Abstract
Aims
Use of comprehensive genomic profiling (CGP) in metastatic colorectal cancer (mCRC) is limited. We estimated impacts of expanded 1 L CGP, using the Tempus xT test, on detection of actionable alterations and testing budgets in a modeled US health plan over two-years.
Materials and methods
A decision analytic model was developed to estimate the impact of replacing 20% of usual testing (a mix of CGP and non-CGP) with Tempus xT CGP. Actionable alterations for matched treatments or clinical trial included KRAS, NRAS, RAF, BRAF, deficient mismatch repair (dMMR)/microsatellite instability (MSI), NTRK, RET, EGFR, HER2, MET, PIK3CA and POLE1. Costs included initial and repeat testing, physician-associated and administrative costs.
Results
In a hypothetical five-million-member plan, 50% Medicare and 50% commercial, 1,112 new cases of mCRC were expected per year. Of these, 566 (51%) would undergo 1 L molecular testing, with 55 re-tested upon progression. Based on current testing rates, there were an expected 521 missed opportunities for genomically informed treatment (47% of new cases), with 442 missed due to lack of testing and 79 due to testing without CGP. Replacing 20% of usual testing with Tempus xT CGP was associated with up to a $0.003 per member per month testing cost increase (net total cost of $202,102 for the five-million-member plan) and 15.5 additional patients with an opportunity for genomically informed care (12.7 patients for treatment and 2.8 for clinical trial). The testing total cost (initial test, repeat test, biopsy and physician services, and administrative cost) to put one additional patient with mCRC on matched therapy or matched clinical trial was estimated to be $13,005. Number needed to test to identify one actionable alteration with Tempus xT CGP versus usual testing was 7.8 patients.
Limitations
Conservative assumptions were made for inputs with limited evidence. Based on high concordance rates with dMMR/MSI status, tumor mutational burden (TMB) status was not calculated separately.
Conclusions
Replacing 20% of usual testing with Tempus xT CGP was associated with a small incremental testing cost and can identify meaningfully more actionable alterations.
Introduction
Colorectal cancer (CRC) is the fourth most common cancer in the United States (US), with annual incidence estimated at 147,950 casesCitation1. Approximately, 22% of CRC patients present in the advanced/metastatic stage, with an expected five-year survival of 14.7%Citation1. A growing number of genomic alterations inform treatment of advanced/metastatic colorectal cancer (mCRC). Identifiable oncogenic drivers for mCRC include KRAS, NRAS, BRAF, NTRK, RET, EGFR, HER2, MET, PIK3CA, POLE1, deficient mismatch repair (dMMR), microsatellite instability (MSI), and tumor mutation burden (TMB) statusCitation2. A study which assayed over 4,000 advanced CRC cases from 2012 to 2015 using comprehensive genomic profiling (CGP) estimated that 73.4% of patients have tumors harboring potentially actionable driver alterationsCitation3. This percentage is likely to be higher now and to continue to rise, given further research on alterations and novel therapies.
Characterizing tumors by identifying genomic alterations has become increasingly important to improving patient care in mCRC, creating opportunities to personalize therapies to patient tumor genetics or to avoid treatments known to be ineffective for patients with specific markers. In mCRC patients, a common example is testing for dMMR/MSI-H status to determine whether immune checkpoint inhibitor therapy can be an optimal treatment (optimal as defined by patient health outcomes)Citation4. Additionally, KRAS, NRAS, or BRAF mutation status information helps patients avoid treatment with unresponsive anti-epidermal growth factor receptor (EGFR) antibody therapy including cetuximab or panitumumabCitation5–14. As genomically informed treatment options continue to expand, it is likely to be increasingly important to capture genetic markers via broad comprehensive genomic profiling (CGP) that enables simultaneous examination of multiple actionable genetic alterations, thereby informing optimal treatment selection and providing additional options for clinical trial enrollmentCitation2,Citation15,Citation16 CGP assays use next-generation sequencing (NGS) to detect and quantify relevant genetic markers.
However, molecular testing is currently not conducted universally, despite NCCN guidelines recommending RAS and BRAF testing for all patients with mCRCCitation17,Citation18, and the use of CGP as a proportion of overall testing is low. As a result, many mCRC patients who are candidates for alteration-targeted therapy have been “undergenotyped”, meaning they are at risk of receiving ineffective anti-EGFR therapy in the first-line (1 L) setting and missing the opportunity to receive appropriate immunotherapy optionsCitation17. A recent study conducted in Italy on testing costs evaluated that an NGS-based approach may be less costly than a single-gene testing approach for advanced NSCLC and metastatic CRCCitation19. A US-based analysis found similar results, where NGS was found to have the lowest total cost of testing compared with PCR-based testing methods and also associated with the fastest time to appropriate targeted therapy initiation for newly diagnosed metastatic NSCLC patientsCitation20. The total cost of care for mCRC in the US is very high, estimated at $140,260 (2020 USD) per patient per year, including genomically-matched or unmatched targeted therapy, chemotherapy and medical costsCitation21,Citation22. Given the large economic burden of mCRC treatment, there may be opportunities to improve the economics of care and expand access to clinical trials through genomically informed treatment with CGP-based precision medicine.
The Tempus xT CGP assay is a laboratory developed test, with hybrid capture NGS-based targeted oncology panel which interrogates 648 genes and performs genome-wide unbiased fusion detection, while also assessing MSI status and TMBCitation16,Citation23. Using a small formalin-fixed, paraffin-embedded (FFPE) tissue sample along with blood testing, the xT CGP assay provides a comprehensive overview of somatic genomic alterations for patients diagnosed with mCRC.
CGP and other NGS methods were historically high cost, although advances in technology have reduced this cost to allow routine use where appropriate. Cost-effectiveness evidence for NGS in mCRC has not been directly investigated to our knowledge. Economic modeling can help inform estimates of financial and other consequences, allowing decision-makers to assess the budget impact and potential value of increased penetration of new technologies. The objective of this model was to estimate impacts associated with expanded 1 L CGP testing (via Tempus xT), compared with standard testing, on the testing cost budget, as well as detection of currently actionable alterations (actionable s matched therapy or matched clinical trial) in mCRC in a hypothetical five-million member US commercial and Medicare health plan.
Methods
Model overview and design
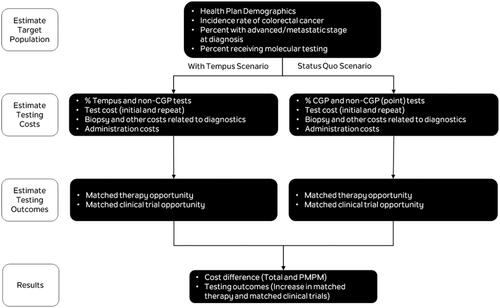
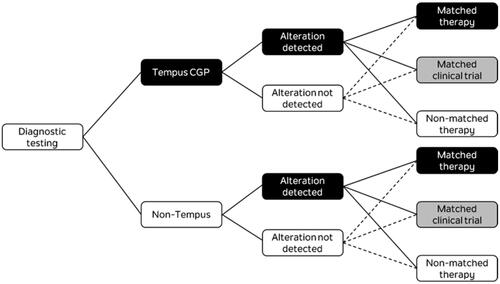
A decision analytic model was developed to compare two 1L mCRC molecular testing scenarios and estimate the budget impact of increased utilization of CGP using the 648-gene, NGS-based Tempus xT targeted oncology panelCitation16,Citation23. The model was used to estimate diagnostic testing costs and opportunities for genomically informed matched therapy or matched clinical trials over a two-year time horizon for a hypothetical health plan covering five million lives. After estimating the incident number of mCRC cases in the hypothetical health plan, the target population was run through two separate testing scenarios () and the decision tree shown in . The base case analysis estimated the annual cost to a health plan of replacing 20% of the current average mix of molecular testing (represented by a mix of CGP and non-CGP diagnostics) with Tempus xT CGP, with 20% estimated to be a reasonable number for uptake of the Tempus test over a 2-year period.
Testing costs were estimated and compared in both scenarios to calculate the budget impact from testing. This analysis considered costs for diagnostic testing (both initial and repeat), biopsy and physician services related to testing, and administrative costs. Costs were inflation-adjusted to 2021 USD where necessary, using the Consumer Price Index for Medical Services in the USCitation22.
A two-year time horizon (with no population growth) was chosen, to reflect the time it takes for repeat testing to reach a steady state as a proportion of initial testing. The average patient was assumed to be diagnosed and tested halfway through the year, and a simplifying assumption was made that their first 6 months with disease occurred in their first year of diagnosis, and months 7–18 occurred in their second year. Across scenarios (status quo scenario and scenario with Tempus CGP testing), the overall rate of testing was unchanged. After 1 L molecular diagnostic testing, alteration rates were estimated in the population, and patients were allocated to either matched therapy, matched clinical trial, or non-matched therapy depending on whether an alteration was detected and which testing method was used. The model calculated differences between the two scenarios in both the numbers of patients allocated to matched treatment and the numbers of patients needed to test to place an additional patient on either matched therapy or matched clinical trial.
An additional calculation was performed to estimate the total number of missed opportunities, by setting the 1L testing rate to 100% CGP.
Model inputs
Patient population
The model used a hypothetical US health plan perspective, with five million covered lives and a population mix of 50% Medicare and 50% commercial plan members, age matched to the 2018 US insured populationCitation24,Citation25. Five million members were chosen to illustrate the effect on a large plan, as the numbers in the overall results were expected to be small. The number of members does not affect per member per month results as the number of members is in the denominator. A 50%:50% split was chosen to give an equal weighting for a mixed plan, without overly weighting either plan type. The model makes no assumptions about the type of Medicare plan (e.g. Medicare Advantage or traditional Medicare). Age-specific (≥65 or <65) incidence rates were sourced from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program, assumed to be constant over the two-year modeling period, and were applied to the corresponding US Census Bureau national population projections (≥65 or <65) to estimate the number of incident patients with CRC in the hypothetical health planCitation1,Citation26. The proportion of patients presenting with advanced/metastatic disease at diagnosis was then applied to the incident population to provide the number of incident mCRC patients eligible for 1 L molecular testingCitation1. The characteristics of the base case health plan used to estimate the incremental budget impact due to Tempus CGP molecular testing are summarized in .
Table 1. Genomic alteration and testing inputs by molecular diagnostic testing type (base case inputs).
Testing, alterations, and treatment allocations
All patients with mCRC were assumed to be eligible for 1L initial testing, of whom 51% were currently tested by any molecular testing, according to a 2013–17 review of US mCRC patient electronic medical recordsCitation17. The number of patients eligible for repeat testing was estimated from progression-free and overall survival curves from a 2011 trial of standard of care FOLFOX + bevacizumab in mCRC, comprising 13% of patients diagnosed in a typical year and 26% of patients diagnosed in the previous yearCitation27. Twenty-five percent of patients eligible for repeat testing were assumed to receive repeat testing based on the authors’ clinical expert opinion. Increased availability of Tempus xT CGP was assumed to have no effect on the rate of patients receiving testing. The model assumed that Tempus xT CGP replaced 20% of current testing. The uptake of Tempus xT CGP was assumed to draw proportionally from non-CGP (point) and other (non-Tempus) CGP testing, with the current ratio of non-CGP to other CGP utilization assumed to be 83:17.
The proportions of patients with an actionable genomic alteration detected were calculated as the product of alteration prevalence, testing rate of each alteration for each testing type (Tempus CGP/non-CGP/Other CGP), and test sensitivity for each alteration according to the testing type (). Tempus and other CGP were assumed to have the same to have the same diagnostic accuracy and range of alterations based on similar validation data, albeit parallel tests have not as yet been publishedCitation16,Citation35–37. Prevalence of alterations of interest were sourced from a 2016 study which assayed over 4,000 advanced CRC cases from 2012 to 2015, and a 2018 Memorial Sloan Kettering Cancer Center study which used targeted sequencing of 1,134 mCRC samples and was subsequently shared on cBioPortalCitation3,Citation28–30. KRAS, and NRAS are typically the first alterations tested for under non-CGP tests and alteration rates used the whole patient population as the denominator and alteration rate inputs other than KRAS and NRAS accounted for overlap between alterations in order to avoid the overestimation of total actionable alterations: BRAF alteration rates were exclusive of RAS-positive patients, and all other alteration rates were exclusive of RAS- and RAF-positive patients, except for POLE1, where data were only available on a whole patient population basisCitation28–30. TMB status was not calculated separately in this model but could be inferred to be concordant with dMMR/MSI status. A 2018 study in CRC showed that TMB-high status had high overlap with MSI-high status, with the majority of TMB cases capturedCitation38.
After testing with either Tempus xT CGP or non-Tempus methods, mCRC patients were allocated to either matched therapy, matched clinical trial, non-matched/no treatment or non-matched clinical trials. Rates of these treatment allocations were based on whether an alteration was detected, and were applied to the aggregate number of alterations detected in each scenario (). For a patient with an alteration detected, rates of allocation to matched clinical trials were assumed to be slightly higher for Tempus xT CGP testing than non-Tempus tests, based on the expectation of detection of rarer alterations more likely to be subject to clinical trial. Rates of allocation to matched therapy for a given alteration were assumed to be the same for Tempus and non-Tempus tests. Treatment allocation rates were based on clinical expert opinion for all types of tests.
Table 2. Results–missed opportunities under usual testing (calculated).
Costs
Testing and procedural costs were sourced from the Centers for Medicare and Medicaid Services’ (CMS) Clinical Diagnostic Laboratory and Physician Fee SchedulesCitation39.
Total non-Tempus test costs per patient tested (both initial and repeat) were constructed from the cost of a point test for each alteration locus included in the model and weighted by the testing rate for that alteration locus (costs for other CGP testing were assigned a 100% testing rate at all loci)Citation39. Tempus initial test cost was assumed to be the full list price for the panel, with no rebates or discounts. Cost for repeat non-Tempus tests were assumed to be the same as initial test costs, while Tempus CGP repeat test cost was zero, as Tempus provides repeat testing within the first 18 months at no cost.
Biopsy and physician costs were calculated as a sum of colonoscopy/biopsy procedure fees, plus physician fees for outpatient office visit, molecular pathology interpretation, and review of tumor tissue. Additionally, the possibility of repeated biopsy or procedure was factored in. Based on clinical opinion, with non-Tempus test biopsy assumed to be repeated in 5% of cases due to potential for tissue sample being insufficient when multiple point tests are conducted.
Administrative costs included processing fees per code submitted and review costs per claim submitted. Processing fees per code, which are now primarily processed electronically, were sourced from publicly available figures in the Council for Affordable Quality Healthcare (CAQH) 2019 Index ReportCitation40. Review cost inputs and rates were based on conservative assumptions for manual review, in the absence of literature on claim review costs. Review costs were calculated as the product of per-claim costs and rates of prior authorization cost, first-level appeal cost, second-level appeal cost, and external review board review. Tempus testing was assumed to use a single panel code processed for CGP testing with a single claim submission (one code) per patient tested, and one review per patient tested. Non-Tempus CGP was assumed to use stacked coding (separate code processing for each alteration claim in the stack), but a single claim submission (one code), with only one review per patient tested. Non-CGP testing was assumed to require separate claim processing and review for each test.
Further details of cost inputs are shown in .
Table 3. Results – impacts on costs and opportunities for matched care.
Outcomes
The model was used to estimate annual total and per member per month (PMPM) diagnostic testing costs and budget impact associated with the increased use of Tempus xT CGP. Annual incremental value outcomes, including opportunities for genomically informed-matched therapy and matched clinical trials, were also estimated. The number of patients who needed to be tested with Tempus vs. the current mix of tests to gain one actionable match (either matched therapy or matched clinical trial) was also assessed. An estimate of the missed opportunities to put patients on matched therapy or matched clinical trial had they received Tempus xT CGP was also calculated.
Sensitivity analysis
A one-way deterministic sensitivity analysis for the diagnostic budget impact (in $PMPM) was conducted, varying key inputs by +/- 20%. Plan composition sensitivity was tested using 100% commercial and 100% Medicare membership. A scenario analysis with 100% of testing using Tempus CGP was also conducted.
Results
In a hypothetical health plan with five million covered lives (50% Medicare, 50% commercial) and base case inputs, 1,112 incident cases of mCRC (22 per 100,000 patients) were estimated in a typical year (represented by the second year in the model’s two-year time horizon). The model estimated that 566 (51%) 1 L molecular diagnostic tests would be performed and 55 repeat tests would be performed upon progression within the following 18 months. Based on current real-world testing rates of 51%,Citation17 there were an expected 521 missed opportunities for genomically informed treatment or clinical trial decisions (47% of the incident mCRC population), with 442 missed due to lack of testing and 79 due to testing without CGP.
The proportion of patients with actionable alterations, as detected by Tempus xT CGP, non-CGP, and other CGP testing were estimated to be 77.2, 62.7, and 77.2% of patients respectively (out of a maximum possible of 78.8%, with 21.2% of patients having no actionable alterations).
The number needed to test (NNT) with CGP versus the current usual mix of testing was 7.8 1 L patients to identify one actionable alteration ().
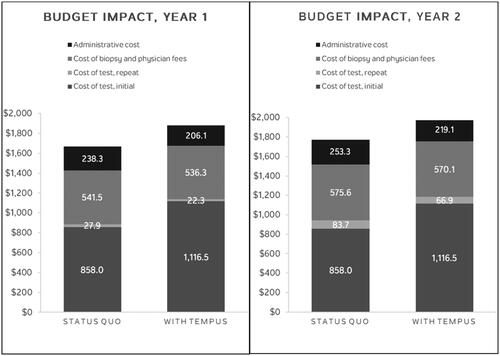
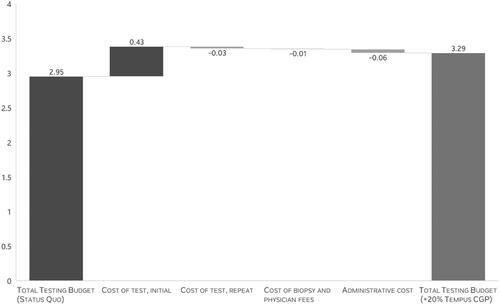
Replacing 20% of usual 1 L mCRC testing with Tempus xT CGP in the modeled scenario was associated with up to a $0.003 per member per month (PMPM) testing cost increase and an additional 15.5 patients with an opportunity for genomically informed care (12.7 patients for guideline-recommended treatment and 2.8 for a clinical trial). The net total cost for the five-million-member plan was of $202,102. This budget impact result was driven by an increase in initial test cost with Tempus xT CGP, with offsets from savings on repeat testing, biopsy and physician services, and administration costs ( and Citation4). The testing total cost (initial test, repeat test, biopsy and physician services, and administrative cost) to put one additional patient with mCRC on matched therapy or matched clinical trial was estimated to be $13,005 (). On a per diagnosed 1 L mCRC patient basis, the annual budget impact was $182 (). When compared to the total cost of care per mCRC patient, the increase in costs due to testing with Tempus xT CGP is negligible in the grand scheme of a patient’s journey from diagnosis to treatment to post-treatment care.
Figure 4. Budget impact results, by category (year 2), PMPM, in US Cents. Abbreviations. NNT, number needed to test; PMPM, per member per month.
Sensitivity analysis
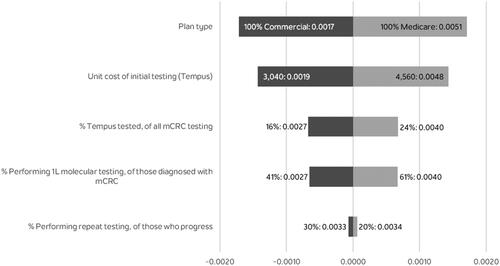
The results of the sensitivity analysis are shown in . The model was most sensitive to type of plan, proportion of 1L patients receiving testing and the list price of the Tempus test. The size of the plan (number of plan members) had no effect on the PMPM results. In addition, the scenario with 100% of testing conducted with Tempus CGP testing resulted in a testing budget impact of $0.017 PMPM.
Discussion
This paper adds to the growing body of evidence from different tumor types that increased CGP is associated with small diagnostic budget impact and notable clinical benefits, including in early-line settings for patients with advanced cancerCitation41–46. At current rates of testing in the 1 L setting, the number of missed opportunities to put patients on matched therapy or matched clinical trials in mCRC is meaningfully high. These opportunities can be captured by increasing overall testing rates and increasing the usage of 1L CGP testing. However, the utilization of broader molecular testing is likely to vary across care centers. The cost implications of broader testing can also impact reimbursement and utilization.
The present study found that increasing the use of CGP testing in mCRC, via the Tempus xT test, by 20% would result in a relatively small budget impact of up to $0.003 PMPM, while the NNT to identify one additional opportunity for genomically informed therapy is relatively low, at 7.8 patients. The budget impact result was driven by higher cost of initial testing with Tempus xT CGP, with cost offsets from lower repeat test cost (as Tempus offers repeated testing for no cost), lower physician-associated cost (due to reduced rate of repeat biopsy for CGP testing), and lower administration cost (due to assumed use of one single panel code). On a per patient basis, the budget increase estimated here at $182 per diagnosed mCRC patient per year would be a small fraction (0.13%) of reported estimates of the total cost of care per mCRC patient of $140,260 per yearCitation21.
Within the additional opportunities for genomically informed therapy captured by the increase in use of Tempus xT CGP, almost one in five of these were predicted to be opportunities to enroll in matched clinical trials. These additional clinical trial opportunities were driven by greater detection of alterations as well as a higher rate of allocation to clinical trial, assumed because the CGP test may detect rarer mutations suitable for clinical trial enrollment. Clinical trial allocation rate assumptions were conservative and real-world rates could be slightly higher, especially in later lines of therapy than the 1L setting that was the focus of this study.
Limitations associated with our analyses include those typical for health economic models, such as limited availability or high variability of published data. Given variations in cost between different US-based plans, different commercial plans and different types of Medicare plans may have different cost results and this will also have implications for how much of the costs the overall health system will bear. Within the alterations, dMMR/MSI-high was assumed to capture the majority of TMB-high cases in CRC as a simplifying and conservative assumption, however, inclusion of TMB separately from dMMR/MSI-high would be likely to slightly improve the economics of CGP testing. Assumptions made to simplify treatment allocation resulted in the model being primarily driven by differences in testing rates and sensitivities between CGP and non-CGP tests. However, the potential for CGP to enhance matched therapy opportunities may have been underestimated by the model, given the potential for decision support tools provided to clinicians by suppliers of CGP tests such as Tempus to lead to improved treatment allocations.
One of the features of this analysis was that it illustrated administrative costs to the plan sponsor, which are costs that are often overlooked in economic models, in part due to difficulty sourcing inputs. These costs are important for the overall health system budget: more than 10% of estimated testing costs in this model were estimated to be driven by administrative costs. The model illustrates the potential for CGP testing to reduce administrative costs through a single review and panel code, compared to non-CGP single point tests, which may require separate administrative review. While automated processing cost inputs could be sourced from publicly available data, most of the cost of administration is in manual review and inputs for this were based on estimates from real-world experience.
The goal of the model was to calculate changes in testing costs and therefore it did not include any costs due to mCRC drug therapy or healthcare utilization. The model was also not intended to be a cost-effectiveness model for testing, but such a model would be useful for a future study. Changes in treatment allocation, based on information from testing, may alter treatment cost and have a significant effect on overall budget impact. This change in pharmaceutical costs could be economically significant, however that would mainly be attributable to the cost of such treatments and increases in total costs due to prolonged survival, rather than the cost of diagnostic testing. Furthermore, there are many treatment options for metastatic colon and rectal cancer (and the mix of these can depend on the setting), which would make the consideration of treatment an extensive exerciseCitation46,Citation47. Given the potential in mCRC to avoid the use of high-cost anti-EGFR therapy that is contraindicated for certain alterations, it is possible that increases in testing could reduce overall healthcare cost, although this would require further investigation to determine.
Conclusions
This economic modeling analysis supports broader use of CGP in mCRC. Replacing 20% of usual testing with Tempus CGP CGP was associated with a small incremental testing cost. Additionally, increased testing with Tempus xT CGP can identify actionable alterations for a meaningful number of patients. For every eight 1L mCRC patients tested with Tempus xT CGP rather than the usual mix of molecular testing, one additional patient was expected to be identified as having currently actionable alterations. While molecular testing with CGP via Tempus xT is costly upfront, the cost of testing within the total cost of mCRC care is small and the budget impact is minor. There may be opportunities to improve the economics of this care and access to clinical trials through CGP-based precision medicine.
Transparency
Declaration of funding
This study was funded by Tempus Labs, Inc.
Declaration of financial/other relationships
SB and MA are employees of Tempus Labs, Inc. DP, JS, and EM are employees of Analysis Group Inc., which has received consultancy fees from Tempus Labs, Inc. ND and AP have no conflict of interest to report.
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Author contributions
DP, JS, EM, SB, and MAA all participated in model conceptualization. All authors participated in the drafting and editing of the manuscript.
Acknowledgements
None stated.
Previous presentations
Parts of the work including results were previously published as an e-abstract at ASCO 2021 and was presented at AMCP Nexus October 2021.
Supplemental Material
Download MS Word (26.8 KB)References
- National Cancer Institute. SEER*Explorer: an interactive website for SEER cancer statistics [Internet]; 2020 [cited 2020 April]. Available from: https://seer.cancer.gov/explorer/.
- Sveen A, Kopetz S, Lothe RA. Biomarker-guided therapy for colorectal cancer: strength in complexity. Nat Rev Clin Oncol. 2020;7(1):817–32.
- Rankin A, Klempner SJ, Erlich R, et al. Broad detection of alterations predicted to confer lack of benefit from EGFR antibodies or sensitivity to targeted therapy in advanced colorectal cancer. Oncologist. 2016;21(11):1306–1314.
- Shiu K-K, Andre T, Kim TW, et al. KEYNOTE-177: Phase III randomized study of pembrolizumab versus chemotherapy for microsatellite instability-high advanced colorectal cancer. J Clin Oncol. 2021;39(3_suppl):6–6.
- Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5(1):22.
- Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(10):1626–1634.
- Baselga J, Rosen N. Determinants of RASistance to anti-epidermal growth factor receptor agents. J Clin Oncol. 2008;26(10):1582–1584.
- De Roock W, Piessevaux H, De Schutter J, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008;19(3):508–515.
- Khambata-Ford S, Garrett CR, Meropol NJ, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25(22):3230–3237.
- Lièvre A, Bachet JB, Boige V, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26(3):374–379.
- Tejpar S, Celik I, Schlichting M, et al. Association of KRAS G13D tumor mutations with outcome in patients with metastatic colorectal cancer treated with first-line chemotherapy with or without cetuximab. J Clin Oncol. 2012;30(29):3570–3577.
- Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–1417.
- McGregor M, Price TJ. Panitumumab in the treatment of metastatic colorectal cancer, including wild-type RAS, KRAS and NRAS mCRC. Future Oncol. 2018;14(24):2437–2459.
- Pietrantonio F, Petrelli F, Coinu A, et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer. 2015;51(5):587–594.
- Kondo T, Matsubara J, Quy PN, et al. Comprehensive genomic profiling for patients with chemotherapy-naïve advanced cancer. Cancer Sci. 2021;112(1):296–304.
- Beaubier N, Tell R, Lau D, et al. Clinical validation of the tempus xT next-generation targeted oncology sequencing assay. Oncotarget. 2019;10(24):2384–2396.
- Gutierrez ME, Price KS, Lanman RB, et al. Genomic profiling for KRAS, NRAS, BRAF, microsatellite instability, and mismatch repair deficiency among patients with metastatic Colon cancer. J Clin Oncol Precision Oncology. 2019;3(3):1–9.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology, colon cancer, version 4.2020. 2020.
- Pruneri G, De Braud F, Sapino A, et al. Next-Generation sequencing in clinical practice: is it a cost-saving alternative to a single-gene testing approach? Pharmacoecon Open. 2021;5(2):285–298.
- Vanderpoel J, Stevens AL, Emond B, et al. Total cost of testing for genomic alterations associated with next-generation sequencing versus polymerase chain reaction testing strategies among patients with metastatic non-small cell lung cancer. J Med Econ. 2022; 25(1):457–468.
- Chawla A, Peeples M, Li N, et al. Real-world utilization of molecular diagnostic testing and matched drug therapies in the treatment of metastatic cancers. J Med Econ. 2018; 21(6):543–552.
- United States Federal Reserve. Consumer price index for all urban consumers: medical care services in U.S. city average, index; 1982. 1984 = 100, Annual, Seasonally Adjusted; 2020 [cited 2020 July 20]. Available from: https://fred.stlouisfed.org/series/CUSR0000SAM2#0.
- Beaubier N, Bontrager M, Huether R, et al. Integrated genomic profiling expands clinical options for patients with cancer. Nat Biotechnol. 2019;37(11):1351–1360.
- Centers for Medicare and Medicaid Services. Total medicare enrollment: part A and part B enrollees, by demographic characteristics, calendar year 2018. 2018. Available from: https://www.cms.gov/files/document/2018-mdcr-enroll-ab-5.pdf.
- Berchick ER, Barnett JC, Upton RD. Current population reports, Health Insurance Coverage in the United States: 2018, P60-267(RV) Washington (DC): U.S. Government Printing Office; 2019. Available from: https://www.census.gov/content/dam/Census/library/publications/2019/demo/p60-267.pdf.
- U.S. Census Bureau. Main projections series for the United States (2017-2060): projected age groups and sex composition of the population 2017. Available from: https://www.census.gov/data/tables/2017/demo/popproj/2017-summary-tables.html.
- Bendell JC, Bekaii-Saab TS, Cohn AL, et al. Treatment patterns and clinical outcomes in patients with metastatic colorectal cancer initially treated with FOLFOX-bevacizumab or FOLFIRI-bevacizumab: results from ARIES, a bevacizumab observational cohort study. Oncologist. 2012;17(12):1486–1495.
- Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404.
- Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1.
- Yaeger R, Chatila WK, Lipsyc MD, et al. Clinical sequencing defines the genomic landscape of metastatic colorectal cancer. Cancer Cell. 2018;33(1):125–136.e3.
- Tempus. Tempus xT (v4) gene panel reports; 2020 [cited 2021 Jan 12]. Available from: https://www.tempus.com/wp-content/uploads/2020/10/xTGene-List_100620-1.pdf.
- Tempus. Tempus xT (v4) validation summary: Tempus; 2020 [updated September 28; cited 2021 Jan 12]. Available from: https://www.tempus.com/wp-content/uploads/2020/10/xTv4-Validation_100620.pdf.
- Dufraing K, Keppens C, Tack V, et al. Evolution of RAS testing over time: factors influencing mutation rates in metastatic colorectal cancer patients. Colorectal Cancer. 2020;9(1):CRC14.
- Snowsill T, Coelho H, Huxley N, et al. Molecular testing for lynch syndrome in people with colorectal cancer: systematic reviews and economic evaluation. Health Technol Assess. 2017;21(51):1–238.
- Caris. MI Tumor Seek™ technical specifications: Tempus; 2020 [updated June 29; cited 2022 May 13]. Available from: https://www.carismolecularintelligence.com/wp-content/uploads/2018/10/MI-Tumor-Seek-Profile-Menu.pdf.
- Genetic Testing Registry (GTR). Caris MI TumorSeek comprehensive genomic profile: performance characteristics; 2022 [updated April 8; cited 2022 May 13]. Available from: https://www.ncbi.nlm.nih.gov/gtr/tests/560898/performance-characteristics/.
- Milbury CA, Creeden J, Yip WK, et al. Clinical and analytical validation of FoundationOne®CDx, a comprehensive genomic profiling assay for solid tumors. PLOS One. 2022;17(3):e0264138.
- Fabrizio DA, George TJ, Jr Dunne RF, et al. Beyond microsatellite testing: assessment of tumor mutational burden identifies subsets of colorectal cancer who may respond to immune checkpoint inhibition. J Gastrointest Oncol. 2018;9(4):610–617.
- Centers for Medicare & Medicaid Services. Clinical laboratory fee schedule; 2020 [cited 2021 Jan 12]. Available from: https://www.cms.gov/Medicare/Medicare-fee-for-service-Payment/clinicallabfeesched/index.html.
- CAQH. Conducting electronic business transactions: why greater harmonization across the industry is needed: CAQH; 2020 [cited 2021 Janu 12]. Available from: https://www.caqh.org/sites/default/files/explorations/index/report/2019-caqh-index.pdf?
- Chawla A, Janku F, Wheler JJ, et al. Estimated cost of anticancer therapy directed by comprehensive genomic profiling in a single-center study. J Clin Oncol Precision Oncology. 2018;2:PO.18.00074.
- Signorovitch J, Zhou Z, Ryan J, et al. Budget impact analysis of comprehensive genomic profiling in patients with advanced non-small cell lung cancer. J Med Econ. 2019; 22(2):140–150.
- Yencho S, Austin J, Betka E, et al. Cancer healthcare utilization impact of precision therapeutics: hospitalization/emergency visits. Cancer Sci Res. 2020;3(3):1–3.
- Hirshfield KM, Tolkunov D, Zhong H, et al. Clinical actionability of comprehensive genomic profiling for management of rare or refractory cancers. Oncologist. 2016;21(11):1315–1325.
- Cobain EF, Wu Y-M, Vats P, et al. Assessment of clinical benefit of integrative genomic profiling in advanced solid tumors. JAMA Oncol. 2021;7(4):525–533.
- Nesline MK, DePietro P, Dy GK, et al. Oncologist uptake of comprehensive genomic profile guided targeted therapy. Oncotarget. 2019;10(45):4616–4629.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology, rectal cancer, version 6.2020; 2020.