity analysis are presented where the values of model parameters were varied simultaneously within their individual uncertainty distributions. In case distributional information was not available from the original source, a standard error of 20% was used as a proxy.
A univariate sensitivity analysis was conducted where model parameters were varied one at a time to the lower and upper 95% confidence interval. Results of the analysis are presented in tornado diagrams including the ten most influential parameters and depicting their impact on the incremental costs, effects and the ICUR. The probabilistic sensitivity analysis is presented on a cost-effectiveness plane and the probability that each treatment is cost-effective at different levels of willingness-to-pay per QALY is presented using a cost-effectiveness acceptability curve (CEAC).
Scenario analyses
Scenario analyses were conducted to explore the main assumptions of the model, which included:
Removing the long-term remission assumption regarding utilities and costs, and adjusting the long-term remission point to 2 years and 10 years,
Use of SCHOLAR-2 survival data matched to the ZUMA-2 safety set,
Use of ZUMA-2 survival data based on the intention-to-treat set i.e. including patients who received conditioning chemotherapy but did not receive KTE-X19 (see Supplementary Figures 6 and 7, and Supplementary Table 6),
Use of ZUMA-2 survival data based on the intention-to-treat set and use of SCHOLAR-2 survival data matched to the ZUMA-2 ITT set, (see Supplementary Figures 6 and 7)
Use of standard parametric survival models for KTE-X19,
Inclusion of an adjustment of the background mortality,
Lowering discount rates to 1.5% for health outcomes and costs,
Assuming KTE-X19 acquisition and leukapheresis costs apply upon retreatment with KTE-X19,
Assuming a 70% dose intensity for SoC treatments,
Increasing the costs of neurotoxic AEs encephalopathy and confusional state,
Reducing the time horizon to 10, 20 and 30 years.
Results
Base case results
outlines the deterministic base case results. Survival at 24 months was 66.8% with KTE-X19 and 39.2% with SoC, and survival at 60 months was 52.0% and 9.3%, respectively. The total discounted LYs were larger for KTE-X19 than for SoC, with 8.27 and 1.98 LYs, respectively. Similarly, the total QALYs were 5.99 and 1.48, respectively, with an incremental QALY of 4.52. Survival and QALYs, as well as incremental benefits with KTE-X19, were largely accrued in the pre-progression health state. Total costs were higher for KTE-X19 (£385,765) than for SoC (£79,742), driven by drug acquisition costs of KTE-X19. Acquisition costs contributed 82% and 64% of KTE-X19 and SoC total costs and represented 87% of the incremental costs. The resulting ICUR was £67,713/QALY gained and the resulting ICER was £48,645/LY gained.
Table 2. Base case results (discounted).
Uncertainty analyses
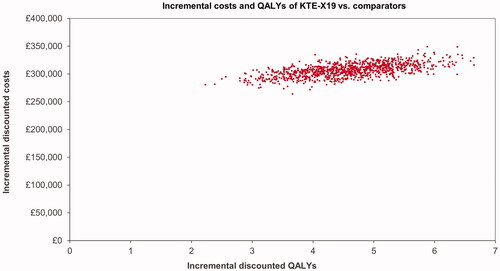
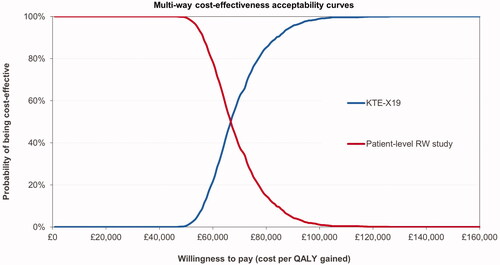
Results of the probabilistic sensitivity analysis showed costs and effects comparable to the deterministic results, see . The resulting ICUR was £68,733. The CEAC shows the probability of KTE-X19 being cost-effective at different willingness-to-pay thresholds; see .
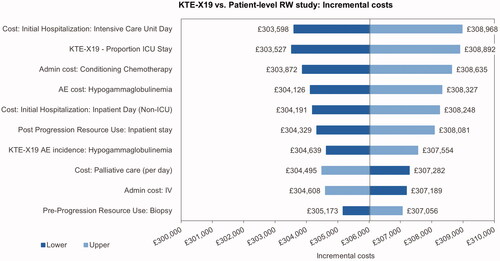
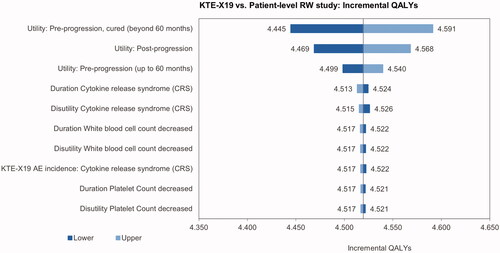
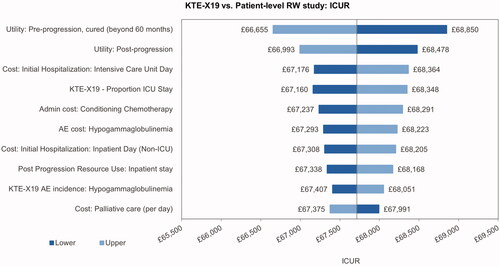
The univariate sensitivity analysis indicated that the incremental cost results were most sensitive toward the ICU day costs, the proportion of ICU days, the costs of conditioning chemotherapy and the cost of treatment for hypogammaglobulinemia (). The main driver of the incremental utilities was the pre-progression utility for patients with long-term remission, followed by the utility for post-progression and pre-progression prior to long-term remission (). The most influential parameters on the ICUR were the pre-progression utility for patients in long-term remission and the utility post-progression ().
Scenario analyses
Detailed results of all scenario analyses can be found in Supplementary Table 7. The most impactful scenario analysis on the ICUR included the use of standard parametric survival curves for the modelling of KTE-X19 effectiveness, which increased the ICUR by £13,566. Reducing the time horizon to 10 years increased the ICUR to £131,804 and reducing discount rates decreased the ICUR by £12,804. The remaining scenarios impacted the ICUR by less than 10%.
Discussion
The analyses investigated the comparative effectiveness and cost-effectiveness of KTE-X19 (brexucabtagene autoleucel) versus SoC for the treatment of patients with R/R MCL post-BTKi in England. This cost-effectiveness model suggests that in real-world use, compared with SoC, KTE-X19 may extend median survival by 4.58 years (5.96 vs. 1.38 years) and result in 4.52 additional discounted QALYs over a lifetime horizon (5.99 vs 1.48 QALYs). The majority of these gains (85%) were made pre-progression. The incremental costs of KTE-X19 were £306,023, with drug acquisition costs of KTE-X19 being the main cost driver. The cost per QALY gained was £67,713 and the cost per LY gained was £48,645. The results provide evidence that KTE-X19 may be a valuable use of resources for the treatment of R/R MCL post-BTKi in England with the potential to provide significant QALY gains at an acceptable cost level. Sensitivity analyses showed the robustness of the results against changes in model parameters and alternative assumptions.
The utility of patients in long-term remission had the largest impact on health outcomes and on the ICUR. This can be explained by the difference in proportions of patients in long-term remission between KTE-X19 and SoC, which determines that changes in long-term remission utilities promptly translate into changes in incremental QALYs. The cost per ICU day had the largest impact on incremental costs and the third largest impact on the ICUR, explained by the fact that ICU costs only apply to the KTE-X19 arm whereby any changes in ICU costs translate into changes in incremental costs. Scenario analyses indicated that results were stable with the use of most alternative assumptions, the use of shorter time horizons increased the ICER, and the use of standard parametric survival curves for KTE-X19 and the use of lower discount rates had a larger impact on the ICUR, increasing it by £13,500 and decreasing it by £13,000, respectively.
Previous studies have assessed the cost-effectiveness of CAR T in other indications and in other settingsCitation23,Citation28,Citation29,Citation40–42, whereby this is the first cost-effectiveness analysis of a CAR T therapy in R/R MCL in England. A previous US-based cost-effectiveness analysis of KTE-X19 for the treatment of R/R MCL using post-BTKi data had shown survival outcomes in the form of 8.99 LYs and 7.39 QALYs, and 4.52 incremental LYs and 3.74 incremental QALYs obtained with KTE-X19 treatment compared to SoCCitation23. In line with the findings of the present study, a previous cost-effectiveness analysis of axicabtagene ciloleucel in adult patients with R/R large B-cell lymphoma following two or more prior therapies showed a substantial survival gain with CAR T treatment in the form of 6.90 incremental LYs and 6.54 incremental QALYs gainedCitation28. ICERs were not compared due to the differences in costs and other settings as the analyses were US-based. Similar assumptions were made regarding patients in long-term remission after 5 years being subject to age- and sex-matched general population mortality. The estimated incremental costs closely align with estimates of other recent cost-effectiveness analysis of CAR T therapiesCitation43.
This analysis used uniquely granular inputs which contribute to the robustness of the results. Deterministic, probabilistic and scenario analyses confirmed the results of the base case analysis, and showed the results were relatively stable with regard to changes in inputs and assumptions. The valuable insights into the comparative effectiveness and cost-effectiveness of KTE-X19 versus SoC support the advancement of treatment in this population of R/R MCL patients post-BTKi for whom SoC offers very limited hope.
The study is also subject to uncertainties and limitations. The extrapolation of short-term results over a lifetime horizon inherently introduces uncertainty into the results. Long-term survival outcomes with KTE-X19 are subject to substantial uncertainty considering the relatively low sample size (N = 68), the limited duration of follow-up of the trial data (median of 25.5 months), and the lack of long-term clinical experience with KTE-X19. The results of the analysis should be confirmed with long-term follow-up data and data from real-world application of KTE-X19.
Patients enrolled in ZUMA-2 had failed multiple prior therapies and a placebo control arm would have been deemed unethical given their poor prognosis. SCHOLAR-2 data was used for the analysis of SoC effectiveness in the absence of prospective SoC OS and PFS data in the post-BTKi setting. While the SoC survival outcomes are more mature and better validated against clinical experience than the ZUMA-2 outcomes, SoC survival outcomes should be cautiously interpreted considering the low sample size of SCHOLAR-2 (N = 59). Additionally, the use of SCHOLAR-2 data for survival with SoC treatment prevented an anchoring of the treatment comparison between KTE-X19 and SoC. The unanchored and unadjusted comparison between the treatments gives rise to potential selection bias stemming from potential differences in population characteristics, which may prevent a fair comparison between KTE-X19 and SoC and impact the relative treatment effect. A scenario analysis using propensity score-matched SCHOLAR-2 survival data showed similar results with somewhat lower SoC health outcomes and costs, and an ICUR of £66,760/QALY, indicating the adjustment of key patient characteristics between ZUMA-2 and SCHOLAR-2, including age and number of prior treatments, had little impact on the results. A further limitation and potential source of bias is that the composition of SoC treatment and thus the costs of SoC treatment acquisition was based on a literature-based meta-analysis, while the effectiveness of SoC was obtained from the SCHOLAR-2 study.
While in line with cure assumptions made in previous CAR T models, a universally accepted definition of long-term remission is lacking in R/R MCL, and uncertainty remains surrounding the 5-year cut-off used. Maurer, GhesquièresCitation32 suggested that in patients with diffuse large B-cell lymphoma, event-free survival 24 months after the completion of first-line treatment should be considered a robust end point for the disease-related outcome. No definition of cure in MCL was identified, and clinical expert opinion indicated that a 24-months cut-off would likely not apply to MCL due to the occurrence of late relapses. Given the lack of evidence, assumptions were made regarding the utility and costs of long-term remission. These assumptions were explored in sensitivity analyses, which showed a limited impact of long-term remission utility and cost on the absolute outcomes. A scenario analysis removed the long-term remission assumption and increased the ICUR from £67,713 to £73,203. Clinical evidence on the timing of long-term remission post CAR T treatment for R/R MCL and its impact on costs and utility would be welcomed to address these evidence gaps.
Omitting AEs with SoC as a conservative assumption in the absence of evidence likely underestimates costs of SoC, and to a lesser degree, overestimates SoC QALYs. This likely increases the incremental costs of KTE-X19 and inflates ICER and ICUR.
Conclusions
KTE-X19 (brexucabtagene autoleucel) for the treatment of R/R MCL post-BTKi has shown significant survival benefits over SoC based on clinical and real-world survival data. This cost-effectiveness model adds to the growing evidence supporting KTE-X19 use in R/R MCL, and further research is warranted to address uncertainties regarding the long-term survival and long-term remission of R/R MCL patients post-BTKi treated with KTE-X19.
Transparency
Declaration of funding
This study was funded by Kite, a Gilead Company.
Declaration of financial/other interests
SP is employed by OPEN Health Company and is a consultant for or has consulted for Kite, a Gilead Company, Galderma, PTC therapeutics, Bristol Myers Squibb, Celgene Ltd, a Bristol Myers Squibb Company, GlaxoSmithKline, Otsuka, Merck & Co, and AbbVie in the past two years.
CLS is employed by OPEN Health Company and is a consultant for or has consulted for Kite, a Gilead Company, Galderma, PTC therapeutics, Takeda, Bristol Myers Squibb, EMD Serono, Grifols and AbbVie in the past two years.
CB is employed by OPEN Health Company and is a consultant for or has consulted for Kite, a Gilead Company, AbbVie, Amgen, TEVA, Takeda, PTC therapeutics, Galderma, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Tesaro, Gilead and Otsuka in the past two years.
GC is an employee of Kite, a Gilead Company, was employed previously by Amgen, Mundipharma and Janssen Cilag.
JW is an employee of Kite, a Gilead Company, was employed previously by Amgen, Abbott, Genentech and Elan, and is a stakeholder of Gilead, Amgen, Abbott, AbbVie, Avid Bioservices, Curis, Moderna and Viracta Therapeutics.
SW is employed by Wade Outcomes Research and Consulting and is a consultant for or has consulted for Kite, a Gilead Company, AbbVie/Allergan, Johnson & Johnson, and Amgen in the past 2 years.
RS is an employee of Kite, a Gilead Company and is a stakeholder of Gilead and Amgen.
IK is an employee of Kite, a Gilead Company and is a stakeholder of Gilead.
WP is an employee of Kite, a Gilead Company.
GS has been advising Abbvie, Beigene, BMS/Celgene, Debiopharm, Epizyme, Genentech/Roche, Genmab, Incyte, Ipsen, Janssen, Kite/Gilead, Loxo, Milteniy, Morphosys, Novartis, Rapt, Regeneron, Takeda and Velosbio in the past two years.
MW has been advising Pharmacyclics, Celgene, Janssen, AstraZeneca, MoreHealth, Pulse Biosciences, Nobel Insights, Guidpoint Global, InnoCare and BioInvent over the past two years.
GH has been advising Janssen, Genmab, Gilead/Kite, AstraZeneca, Lilly, Roche, Abbvie and Morphosys/Incyte over the past two years.
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Author contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this manuscript.
Supplemental Material
Download MS Word (840.2 KB)Acknowledgements
The authors would like to thank Nour Chami, (employee of OPEN Health) for the collection of costing inputs used in this study. The authors would also like to thank Greg Maglinte for his contributions during the conception and design of this work.
References
- Smith A, Crouch S, Lax S, et al. Lymphoma incidence, survival and prevalence 2004-2014: sub-type analyses from the UK's haematological malignancy research network. Br J Cancer. 2015;112(9):730–1584.
- McKay P, Leach M, Jackson B, et al. Guideline for the management of mantle cell lymphoma. Br J Haematol. 2018;182(1):46–62.
- NICE. Non-Hodgkin’s lymphoma: diagnosis and management [NG52]. 2016.
- Rule S. The modern approach to mantle cell lymphoma. Hematol Oncol. 2019;37(S1):66–69.
- Eyre TA, Follows G, Hodson A, et al. Efficacy of venetoclax monotherapy in patients with relapsed, refractory mantle cell lymphoma after Bruton tyrosine kinase inhibitor therapy. haematologica. 2019;104(2):e71–e71.
- Cohen BJ, Moskowitz C, Straus D, et al. Cyclophosphamide/fludarabine (CF) is active in the treatment of mantle cell lymphoma. Leuk Lymphoma. 2001;42(5):1015–1022.
- Wang M, Schuster SJ, Phillips T, et al. Observational study of lenalidomide in patients with mantle cell lymphoma who relapsed/progressed after or were refractory/intolerant to ibrutinib (MCL-004). J Hematol Oncol. 2017;10(1):171.
- Arcaini L, Lamy T, Walewski J, et al. Prospective subgroup analyses of the randomized MCL-002 (SPRINT) study: lenalidomide versus investigator's choice in relapsed or refractory mantle cell lymphoma. Br J Haematol. 2018;180(2):224–235.
- Smith A, Roman E, Appleton S, et al. Impact of novel therapies for mantle cell lymphoma in the real world setting: a report from the UK's Haematological Malignancy Research Network (HMRN). Br J Haematol. 2018;181(2):215–228.
- Kumar A, Sha F, Toure A, et al. Patterns of survival in patients with recurrent mantle cell lymphoma in the modern era: progressive shortening in response duration and survival after each relapse. Blood Cancer J. 2019;9(6):50.
- Reyes Arranz Jose Luis B, Miguel Ángel C, et al. editors. Management of relapsed/refractory mantle cell lymphoma (MCL) in routine clinical practice in Spain (IMORS study). Descriptive Data and Efficacy Results; 2018. Available from: https://library.ehaweb.org/eha/2018/stockholm/216049/reyes.arranz.management.of.relapsed.refractory.mantle.cell.lymphoma.28mcl29.in.html?f=menu=6*ce_id=1346*ot_id=19046*media=3*marker=168
- McCulloch R, Lewis D, Crosbie N, et al. Ibrutinib for mantle cell lymphoma at first relapse: a United Kingdom real-world analysis of outcomes in 211 patients. Br J Haematol. 2021;193(2):290–298.
- McCulloch R, Rule S, Eyre TA, et al. Ibrutinib at first relapse for mantle cell lymphoma: a United Kingdom real world analysis of outcomes in 169 patients. Blood. 2019;134(Supplement_1):3993–3993.
- Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382(14):1331–1342.
- Wang ML, Munoz J, Goy A, et al. editors. KTE-X19, an Anti-CD19 Chimeric Antigen Receptor (CAR) T Cell Therapy, in Patients (Pts) With Relapsed/Refractory (R/R) Mantle Cell Lymphoma (MCL): Results of the Phase 2 ZUMA-2 Study. ASH; 2019. Blood.
- Wang Y, Lin R, Xu L-P, et al. One-Year follow-up of ZUMA-2, the multicenter, registrational study of KTE-X19 in patients with relapsed/refractory mantle cell lymphoma. Blood. 2020;136(Supplement 1):20–22.
- NICE. Autologous anti-CD19-transduced CD3+ cells for treating relapsed or refractory mantle cell lymphoma. 2021.
- NICE Decision Support Unit. NICE DSU Technical Support Document 19 Partitioned Survival Analysis for Decision Modelling in Health Care: A Critical Review; 2017.
- Hess G, Dreyling M, Oberic L, et al. editors. KTE-X19 versus standard of care for relapsed/refractory mantle cell lymphoma previously treated with Bruton tyrosine kinase inhibitors: Real-World evidence from Europe. EHA; 2021.
- Dreyling M, Shah B, Wu JJ., et al. Efficacy Outcomes Following Treatment with BTKi for Relapsed/Refractory Mantle Cell Lymphoma (R/R MCL): A Literature-Based Meta-Analysis. Abstract accepted, ISPOR library; 2022-05;SA29 [Internet]. Available from: https://www.ispor.org/heor-resources/presentations-database/presentation/intl2022-3461/116051
- Gauthier J, Maloney DG. Allogeneic transplantation and chimeric antigen receptor-engineered T-cell therapy for relapsed or refractory mantle cell lymphoma. Hematol Oncol Clin North Am. 2020;34(5):957–970.
- NICE. Guide to the methods of technology appraisal 2013. Available from: https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781.
- Simons CL, Malone D, Wang M, et al. Cost-effectiveness for KTE-X19 CAR T therapy for adult patients with relapsed/refractory mantle cell lymphoma in the United States. J Med Econ. 2021;24(1):421–431.
- Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19(6):716–723.
- NICE. Axicabtagene ciloleucel for treating diffuse large B-cell lymphoma and primary mediastinal B-cell lymphoma after 2 or more systemic therapies [ID1115]. 2018.
- Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398(10299):491–502.
- NICE. Tisagenlecleucel-T for treating relapsed or refractory diffuse large B-cell lymphoma [ID1166]: Committee Papers. 2018.
- Roth JA, Sullivan SD, Lin VW, et al. Cost-effectiveness of axicabtagene ciloleucel for adult patients with relapsed or refractory large B-cell lymphoma in the United States. J Med Econ. 2018;21(12):1238–1245.
- ICER. CAR-T therapies: final evidence report. [Internet]; ICER. 2018 [cited 2021 Dec 9]. Available from: https://icer-review.org/material/car-t-final-report/.
- Lambert PC, Dickman PW, Weston CL, et al. Estimating the cure fraction in population-based cancer studies by using finite mixture models. J Royal Stat Soc C. 2010;59(1):35–55.
- Rutherford MJ, Lambert PC, Sweeting MJ, et al. NICE DSU technical support document 21. Flexible Methods for Survival Analysis. [Internet]; 2020 [cited 2021 Dec 14]. Available from: http://nicedsu.org.uk/.
- Maurer MJ, Ghesquières H, Jais J-P, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol. 2014;32(10):1066–1073.
- British National Formulary. [Internet]; [cited 2021 Oct 8]. Available from: https://bnf.nice.org.uk.
- NHS. NHS National Reference Cost schedule 2018-19 2019. Available from: https://www.england.nhs.uk/national-cost-collection/#ncc1819.
- Hess G, Herbrecht R, Romaguera J, et al. Phase III study to evaluate temsirolimus compared with investigator's choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27(23):3822–3829.
- NICE. Ibrutinib for treating relapsed or refractory mantle cell lymphoma. Technology appraisal guidance [TA502] – Committee Papers. 2016.
- Bennett MI, Ziegler L, Allsop M, et al. What determines duration of palliative care before death for patients with advanced disease? A retrospective cohort study of community and hospital palliative care provision in a large UK city. BMJ Open. 2016;6(12):e012576.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2020; 2021. Available from: https://www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2020/.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health. 2010;13(5):509–518.
- Hettle R, Corbett M, Hinde S, et al. Review of cost-effectiveness evidence for chimeric antigen receptor T-cell therapy and other interventions for acute lymphocytic leukaemia. [Internet]. The assessment and appraisal of regenerative medicines and cell therapy products: an exploration of methods for review, economic evaluation and appraisal. NIHR Journals Library. 2017 [cited 2021 Aug 11]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK424721/.
- Sarkar RR, Gloude NJ, Schiff D, et al. Cost-Effectiveness of chimeric antigen receptor T-Cell therapy in pediatric relapsed/refractory B-Cell acute lymphoblastic leukemia. J Natl Cancer Inst. 2019;111(7):719–726.
- Liu R, Snider JT, Diakite I Cost effectiveness of axicabtagene ciloleucel (axi-cel) and tisagenlecleucal (tisa-cel) for adult patients with relapsed or refractory large B-cell lymphoma (RR LBCL) in the US. EHA library; 06/12/20; 294211; EP1731. 2020, et al. 2020.
- Heine R, Thielen FW, Koopmanschap M, et al. Health economic aspects of chimeric antigen receptor T-cell therapies for hematological cancers: present and future. Hemasphere. 2021;5(2):e524.