Abstract
Aim
Recent studies have compared the efficacy and safety of direct-acting oral anticoagulants (DOAC) and low-molecular-weight heparin (LMWH) for cancer-associated venous thromboembolism (VTE). However, there is no available cost-effectiveness analysis comparing DOAC and LMWH. The study aimed to conduct a cost-effectiveness analysis of DOAC (apixaban, edoxaban, and rivaroxaban) vs. LMWH for the treatment of cancer-associated VTE in Spain from the Spanish healthcare system perspective.
Methods
We developed a Markov model with a 12-month time horizon. The states included pulmonary embolism, deep vein thrombosis, major and non-major bleeding, chronic thromboembolic pulmonary hypertension, post-thrombotic syndrome, and death. The use of medical resources and drug costs were obtained from the 2021 Spanish Ministry of Health database, and the main references for obtaining the outcomes were derived from Caravaggio, Hokusai VTE Cancer, ADAM VTE, and SELECT-D trials. We performed a deterministic and probabilistic sensitivity analysis to validate the robustness. The Incremental Cost-Effectiveness Ratio (ICER) scores cost per life-year (€/LY) gained and cost per quality-adjusted life-year (€/QALY) gained.
Results
The 12-month cost of DOAC was 1,994€ (apixaban 1,944€, edoxaban 1,968€, rivaroxaban 2,122€) and 2,152€ for LMWH. The amount of QALY for DOAC was 0.54 (apixaban 0.55, rivaroxaban 0.53, and edoxaban 0.52) and 0.53 for LMWH. We observed similar results for LYs. ICER scores in terms both of €/LY and €/QALY show that DOAC is dominant over LMWH and apixaban showed the best profile.
Limitations
Our research is based on an indirect comparison of a short-term clinical trial.
Conclusion
Our results suggest that DOAC is cost-effective and cost-saving compared to LMWH in treating VTE.
Introduction
Cancer is associated with an increased risk of venous thromboembolism (VTE) which includes deep vein thrombosis (DVT) and pulmonary embolism (PE)Citation1,Citation2. Cancer-related treatments, such as chemotherapy, surgical interventions, or a permanent central venous catheter also increase the risk of suffering VTECitation1,Citation3,Citation4. Cancer‐associated thrombosis patients have a higher risk of death than non-cancer patients with VTE, and cancer patients without VTECitation5–9.
Several studies show that direct oral anticoagulants (DOAC) are a convenient and effective treatment alternative to low molecular weight heparins (LMWH) in patients with VTECitation10–12. More recently, DOAC has been compared to LMWH in randomized clinical trials in patients with cancer where apixaban, edoxaban, and rivaroxaban showed to be non-inferior to dalteparin in preventing VTE recurrenceCitation7,Citation9. Edoxaban and rivaroxaban were associated with an increased risk of bleeding in comparison with dalteparin while this was not the case for apixabanCitation13. A direct and indirect meta-analysis confirmed that DOAC may also have at least similar efficacy and safety as LMWH for the management of cancer‐associated thrombosisCitation14,Citation15.
VTE causes substantial morbidity and mortality, and has a significant adverse impact on the quality of life in cancer patientsCitation16. The guidelines for the treatment of VTE in cancer patients recommend LMWH or DOAC for the initial treatment of VTE, DOAC for the short-term treatment of VTE, and LMWH or DOAC for the long-term treatment of VTE in patients with cancerCitation17. Considering the burden associated with daily subcutaneous injections and the potential low adherence in a long-term treatmentCitation18,Citation19, DOAC may provide a convenient and effective alternative to LMWH. Currently, only a cost-effectiveness analysis was performed, not including all DOACs, showing potential benefit vs. LMHWCitation20.
The objective of this study was to develop a cost-effectiveness analysis in Spain comparing DOAC to LMWH for the treatment of cancer patients suffering from VTE and its related complications.
Methods
We used a transition state Markov model with monthly cycles and a time horizon of 12 months since the optimal duration of therapy for cancer‐associated thrombosis remains unclear after 6 monthsCitation21. A hypothetical active cancer outpatient cohort based on the clinical trial patient profile was used. Active cancer was defined as cancer that had been diagnosed within the past 6 months or under anticancer treatment. The profile of the patients in our study is the result of combining the patients from Hokusai VTE CancerCitation11, SELECT-DCitation7, ADAM VTECitation22, and CaravaggioCitation5,Citation23. We evaluated the cost-effectiveness relationship between DOAC (apixaban, rivaroxaban, and edoxaban) and LWMHs (bemiparin, dalteparin, enoxaparin, nadroparin, and tinzaparin) for VTE treatment for both a 6-month interval and 1-year interval in the Spanish healthcare system. The same effectiveness was assumed for all the LMWH commercialized in Spain, and we weighted the cost of each LMWH in terms of their market share, which is 42.4% for enoxaparin, 39.3% for bemiparin, 17.8% for tinzaparin, 0.5% for dalteparin, and 0.0% for nadroparinCitation24. The Markov model structure is represented in Each health state is illustrated with a black ellipse, and each state transition is depicted by an arrow. The patients of this model can experience PE, DVT, clinically relevant non-major bleeding, non-intracranial major bleeding, intracranial bleeding (ICB), death related to PE, death related to major bleeding, and death related neither to PE nor to major bleeding. Additional states include complications after ICB (post-ICB), chronic thromboembolic pulmonary hypertension after PE, and post-thrombotic syndrome after experiencing DVT.
Figure 1. Model structure. For each simulated cancer patient, the different VTE treatment states are represented by ellipses and each transition between two states is represented by an arrow between those states.
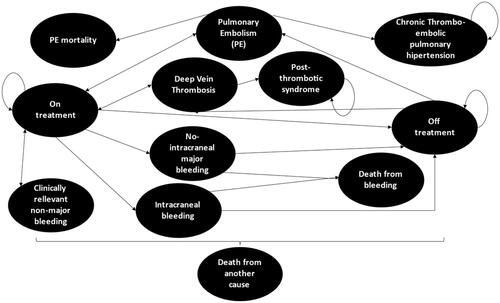
The computational model was dynamic, patients started at the health state: “on treatment” and transferred to adjacent states according to the possible transition probabilities, which try to simulate the practical complications that occur when a patient is on treatment. The simulated patient transitioned within the different health states until they arrived at a state labeled “death” or until they recovered. Moreover, during the simulation, every patient had a global chance of discontinuing the treatment and a global chance of suffering PE or DVT regardless of their current state. Patients in each health state had a global chance of dying due to cancer or any other cause. Moreover, patients with PE and patients with major bleeding or ICB had an additional risk of dying. Simulated patients could only stay in one health state each time. The Incremental Cost-Effectiveness Ratio scores cost per life-year (€/LY) gained and cost per quality-adjusted life-year (€/QALY) gained.
Probabilities
VTE and major bleeding probabilities directly come from Hokusai VTE CancerCitation7, SELECT-DCitation9, ADAM VTECitation22, and CaravaggioCitation23. We estimated the Hazard Ratios of the different treatments based on a Matching-adjusted indirect comparisonCitation25.
The percentage of VTEs that was a PE at 6 months was 58% for apixaban, 50% rivaroxaban, 68% edoxaban, and 55% LMWH based on studiesCitation7,Citation9,Citation22,Citation23. Due to the lack of information for apixaban and rivaroxaban after 6 months, we assumed the distribution from Hokusai VTE Cancer: 57% for all DOAC and 31% for the LMWHCitation7. Also, we considered that PE patients could have suffered chronic thromboembolic pulmonary hypertension at 0.9% at 12 monthsCitation26 and DVT patients could have suffered post-thrombotic syndrome at 6.7%Citation27 ().
Table 1. Probabilities.
In the case of major bleeding, we subdivided the analysis into the subcases of ICB or non-intracranial major bleeding. The distribution of non-intracranial major bleeding over major bleeding is 100% apixaban, 100% rivaroxaban, 93.1% edoxaban, and 87.5% LMWHCitation7,Citation9,Citation22,Citation23. After 6 months, and in agreement with what Hokusai VTE showsCitation7, we assumed that all bleedings were non-intracranial for DOAC.
The distribution of clinically relevant non-major bleeding was 8.5% apixaban, 12.3% rivaroxaban, 12.3% edoxaban, and 6.4% LMWHCitation7,Citation9,Citation22,Citation23. After 6 months all DOAC had a 3.1% chance of clinically relevant non-major bleeding and LMWH showed a 3.8% chanceCitation7.
Mortality
We included the mortality risk by gender and age in any health stateCitation28. The mortality risk of patients with cancer, PE, ICB, and major bleeding for each treatment was also considered. We assumed a cancer mortality at 6-months of 19% apixaban, 23% rivaroxaban, 26% edoxaban and 23% LMWHCitation7,Citation9,Citation22,Citation23, and a PE mortality of 21% apixaban, 25% rivaroxaban, 22% edoxaban and 11% LMWHCitation7,Citation9,Citation22,Citation23. We selected an ICB mortality of 0% for all DOAC and of 33% for LMWHCitation7,Citation9,Citation22,Citation23, and a non-intracranial major bleeding mortality of 9% apixaban, 0% rivaroxaban, 4% edoxaban, and 5% LMWHCitation7,Citation9,Citation22,Citation23. After 6 months, we assumed that cancer mortality was 17% for DOAC and 16% for LMWHCitation7,Citation9,Citation22,Citation23, PE mortality was 25% for all treatments, and we considered an ICB and non-intracranial major bleeding mortality of 0% for all treatmentsCitation7.
Utilities
Healthcare outcomes were expressed in terms of the QALYs, which are the quality-adjusted life years of a person depending on each health state they are in. Utilities are essential since they tell us the preferences of society for being in the various health statesCitation29. They represent the ideal health state when it attains a value of 1, and death when it has a value of 0. In our study, we determined the utility by assuming a life quality of 0.6925 for cancer patientsCitation19,Citation20, from which we subtracted the disutility of each eventCitation30–34 ().
Table 2. Utilities table.
Use of resources and costs
To estimate the cost of each treatment per patient, we approximated the pharmacological cost of each therapy, the days the patient stays in the hospital, and the follow-up visits to different doctors while the patient is under medication.
The pharmacological costs were calculated from the drug costCitation35 and predicted posology by the standard studiesCitation7,Citation9,Citation22,Citation23 (). In this way, we applied a dose of 115 IU/kg per day of bemiparin, 200 IU/kg per day of dalteparin, 100 IU/kg per day of enoxaparin, 85.5 IU/kg every 12 h of subcutaneous nadroparin, and a dose of 175 IU/kg per day of subcutaneous tinzaparin. We also applied a posology of 10 mg/12h for 7 days for apixaban, 60 mg/24h for 25 days of edoxaban after receiving a 5-day treatment with an LMWH, and a dose of 15 mg/12h for 21 days of rivaroxaban. After the initial 7 days, the posology of apixaban became 5 mg/12h, and the posology for rivaroxaban became 20 mg/24h after the initial 21 days.
Table 3. Pharmacological cost for short and long term.
For computing the drug costs (€2,021) we took into consideration the ex-factory price and the government discount contained in the Spanish Royal Decree 8/2010Citation36, which is 15% for enoxaparin, dalteparin, and nadroparin, and a 7.5% discount for biosimilar enoxaparin, bemiparin, tinzaparin, and for the DOAC.
If any simulated patient suffered from VTE during or after treatment, then treatment was resumed with the initial posology for the first month, and then the maintenance treatment as indicated in the previous paragraph was applied. In the case that the simulated patient suffered major bleeding of any sort, the treatment was stopped, and they were transferred to the off-treatment state. If the bleeding was clinically relevant to non-major bleeding, then the treatment was continued.
We obtained the amount and proportion of patients that needed each resource to attend follow-up appointments from a set of experts and their cited studies. Resource unit costs were obtained from the autonomous region tariffs in Spain.
All patients were assessed at 4 weeks, 3 months, and 6 months’ time, and then once more at the end of the time horizon. Physical examination (as clinically indicated) and routine hematology and biochemistry were performed at each visit.
Patients with clinically relevant non-major bleeding followed a nurse and primary care visit and had a blood and biochemistry test, 10% of them visited the emergency room.
The costs of each health state were based on the International Classification of Diseases (ICD 10) from the 2018 Spanish Ministry of HealthCitation37. PE with code I26-EP is 4,224.15 (SD of 431.04), DVT with code i80.9 is 2,014.60 (SD of 205.58), major bleeding with code D68.32 is 5,281.96 (SD of 538.99), ICB with code I62 is 7,817.94 (SD of 797.76), post-thrombotic syndrome with code i87.0 is 3,031.28 (SD of 309.32) and the chronic thromboembolic pulmonary hypertension with code I27.82 is 5,837.72 (SD of 595.70).
Discontinuation
Patients that continued with the therapy might have discontinued treatment at some point for various reasons. The median duration of the treatment was 185 days for LMWH (lambda: 0.0038), and 211 days for DOAC (lambda: 0.0033)Citation7. We added discontinuation in the model assuming an exponential distribution.
Sensitivity analysis
A univariant sensitivity analysis was performed for every variable by changing the value of each variable used in the model according to the probability range of each of them. In this analysis, the aim was to highlight which variables are more influential in the final results and to detect in which way they ensured the credibility of the results. Univariant sensitivity analysis was represented in the tornado diagram based on Net Monetary Benefit. To check for robustness, a probabilistic sensitivity analysis was performed with a Monte Carlo simulation of second order. We used Dirichlet/Beta distributions for the probabilities, Gamma distribution for the utilities, and log-normal distributions for the costs and hazard ratios.
Results
At 6 months, the DOAC hazard ratio of 0.62 (95% confidence interval [CI] 0.46–0.83, p = .001) showed a significantly lower VTE risk than LMWH. Specifically, the hazard ratios vs. LMWH for apixaban was 0.54 (95% CI 0.36–0.80, p = .001), for rivaroxaban was 0.47 (95% CI 0.23–0.96, p = .038), and for edoxaban was 0.79 (95% CI 0.54–1.15, p = .22). At 6 months, there was no significant VTE risk difference between apixaban and rivaroxaban (p = .726), and between edoxaban and apixaban (p = .118). In terms of major bleeding, DOAC obtained a hazard ratio of 1.24 (CI 0.86–1.82, p-value = .247), with respect to LMWH at 6 months. In particular, the hazard ratios vs. LMWH of apixaban was 0.87 (95% CI 0.52–1.45, p = .593), rivaroxaban was 1.60 (95% CI 0.83–3.07, p = .162), and edoxaban was 1.61 (95% CI 1.02–2.54, p = .04). At 6 months, apixaban showed a significantly lower major bleeding risk than edoxaban (hazard ratio 0.54, 95% CI 0.31–0.94, p = .031), while rivaroxaban did not show significant differences vs. edoxaban (hazard ratio 1.01, 95% CI 0.50–2.02, p = .98) or apixaban (hazard ratios 0.55, 95% CI 0.26–1.13, p = .10). With these outcomes we obtained 0.43 LY for DOAC (apixaban 0.43, rivaroxaban 0.42, edoxaban 0.41) and 0.30 QALYs for DOAC (apixaban 0.30, rivaroxaban 0.30, edoxaban 0.29) and 0.42 LY and 0.30 QALYs for LMWH. DOAC showed the lowest cost per patient with a figure of 1,492€(apixaban 1,429€, rivaroxaban 1,609€and edoxaban 1,502€) compared to the cost of LMWH (1,860€).
At 12 months DOAC obtained 0.77 LY (apixaban 0.79, rivaroxaban 0.76, and edoxaban 0.74) and 0.54 QALYs (apixaban 0.55, rivaroxaban 0.53, and edoxaban 0.52) and LMWH obtained 0.76 LY and 0.53 QALYs (). DOAC showed the lowest cost per patient with a figure of 1,994€ (apixaban 1,944€, rivaroxaban 2,122€, and edoxaban 1,968€) compared to the cost of LMWH 2,512€. The Incremental Cost-Effectiveness Ratio scores in terms of both €/LY and €/QALY show that DOAC is dominant over LMWH ().
Table 4. Costs and effectiveness for each treatment after 12 months.
Sensitivity analysis
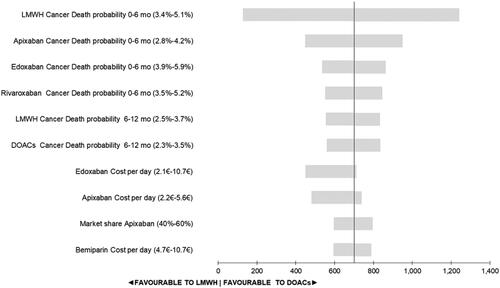
The deterministic univariant sensitivity analysis showed the variability in the percentage of death due to cancer, edoxaban cost per day, apixaban cost per day, the change of market share, and the bemiparin cost per day were the most influential factors in the variability of the results. However, none of the variabilities in the univariate analysis yielded a change in interpretation of the results. In all the scenarios DOAC was the most cost-effective treatment option (). When we maintained the same mortality for all DOAC we had the same results at the base case.
Figure 2. Tornado diagram based on Net Monetary Benefit. The 10 most sensitive factors that account for most of the variability in the model. DOAC: direct oral anticoagulants compared; LMWH: low-molecular-weight-heparins.
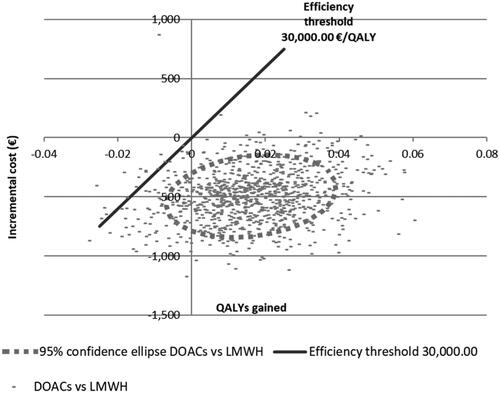
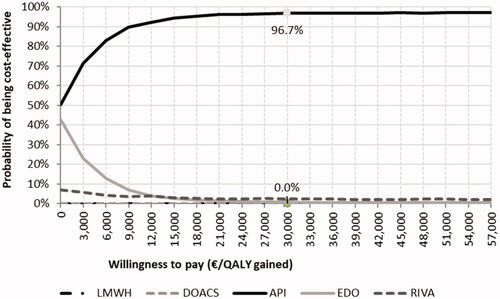
The probabilistic sensitivity analysis showed that the DOAC was cost-effective with respect to LMWH () in most of the 1,000 simulations done by our model. When the different DOAC were evaluated, apixaban had a 96.7% chance of being more cost-effective than the other DOAC and LMWH for a threshold of 30,000€ ().
Discussion
Our results suggest that DOAC is more cost-effective than LMWH. At 6 months, DOAC showed a significantly lower VTE risk than LMWH, and there was no significant difference in major bleeding with respect to LMWH. These clinical results were confirmed after 12 months with a decrease in the cost of treatment with respect to LMWH.
The systematic review conducted by Al Mukdad et al. showed that apixaban is as cost-effective a strategy in the treatment of VTE as the other DOACCitation38. These authors also noted that the benefits concerning the efficacy and safety associated with DOAC exceeded the cost related to these treatment options. Our study shows similar results to those displayed in Al Mukdad et al.Citation38, where clinical pivotal trials with warfarin as the control treatment were used. In our study, we included specific clinical trials of cancer patients with VTE and LMWH as the control treatment.
Cancer treatments are known to be burdensome for cancer patientsCitation39. In addition, subcutaneous injection therapies, such as LMWH are considered painful by the patientsCitation40, which may decrease the utility of LMWH compared to DOAC, since the latter does not require subcutaneous injectionsCitation41. Comparing the disutility associated with injections vs. oral therapy with differences in QALYs, we can expect that the disutility of LMWH achieves a value of 0.02 for some of the recurring administrations of LMWHCitation42. On the other hand, the cost-utility analysis performed by Li et al. found that DOAC is a cost-saving treatment when compared to LMWHCitation14. Their result was associated with no meaningful differences in utilities between the two treatment optionsCitation14. Similar results were obtained by Connell and ConnorsCitation41, whose study suggests that DOAC could be cost-effective with respect to LMWH which is in line with our present work in which we do include a utility difference between DOAC and LMWH. The novelty of our study, when compared to the work of Li et al, is the addition of the apixaban patients that Li et al. did not use. Apixaban has shown to have the lowest total treatment costs among all DOAC and to have significantly decreased the major bleeding events compared to edoxaban. Although there are some slight differences in patient characteristics, probabilistic sensitivity analyses validate the results.
Since DOAC are at least as effective as LMWH according to our analysis, patients would enjoy at least as many life years and quality of life using DOAC as if they were prescribed LMWH. This suggests that it would be reasonable to prescribe DOAC over LMWH for treating VTE associated with cancer in Spain. There are some clinical concerns related to LMWH having very few interactions or DOAC that are contraindicated for Gastro-intestinal tumor patients that will have to be taken into account for the final choice of DOAC treatment.
Although our study was set in a different country, our results were in line with those of the Li et al. studyCitation20. Both performed a cost-utility analysis of DOAC, however, we had the opportunity to include all the new trials of apixaban, and we included a potential consequence after PE and DVT based on literatureCitation26,Citation27. This allowed us to show a better QALY for apixaban vs. LMHW and confirm the dominance of DOAC.
This model assumes a favorable efficacy to LMWH. In other words, to determine the general efficacy of LMWH, we assumed the efficacy of dalteparin for all the LMWH in this cost-effectiveness analysis, which is higherCitation43 than the efficacy of the other LMWHCitation44–47. In other words, if we proportionally weighted the efficacy of dalteparin, enoxaparin, and tinzaparin, the general efficacy of LMWH would be lower than it is in the presented work.
For the correct interpretation of the results, several assumptions must be considered. Firstly, the acquisition of costs of both strategies will vary depending on the country that is under analysis. This is also true for the utilities as country-specific tariffs and utilities have been used. Another assumption concerns the model’s event probabilities which were derived from clinical trials and are expected to present some variability in a real-world evidence study, leading to potential differences in the results.
More research is needed to show the long-term efficacy and safety of DOAC, and further real-world studies need to be developed.
Conclusions
This study demonstrates that DOAC is a more cost-effective strategy and cost-saving for VTE treatment than LMWH from the Spanish healthcare system perspective.
Transparency
Declaration of funding
This study was sponsored by Pfizer and Bristol Myers Squibb.
Declaration of financial/other relationships
GA reports personal fees from Bristol Myers Squibb, Pfizer, Bayer Healthcare, and Daichi Sankyo outside the submitted work.
AM reports personal fees and others from Celgene, personal fees and others from Sanofi, personal fees from Pfizer-BMS, personal fees and others from Leo Pharma, personal fees from Daichii Sankyo, personal fees from Bayer, personal fees from Halozyme, personal fees from AstraZeneca, personal fees from Rovi, personal fees from Merck Sharp & Dohme, personal fees and other from Roche, personal fees from Eli Lilly, personal fees from Servier, other from Merck Serono, personal fees from Incyte, other from Amgen, outside the submitted work; In addition, AM has a patent Risk assessment model in venous thromboembolism in patients with cancer issued to NO.
EG reports personal fees from Sanofi, personal fees from Pfizer, personal fees and non-financial support from Rovi, personal fees and non-financial support from Daiichi Sankyo, personal fees from Techdow, personal fees and non-financial support from Leo Pharma, personal fees from Boehringer Ingelheim, during the conduct of the study; grants from Sanofi, grants, personal fees and non-financial support from Janssen, grants and personal fees from Astellas, grants and personal fees from Bayer, grants, personal fees and non-financial support from Ipsen, grants, personal fees and non-financial support from Roche, grants from Novartis, grants, personal fees and non-financial support from Pfizer, grants, personal fees and non-financial support from Eisai, personal fees from EUSA Pharma, grants, personal fees and non-financial support from BMS, personal fees and non-financial support from AstraZeneca, personal fees from Merck, personal fees from MSD, grants from Ferrer, grants from GSK, outside the submitted work.
JS, SFC, and DA report that they are current employees at Pfizer Spain. Pfizer Spain is the owner of Apixaban.
CC and MF are employees of Axentiva Solutions who were paid consultants to Pfizer and Bristol Myers Squibb in connection with the development of this manuscript. CC was involved in projects with Novartis, Amgen, Angellini, Ferrer, Mallinkcrodt, Boehringer, Biogen, Shire, Almirall, Abbvie, Edwards, Boston Scientific, Medtronic, Dexcom, Takeda, Mundipharma, Pfizer, Fresenius Kabi, Pierre-Fabre, Roche, Vifor, and LeoPharma. CC reports personal fees from Amgen and GSK.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
JS, SFC, and DA developed the conception of the study, supervised the whole study, and were involved in its design. AM, EG, and GA provided background information based on their experience as principal investigators in this field and interpretation of data. CC and MF were involved in the study design, conducted the research and the data analysis, and drafted the manuscript. All the investigators revised the draft and contributed to the final version of the manuscript.
Acknowledgements
None reported.
References
- Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160(6):809–815.
- Kahale LA, Hakoum MB, Tsolakian IG, et al. Anticoagulation for the long-term treatment of venous thromboembolism in people with cancer. Cochrane Database Syst Rev. 2018;6:CD006650.
- Guo JD, Hlavacek P, Poretta T, et al. Inpatient and outpatient treatment patterns of cancer-associated thrombosis in the United States. J Thromb Thrombolysis. 2020;50(2):386–394.
- Soff GA, Mones J, Wilkins C, et al. Rivaroxaban treatment of cancer‐associated venous thromboembolism: memorial sloan kettering cancer center institutional experience. Res Pract Thromb Haemost. 2019;3(3):349–356.
- Agnelli G, Becattini C, Bauersachs R, et al. Apixaban versus dalteparin for the treatment of acute venous thromboembolism in patients with cancer: the Caravaggio study. Thromb Haemost. 2018;118(9):1668–1678.
- Levitan N, Dowlati A, Remick SC, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine. 1999;78(5):285–291.
- Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of Cancer-Associated venous thromboembolism. N Engl J Med. 2018;378(7):615–624.
- Sørensen HT, Mellemkjaer L, Olsen JH, et al. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343(25):1846–1850.
- Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol. 2018;36(20):2017–2023.
- Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799–808.
- Hokusai-VTE Investigators;Büller HR,Décousus H,Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. New Eng J Med. 2013;369(15):1406–1415.
- Kimpton M, Carrier M. Efficacy and safety of Xa inhibitors for the treatment of cancer-associated venous thromboembolism. Expert Opin Drug Saf. 2019;18(4):313–320.
- Guo W-Q, Chen X-H, Tian X-Y, et al. Differences in gastrointestinal safety profiles among novel oral anticoagulants: evidence from a network meta-analysis. Clin Epidemiol. 2019;11:911–921.
- Li A, Manohar PM, Garcia DA, et al. Cost effectiveness analysis of direct oral anticoagulant (DOAC) versus dalteparin for the treatment of cancer associated thrombosis (CAT) in the United States. Thromb Res. 2019;180:37–42.
- Giustozzi M, Agnelli G, del Toro-Cervera J, et al. Direct oral anticoagulants for the treatment of acute venous thromboembolism associated with cancer: a systematic review and meta-analysis. Thromb Haemost. 2020;120(7):1128–1136.
- Marin-Barrera L, Muñoz-Martin AJ, Rios-Herranz E, et al. A case-control analysis of the impact of venous thromboembolic disease on quality of life of patients with cancer: quality of life in cancer (QCA) study. Cancers. 2019;12(1):75.
- Lyman GH, Carrier M, Ay C, et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021;5(4):927–974.
- Noble S, Matzdorff A, Maraveyas A, et al. Assessing patients' anticoagulation preferences for the treatment of cancer-associated thrombosis using conjoint methodology. Haematologica. 2015;100(11):1486–1492.
- Lloyd AJ, Dewilde S, Noble S, et al. What impact does venous thromboembolism and bleeding have on cancer patients' quality of life? Value Health. 2018;21(4):449–455.
- Li A, Carlson JJ, Kuderer NM, Schaefer JK, et al. Cost-effectiveness analysis of low-dose direct oral anticoagulant (DOAC) for the prevention of cancer-associated thrombosis in the United States. Cancer. 2020;126(8):1736–1748.
- Streiff MB, Abutalib SA, Farge D, et al. Update on guidelines for the management of cancer‐associated thrombosis. Oncologist. 2021;26(1):e24–e40.
- McBane RD, Wysokinski WE, Le‐Rademacher JG, et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: the ADAM VTE trial. J Thromb Haemost. 2020;18(2):411–421.
- Agnelli G, Becattini C, Meyer G, et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020;382(17):1599–1607.
- Rubio Salvador A, Perez Segura P. Coste por paciente con heparina de bajo peso molecular en el tratamiento extendido de tromboembolismo venoso y la prevención de recurrencias en pacientes con cáncer activo. Albacete: XXXIX Jornada de la Asociación Economia de la Salud 2019.
- Guyot P, Ades AE, Ouwens MJNM, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9.
- Ende-Verhaar Y, Cannegieter S, Vonk Noordegraaf A, et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a contemporary view of the published literature. Eur Respir J. 2017;49(2):1601792.
- Kahn SR, Shapiro S, Wells PS, Rodger MA, et al. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383(9920):880–888.
- Spanish National Statistics Institute (INE). Mortality Tables by year, sex, age and functions. National results by region and province. Spain, INE; 2018.
- Tramontano AC, Schrag DL, Malin JK, et al. Catalog and comparison of societal preferences (utilities) for lung cancer health states. Med Decis Making. 2015;35(3):371–387.
- Hogg K, Kimpton M, Carrier M, et al. Estimating quality of life in acute venous thrombosis. JAMA Intern Med. 2013;173(12):1067.
- Locadia M, Bossuyt P, Stalmeier P, et al. Treatment of venous thromboembolism with vitamin K antagonists: patients' health state valuations and treatment preferences. Thromb Haemost. 2004;92(6):1336–1341.
- Preblick R, Kwong WJ, White RH, et al. Cost-effectiveness of edoxaban for the treatment of venous thromboembolism based on the Hokusai-VTE study. Hosp Pract. 2015;43(5):249–257.
- Lenert LA, Soetikno RM. Automated computer interviews to elicit utilities: potential applications in the treatment of deep venous thrombosis. J Am Med Inform Assoc. 1997;4(1):49–56.
- Ghofrani H-A, D'Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369(4):319–329.
- General Council of Pharmaceutical Associations. Drug Cost Database. BotPlus. Spain GCPA; 2020.
- Healthcare SM. Real Decreto-Ley 8/2010; 2020.
- Ministry SH. Registro de Atención Especializada (RAE-CMBD). Desde 2016 en adelante; 2021. Available from: https://pestadistico.inteligenciadegestion.mscbs.e
- Mukdad MA, Al-Badriyeh D, Elewa HF. Cost-effectiveness evaluations among the direct oral anticoagulants for the prevention and treatment of venous thromboembolism: systematic review. Clin Appl Thromb Hemost. 2019; 25:107602961984910.
- Given BA, Given CW, Vachon E, et al. Do we have a clue: the treatment burden for the patient with cancer? Cancer Nurs. 2016;39(5):423–424.
- Van Der Wall SJ, Klok FA, Den Exter PL, et al. Higher adherence to treatment with low-molecular-weight-heparin nadroparin than enoxaparin because of side effects in cancer-associated venous thromboembolism. Hemasphere. 2018;2(1):e19.
- Connell NT, Connors JM. Cost-effectiveness of edoxaban versus dalteparin for the treatment of cancer-associated thrombosis. J Thromb Thrombolysis. 2019;48(3):382–386.
- Matza LS, Boye KS, Stewart KD, et al. Health state utilities associated with attributes of weekly injection devices for treatment of type 2 diabetes. BMC Health Serv Res. 2017;17(1):774.
- Lee AYY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146–153.
- Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med. 2002;162(15):1729–1735.
- Deitcher SR, Kessler CM, Merli G, et al. Secondary prevention of venous thromboembolic events in patients with active cancer: enoxaparin alone versus initial enoxaparin followed by warfarin for a 180-day period. Clin Appl Thromb Hemost. 2006;12(4):389–396.
- Hull RD, Pineo GF, Brant RF, et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med. 2006;119(12):1062–1072.
- Lee AYY, Kamphuisen PW, Meyer G, et al. Tinzaparin tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677–686.