 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
While effective asthma control medications reduce the burden of asthma, a significant subgroup of these treatments, namely metered-dose inhalers (MDIs), produce substantial greenhouse gas (GHG) emissions, thus contributing to climate change. This study quantified the global climate impact (i.e. carbon dioxide equivalent [CO2e] emissions) and costs of long-term status quo asthma inhaler use versus alternative scenarios substituting MDIs with propellant-free dry powder inhalers (DPIs).
Methods
Three scenarios were evaluated across 10-year (2020–2030) and 50-year (2020–2070) time horizons: A (status quo inhaler use), B and C (2% and 5% year-over-year substitution of MDIs with DPIs, respectively). Global inhaler volumes and costs at baseline were sourced from IQVIA, then projected using UN and WHO trends in per capita GDP, urbanization, and asthma population growth. Inhaler spending was assumed to fall by 90% following generic entry in 2030. The carbon footprint per inhaler and health damage factors for disability-adjusted life years (DALYs) were derived from literature. The US government’s central and high-impact estimates for the social cost of carbon (SCC) were used to calculate emissions costs.
Results
Over 50 years, scenario A resulted in 826 million tonnes of CO2e emissions globally, with an associated SCC between 21% and 65% of the projected global spending on asthma inhalers. In comparison, CO2e emissions were reduced by 38% and 58% in Scenarios B and C, respectively, and DALYs improved by 33 and 51%. Depending on SCC estimates, Scenarios B and C increased global costs by 7.3% and 16.5%, respectively (central SCC), or decreased costs by 4.2% and 2.6% (high-impact SCC) versus Scenario A. Over 10 years, Scenario A resulted in 97 million tonnes of CO2e emissions globally, with an associated SCC between 4.4% and 12.2% of projected spending. In comparison, Scenarios B and C were associated with 12% and 24% reductions in CO2e emissions and improvements in DALYs by 11.5% and 22.7%, respectively.
Conclusions
Global efforts by environmental and health-policy decision-makers to substitute currently available MDIs with DPIs for asthma control would result in substantial reductions in GHG emissions with manageable costs, or potential cost savings, depending on the SCC. Policies that decrease use of MDIs warrant global attention.
Introduction
Asthma is a chronic respiratory disease of the airways and lungs that affects 339 million people worldwideCitation1. In 2016, asthma contributed 23.7 million disability-adjusted life years (DALYs) globally and was ranked 23rd among the leading causes of premature mortalityCitation1. While effective asthma control medications can reduce the clinical and life-quality burden of asthmaCitation2, access and utilization are heterogenous across the globeCitation1. The opportunity to improve global health outcomes by broadening access to effective asthma control is substantialCitation1. At the same time, some widely used classes of asthma control medications result in substantially higher greenhouse gas (GHG) emissions compared to other classes, thus contributing to global climate changeCitation1. While initial actions have been taken, most notably in England, to notify patients and prescribers of differences in GHG emissions associated with asthma therapy choicesCitation3,Citation4, controlling these emissions is a global collective action problem – with each individual country impacted more by the GHG emissions of all others than by its own. This raises important questions for global population health and environmental health. First, the question of global scale: i.e. what are the expected global clinical benefits, GHG emissions, and costs of increased access to guideline-recommended asthma care if the status quo mix of control medications is maintained? And secondly, how might these impacts change with a shift towards greater use of low-emissions inhalers for asthma control?
Inhalation therapy is the cornerstone of asthma management. Globally, pressurized metered-dose inhalers (MDIs) and dry powder inhalers (DPIs) are the most common types of inhaler devices used for asthmaCitation5. However, MDI use has a disproportionately large carbon footprint compared to DPI use. Specifically, currently available MDIs have a carbon footprint 20–30 times higher than DPIs per equivalent doseCitation6. This difference in carbon footprint is due to MDIs’ reliance on certain propellants (hydrofluoroalkanes, HFAs; Citation7) whereas DPIs are propellant-free. HFAs have high global warming potentials (GWPs), a measure of how much heat a GHG traps in the atmosphere over a specified time relative to carbon dioxide (CO2). Notably, HFAs used in currently available MDIs have GWPs 1,300–3,350 times higher than that of CO2Citation7–9. Annually, 630 million MDIs are used globally for treatment of all respiratory conditions, resulting in an estimated burden of 13 million tonnes of CO2 equivalent emissions (tCO2e), which is comparable to the carbon footprint of 2 million European Union (EU) citizensCitation10.
HFAs are already a target of global efforts to reduce GHG emissions. Unsurprisingly, environmental policy has historically focused on HFA use in industrial and refrigerant applications, which account for over 90% of HFA emissionsCitation6, while delaying or avoiding targeting inhalers due to economic and patient health considerations such as patient-borne costs, patient ability, and patient preferenceCitation11,Citation12. Notably, HFAs in inhalers are currently exempt from the HFA phase-out under the Kigali Amendment to the Montreal Protocol and are excluded from current EU regulations on fluorinated gases (F-gas), which are man-made gases, including hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), sulfur hexafluoride (SF6), that are often used as substitutes for ozone-depleting substancesCitation7,Citation12,Citation13. In the United States, recent legislation requires the Environmental Protection Agency to set necessary allowances for use of hydrofluorocarbons in MDIs to meet quantities needed “based on projected, current, and historical trends”Citation14. Currently, the use of MDIs accounts for only 2.3% of the F-gas contribution to global emissionsCitation15 or less than 0.1% of global GHG emissionsCitation16. Nevertheless, as demand for MDIs increases due to global population growth and increased asthma prevalence, and as F-gases are phased out from other sectors and applications, MDIs will represent a growing proportion of F-gas use across the globe and will continue to contribute to climate change. Thus, understanding the greenhouse gas emissions and costs implications of efforts aiming to reduce the carbon footprint of MDIs is important for future policy.
Recently, there have been increasing calls to consider GHG emissions as one component of prescribing decisions for MDIs and DPIs. England, through the National Health Service (NHS), has developed healthcare measures targeting the carbon footprint of high-emissions inhalers, such as offering patients lower-emissions inhalers when suitable, supporting better prescribing patterns to reduce the over-prescribing of inhalers, and improving the recovery and recycling of used inhalersCitation17. In addition, a patient decision aid developed by the National Institute of Care and Excellence (NICE) encourages consideration of carbon footprints when choosing inhalers and recommends DPIs as the most environmentally friendly optionCitation4. Through a shift to lower-emissions inhalers, such as DPIs, the NHS plans to reduce 4% of its carbon emissions by 2025Citation3. Additionally, since 2019, GINA guidelines no longer recommend SABA use as the only line of treatment for asthma symptom relief, and instead recommend the use of ICS-containing treatmentsCitation18. Given that the majority of SABAs are only available in MDI form while several ICS combinations exist in DPI form, these new guidelines provide an opportunity for substituting DPIs in place of MDIs.
Several studies have analyzed the climate impacts and costs of switching from currently available MDIs to alternative low-emissions inhalers at the national level. Janson et al.Citation6 found that reducing the proportion of respiratory inhalers that are MDIs in England from 70% to 13%, to match that of Sweden, would lead to an annual reduction of 550 kilotonnes CO2eCitation6. Wilkinson et al.Citation19 found that for every 10% of MDIs replaced with DPIs, 58 kilotonnes CO2e could be saved annually in England. They also estimated that for every 10% of MDIs replaced with DPIs following current prescription trends, the total cost would increase by £12.7 M whereas with every 10% of MDIs substituted with the cheapest DPI equivalent, England would save £8.2 M annually on drug costsCitation19.
To complement the growing number of studies on the country-level climate impacts of inhaler use, this study quantifies the global climate impacts of long-term status quo asthma inhaler use and estimates the long-term global CO2e emissions and cost impacts of substituting currently available MDIs with DPIs.
Methods
Market definition and scenarios
In this analysis, the global asthma inhaler market was restricted to MDIs and DPIs, which are the most used inhalation devices for asthma management. Asthma patients may also be prescribed soft-mist inhalers (SMIs), which are propellant-free handheld devices with a carbon footprint 20 times lower than MDIsCitation20. However, these devices account for less than 1% of the inhaler market and are only available for a few drug classes in a small number of countriesCitation21. Hence, to avoid making assumptions about SMI market share growth across all drug classes and country groups, SMIs are excluded from the analysis. In addition to SMIs, nebulizers are another type of inhalation device that asthma patients may use. Nebulizers are excluded from the analysis because they are not the primary devices recommended by GINACitation22 and BTSCitation23 guidelines and account for less than 1% of the inhaler marketCitation21. Instead, these devices are recommended for the minority of patients, mostly children, who cannot be taught how to properly use a spacer device. In the 2020 patient aid form provided by NICE, for instance, nebulizers are not listedCitation4. Lastly, asthma patients may also be prescribed low-GWP MDIs in the future. The development of these new devices, which use HFA152a and HFO1234a, is underwayCitation19. However, these technologies are not expected to enter the market until 2025Citation24 and are also excluded from this analysis.
In this analysis, three scenarios for global asthma inhaler use were investigated over a 50-year time horizon from 2020 to 2070. As a secondary analysis, the same three scenarios were investigated over a 10-year time horizon. Scenario A considered the continuation of status quo asthma inhaler use for DPIs and MDIs over the 10-year and 50-year time horizons. Scenario B considered a 2% year-over-year (YOY) increase in DPI market shares over the time horizons, and Scenario C considered a 5% YOY increase in DPI market shares over the time horizons. The choice of a 50-year time horizon was informed by current practice by agencies such as the Intergovernmental Panel on Climate Change (IPCC), which primarily uses a 100-year time horizon. Since greenhouse gases have long-lasting effects on global warming, which can extend for decades, understanding emissions and cost pathways over a longer timeframe is important for policy making. In addition to the 50-year time horizon, the analysis investigated a 10-year time horizon to reflect the effects of short-term global asthma inhaler consumption on global GHG emissions and costs, and to complement findings based on a 50-year time horizon. While from a climate perspective, this shorter time horizon may be less informative than a 50- or 100-year time horizon, it answers questions related to the short-term effects of global asthma inhaler consumption and is aligned with recent policy by agencies such as the United Nations, which have adopted a climate action plan for 2020–2030 to “respond to the climate emergency, as well as the serious consequences of continued inaction” Citation25.
The 2% and 5% YOY displacement scenarios model gradual shifts in market shares at the time of therapy initiation and switching within drug classes that exist in both MDI and DPI forms. These scenarios were chosen to investigate the effects of realistic policy interventions for asthma that are aligned with current commercial commitments and governmental climate goals to address the global climate crisis. For instance, in the United States, the Environmental Protection Agency has proposed a new rule directed at phasing down the production and consumption of HFCs by 85% over the next 15 yearsCitation14. In the automotive industry, companies such as Ford, General Motors and Volkswagen have committed to phasing out fossil-fuel vehicles in the next 20-years. Given these trends, it is likely that policy interventions aimed at reducing the carbon footprint of asthma care via substitution to lower-emissions inhalers are forthcoming.
Asthma inhaler volumes
For all scenarios, asthma inhaler volumes from year 2020 were derived from IQVIA market share data by inhaler type (i.e. DPI, MDI) and drug class (i.e. inhaled corticosteroids [ICS], long-acting beta-agonist [LABA], ICS/LABA, long-acting muscarinic antagonist [LAMA], ICS/LABA/LAMA, short-acting beta-agonist [SABA], short-acting muscarinic antagonist [SAMA], SAMA/SABA). Present-day (i.e. year 2020) asthma inhaler volumes in pack units and standard units were sourced from the IQVIA market share database for the 56 countries with available data. Volumes for countries not found in IQVIA were extrapolated by inhaler type and drug class using derived asthma inhaler consumption rates and asthma population estimates. To do this, average consumption rates were calculated for four income groups, reflecting groups of countries (i.e. high-income, upper-middle income, lower-middle income, low-incomeCitation26, using the World BankCitation26 classification) by dividing (1) overall 2020 inhaler volumes for each country with available market share data in each income group by (2) these countries’ total estimated asthma population size. Countries’ asthma population sizes were derived by multiplying total population estimates from the United Nations (UNCitation27) by asthma prevalence rates from the 2016 Global Burden of Disease studyCitation28 which were projected to 2020 using urbanization rates from the UN.Citation29 Then, 2020 volumes for countries not listed in IQVIA were imputed by multiplying the average consumption rate of the country’s income group by the country’s estimated 2020 asthma population size. Finally, to arrive at 2020 inhaler volumes for each income group by inhaler type and drug class, the average asthma inhaler consumption rate (updated with the imputed country estimates) of each income group was multiplied by the total estimated asthma population for that income group. Global volumes were then calculated by summing the volumes across income groups.
For all scenarios, total asthma inhaler volumes were projected from 2021 to 2070 by income group based on annual trends of the income group’s asthma population and GDP per capita, using the formula below. Asthma populations were projected to 2070 using the trends in urbanization rates from the UNCitation29, and GDP per capita trends were based on projections made by the World Bank and the World Health Organization (WHO).Citation30,Citation31
Market shares
The distribution of total inhaler volumes (i.e. market shares) across inhaler type and drug class in 2020 was calculated by income group for all scenarios using the derived 2020 volumes. Changes in market share across inhaler type and drug class were then projected over the time horizon separately for the three scenarios. For Scenario A, IQVIA market share data from 2015 was used for drug classes with historical data available. It was assumed that the 2020–2025 and 2025–2030 changes in market share for each income group would be equal to the changes in market share from 2015 to 2020 of that income group. After 2030, it was assumed that the market shares remained constant through 2070. For the ICS/LABA/LAMA drug class, there were no historical data available due to its market entry in 2020 for the treatment of asthma. As such, the following was assumed: market shares for ICS/LABA/LAMAs increased by 5% from 2020 to 2025, and by an additional 5% and 10% from 2025 to 2030 and 2030 to 2070, respectively. Furthermore, given current trends in urbanization ratesCitation29 and GDP per capita growthCitation32, it was assumed that between 2020 and 2030, the consumption of ICS/LABA/LAMAs would be highly concentrated in the high income and upper-middle income countries, making up 33% and 50% of the global market share, respectively. However, by 2070, the global market share would decrease to 11% in high-income countries, 33% in upper-middle income countries, and increase to 50% in lower middle-income countries, which were projected to have a larger asthma population; the remaining market shares were then attributed to low-income countries. Based on these, the projected market shares for other drug classes were consequently normalized by the market share for ICS/LABA/LAMAs for 2025, 2030, and 2070. Annual market shares were then derived for all drug classes by inhaler type using linear interpolation.
For Scenario B, a 2% YOY increase was applied by drug class to DPI market shares for each income group. Similarly, for Scenario C, a 5% YOY increase was applied by drug class to DPI market shares for each income group. Any increase in DPI market share in these two scenarios was displaced from the MDI market share. In this way, inhaler volumes over the time horizon were constant across the three scenarios, while DPI and MDI market shares by drug class were varied across scenarios.
CO2e emissions for asthma inhalers
CO2e emissions associated with currently available MDIs and DPIs were calculated for all scenarios using the carbon footprint of inhalers and the derived volume of inhalers in pack units. The carbon footprint associated with the use and disposal of MDIs was extracted by drug class from Wilkinson et al.Citation19 and estimated by multiplying the weight of HFA propellants in each MDI drug class by its 100-year GWPCitation19. For each MDI drug class, the midpoint of the range of carbon footprints was used. For DPIs, a carbon footprint of 1 kg CO2e for the use and disposal of an inhaler was assumed, irrespective of drug class. The annual carbon footprint of asthma inhalers was calculated by multiplying the carbon footprints per inhaler by the corresponding projected inhaler volumes in pack units. Cumulative emissions due to MDIs and DPIs were aggregated at the global level for each scenario.
Costs of asthma inhalers
Costs of asthma inhalers were derived using average costs per inhaler in standard dose units and projected volumes in standard dose units. The average cost per DPI in 2020 was calculated by dividing global (i.e. across income groups) costs for DPIs by the total global volume of DPIs. The same was done for MDIs. For all subsequent years, aggregate global costs for DPIs and MDIs were projected by multiplying average 2020 costs per inhaler by the corresponding inhaler volumes of the corresponding year. Across scenarios, estimated spending on inhalers reflected generic entry. Based on patent expiration dates for the top 10 asthma inhalers in the market in 2020, which were retrieved from the U.S Food and Drug Administration’s Orange BookCitation33 spending on inhalers was assumed to undergo a 50% reduction at year 2030, and a 90% reduction for all subsequent years in the time horizon. Global inhaler costs were discounted at a 3% annual rate. Cumulative inhaler costs were aggregated at the global level for each of the three scenarios.
Social cost of carbon (SCC) due to asthma inhalers
The value of damages associated with an incremental increase in carbon emissions in a given year was monetized using the SCC, which accounts for the societal value of market and non-market climate change impacts, including changes in net agricultural productivity, human health, energy systems, and the environment.Citation34 Two sets of SCC values were sourced from the United States Government Interagency Working Group on Social Cost of Greenhouse Gases: (1) the central SCC estimate and (2) the high-impact SCC estimateCitation34. These estimates derive from three integrated assessment models. The central SCC estimate was based on the average SCC, and the high-impact SCC represents higher-than-expected economic impacts from climate change at the 95th percentile of the SCC distributionCitation34. Both SCC estimates vary by year and are provided for years 2020 − 2050. Linear extrapolation was applied to derive annual SCC estimates for years 2051 − 2070. For each year over the time horizon, the two sets of SCC values were separately multiplied by total CO2e emissions to derive a total annual SCC estimate. Cumulative SCC estimates were aggregated at the global level for each scenario using a 3% annual discount rate.
Per capita costs
Per capita costs were calculated over the time horizon by dividing annual total spending due to inhaler use and the SCC with the total asthma population for each year. The average annual per capita costs were then calculated by taking the mean over each time horizon.
DALYs associated with emissions
CO2e emissions due to inhaler use were converted to DALYs to capture their health impact. In principle, health impacts captured by DALY estimates are captured by the SCC, and thus these impacts are not incremental to the SCC. DALY estimates reflect health damage factors (i.e. changes in DALYs per unit of CO2 emissions) estimated by Tang et al.Citation35, who estimated these factors based on expected temperature change and temperature-dependent disease burdenCitation35. Two damage factors, each corresponding to different 100-year climate change scenarios projected by the Intergovernmental Panel on Climate Change (IPCC) were consideredCitation36. Scenario A1B is characterized by very rapid economic growth with a peak and subsequent decline in the global population mid-century, and its estimated damage factor was 2.0 × 10−7 DALYs/kg CO2eCitation35,Citation36. Scenario A2 is characterized by regionally oriented economic development with a continually increasing global population, and its estimated damage factor was 6.2 × 10−7 DALYs/kg CO2e.Citation35,Citation36. These two damage factors “bookend” the range of expected damage factors associated with the other climate scenarios assembled by the IPCC. DALYs were calculated for Scenarios A, B, and C by separately multiplying annual global CO2e emissions by each damage factor and were discounted at an annual rate of 3%. Cumulative DALYs were aggregated at the global level for each scenario.
Results
DPI market shares
Over the 50-year time horizon, trends in global DPI market shares varied across scenarios. For Scenario A, in both standard dose units and pack units, trends in DPI market shares were relatively flat over the 50-year time horizon. DPI market shares changed slightly from 22.9% in 2020 to 20.6% in 2070 in standard dose units, and from 36.2% to 35.4% from 2020-2070 in pack units. For Scenario B, there was an upward trend in global DPI market shares over the 50-year time horizon. DPI market shares increased from 22.9% in 2020 to 48.4% in 2070 in standard dose units, and from 36.2% in 2020 to 61.5% in 2070 in pack units. Scenario C saw the steepest increase in global DPI market shares over the time horizon. In standard dose units, DPI market shares increased from 22.9% in 2020 to 78.7% in 2070 and increased from 36.2% in 2020 to 83.6% in 2070 in pack units ().
Figure 1. Global DPI market shares across scenarios. Abbreviations. DPI, dry powder inhaler; PU, pack units; SU, standard dose units
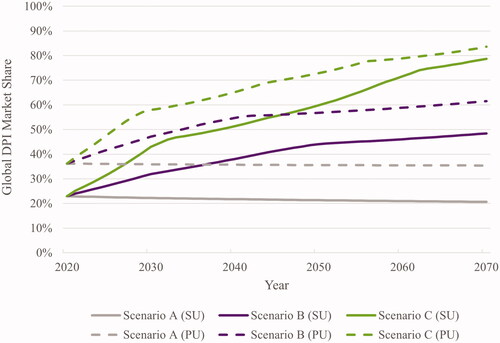
Over the 10-year time horizon, global DPI market shares for Scenario A changed slightly from 22.9% in 2020 to 22.2% in 2030 in standard dose units, and from 36.2% to 35.9% in pack units. For Scenario B, the global DPI market shares increased from 22.9% in 2020 to 31.9% in 2030 in standard dose units, and from 36.2% to 47.1% in pack units. Global DPI market shares increased the most in Scenario C. In standard dose units, DPI market shares increased from 22.9% in 2020 to 42.9% in 2030, and from 36.2% to 57.9% in pack units ().
GHG emissions
Over the 50-year time horizon, Scenario A resulted in approximately 826 million tCO2e, of which 21 million tonnes (2.6%) were attributable to DPIs, and 805 million tonnes (97.4%) were attributable to MDIs (). In Scenario B, total GHG emissions due to inhaler use was approximately 513 million tCO2e, of which 33 million tonnes (6.5%) were attributable to DPIs, and 480 million tonnes (93.5%) were attributable to MDIs (). Of the three scenarios, scenario C resulted in the lowest GHG emissions from inhaler use with a total carbon footprint of approximately 346 million tCO2e. Of the total GHG emissions attributable to inhaler use, 43 million tonnes (12.3%) were due to DPIs, and 303 million tonnes (87.7%) were due to MDIs (). Compared with Scenario A, Scenario B had 38% lower CO2e emissions (). Compared with Scenario A, Scenario C was associated with a 58% reduction in CO2e emissions ().
Table 1. Global CO2e emissions, costs, and DALYs across status quo scenario and scenarios of DPI market share increases (2020–2070) – Central value of social cost of carbon.
Table 2. Differences in global CO2e emissions, costs, and DALYs across status quo scenario and scenarios of DPI market share increase (2020–2070) – Central value of social cost of carbon.
provides GHG emissions estimates over the 10-year time horizon between 2020 and 2030. Scenario A resulted in 97 million tCO2e, of which 94.5 million tonnes (97.3%) were associated with MDI use and the rest to DPI use. In scenario B, total GHG emissions due to inhaler use were estimated at approximately 85 million tCO2e, of which 3 million tonnes (3.6%) were attributable to DPIs and 82 million tonnes (96.4%) to MDIs. Like the 50-year projections, scenario C resulted in the lowest GHG emissions from inhaler use, 74 million tCO2e, with 70 million tonnes (95.2%) due to MDI use and about 4 million tonnes (4.8%) to DPI use. Compared with Scenario A, Scenarios B and C were associated with 12% and 24% reductions in global CO2e emissions, respectively ().
Table 3. Global CO2e emissions, costs, and DALYs across status quo scenario and scenarios of DPI market share increases (2020–2030) – Central value of social cost of carbon.
Table 4. Differences in global CO2e emissions, costs, and DALYs across status quo scenario and scenarios of DPI market share increase (2020–2030) – Central value of social cost of carbon.
Total costs
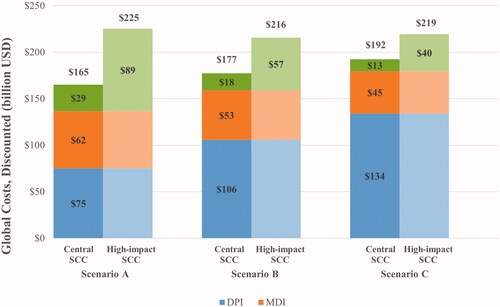
Assuming the central SCC estimate, total discounted costs due to inhalers and the SCC over the 50-year time horizon were $165 billion in Scenario A, $177 billion in Scenario B, and $192 billion in Scenario C (). The percentage of total inhaler costs due to DPIs (rather than MDIs) increased in Scenarios B and C, relative to Scenario A. In Scenario A, DPI inhalers made up 54.9% of the projected global spending on asthma inhalers, whereas in Scenarios B and C, they made up 66.6% and 74.7%, respectively (). The total SCC relative to total inhaler costs decreased in Scenarios B and C, relative to Scenario A. In Scenario A, the total SCC was 21.0% of total projected global spending on asthma inhalers, whereas in Scenarios B and C, it was 11.6% and 7.2%, respectively (). Compared with Scenario A, Scenarios B and C were associated with a 7.3% and 16.5% increase in total costs, respectively ().
Figure 2. Global costs across status quo scenario and scenarios of DPI market share increase (2020–2070). Abbreviations. DPI, dry powder inhaler; MDI, metered dose inhaler; SCC, social cost of carbon; USD, United States dollars.
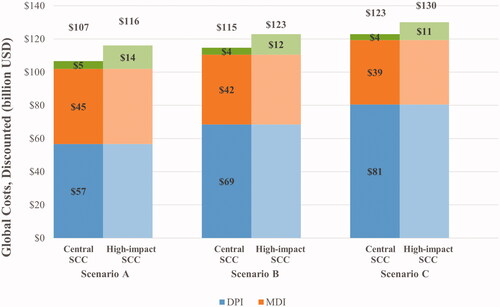
Over the 10-year time horizon, total discounted costs due to inhaler use and the SCC were $107 billion in Scenario A, $115 billion in Scenario B, and $123 billion in Scenario C, assuming the central SCC value (). DPIs accounted for 55.6%, 62.0%, and 67.5% of inhaler costs in Scenarios A, B, and C, respectively. In Scenario A, the total SCC accounted for 4.4% of the total global spending on asthma inhalers and decreased to 3.6% and 2.9% in scenarios B and C, respectively ().
Figure 3. Global costs across status quo scenario and scenarios of DPI market share increase (2020-2030). Abbreviations. DPI, dry powder inhaler; MDI, metered dose inhaler; SCC, social cost of carbon; USD, United States dollars.
Based on the high-impact SCC estimate, total costs due to inhalers and the SCC were $225 billion in Scenario A, $216 billion in Scenario B, and $219 billion in Scenario C, over the 50-year time horizon (). As with the central SCC estimate, total spending on inhalers attributable to DPIs (rather than MDIs) increased in Scenarios B and C, relative to Scenario A. In Scenario A, spending on DPI inhalers was about 54.9% of the projected global spending on asthma inhalers, whereas in Scenarios B and C, they were 66.6% and 74.7%, respectively (). In Scenario A, the total SCC amounted to 65.0% of the projected global spending on asthma inhalers, while it was 35.8% and 22.2% in Scenarios B and C, respectively (). Compared with Scenario A, Scenarios B and C decreased total costs by 4.2% and 2.6%, respectively ().
Table 5. Global CO2e costs between the status quo scenario and scenarios of DPI market share increase (2020–2070) – High-impact social cost of carbon.
Table 6. Differences in global CO2e costs across status quo scenario and scenarios of DPI market share increase (2020–2070) – High-impact social cost of carbon.
Over the 10-year time horizon, total costs due to inhaler use and the SCC were $116 billion in Scenario A, $123 billion in Scenario B, and $130 in Scenario C, assuming the high-impact SCC (). As for the 50-year projections, total costs attributable to DPIs increased in Scenarios B and C, relative to Scenario A (). In Scenario A, the total SCC accounted for 12.2% of the projected global spending on asthma inhalers, while it was 10.1% and 8.3% in Scenarios B and C, respectively. Compared with Scenario A, Scenarios B and C increased total costs by 5.9% and 12.1%, respectively ().
Table 7. Global CO2e costs between the status quo scenario and scenarios of DPI market share increase (2020–2030) – High-impact social cost of carbon.
Table 8. Differences in global CO2e costs across status quo scenario and scenarios of DPI market share increase (2020–2030) – High-impact social cost of carbon.
Per capita costs
Assuming the central SCC estimate, average annual per capita costs of asthma inhaler use over the 50-year time horizon were $7.82 in Scenario A, $8.28 in Scenario B, and $9.07 in Scenario C (). Over the 10-year time horizon, average annual per capita costs of asthma inhaler use were $26, $27, and $29 in Scenarios A, B, and C, respectively ().
Assuming the high-impact SCC estimate, average annual per capita costs of asthma inhaler use over the 50-year time horizon were estimated at $10.3, $10.0, and $10.2 for Scenarios A, B and C, respectively (). Over the 10-year time horizon, average annual per capita costs were $28, $29, and $31 in Scenarios A, B, and C, respectively ().
DALYs
Over the 50-year time horizon, using the damage factors of both the A1B and A2 IPCC scenarios, estimated total DALYS were highest in Scenario A and lowest in Scenario C. Based on the A1B IPCC scenario, 73,848 DALYs were estimated in Scenario A, 49,371 in Scenario B, and 36,045 in Scenario C. Based on the A2 IPCC scenario, estimated DALYs were 228,928 in Scenario A, 153,051 in Scenario B, and 111,740 in Scenario C (). Relative to Scenario A, DALYs decreased in Scenarios B and C by 33.1% and 51.2%, respectively ().
Over the 10-year time horizon, total DALYs were also highest in Scenario A and lowest in Scenario C. Based on A1B IPCC scenario, 16,656 DALYs were estimated in Scenario A, 14,746 in Scenario B, and 12,875 in Scenario C. Based on the A2 IPCC scenario, estimated DALYs were 51,633 in Scenario A, 45,712 in Scenario B, and 39,913 in Scenario C (). Compared to Scenario A, DALYs decreased by 11.5% and 22.7% in Scenarios B and C, respectively ().
Discussion
This study presents the first global, long-term evaluation of CO2e emissions associated with asthma control, and the projected costs and benefits of substituting currently available MDIs with DPIs at a global level. The study presents results across a 10-year time horizon as well as a 50-year time horizon, informed by practices and policy by governmental bodies, which reflect the need for both short and long-term assessments of the climate impacts of status quo operations. Our results indicate that continuation of status quo asthma inhaler use for the next 50 years would result in global emissions of over 800 million tCO2e. Over the 50-year time horizon, 2% and 5% YOY increases in DPI market shares, replacing currently available MDIs, would reduce these GHG emissions by 38% and 58%, respectively, compared with status quo inhaler use. Likewise, 2% and 5% YOY increases in DPI market shares, replacing MDIs, would improve DALYs by 33% and 51%, respectively. When considering the nearest 10 years, 2% and 5% YOY increases in DPI market shares, replacing MDIs, would result in decreases in GHG emissions by 12% and 24%, respectively. These reductions in global GHG emissions over the 10-year time horizon were associated with an improvement in global DALYs by 12% and 23% for Scenarios B and C, respectively. As compared with continuation of the status quo over a 50-year time horizon, YOY increases in DPI market shares can result in cost savings if the SCC equals the high-impact estimate, or cost increases if the SCC equals the central value estimate. For the 10-year time horizon, on the other hand, the 2% and 5% YOY increases in DPI market share would lead to cost increases across both SCC values.
In terms of per capita costs of asthma inhaler use, the study shows that scenarios favoring substitution of MDIs with DPIs result in similar per patient costs in absolute terms. Over the 10-year time horizon, per capita costs were $26, $27, and $29 assuming central SCC and $28, $29, $31 assuming high-impact SCC for Scenarios A, B and C, respectively. Over the 50-year time horizon, the average per capita costs decreased to $7.8, $8.4, and $9.1 (central SCC) and $10.3, $10.0, and $10.2 (high-impact SCC) for Scenarios A, B, and C, respectively, due to the availability of generic alternatives. This shows that despite the global increases in spending incurred from substituting MDIs with DPIs, differences in per patient costs across scenarios are modest.
We found that for the status quo scenario and across the 50-year time horizon, MDIs represented about 80% of the global inhaler market in standard dose units and were responsible for 97% of estimated GHG emissions due to inhalers. Continuation of status quo MDI inhaler use for treatment of asthma over 50 years would result in GHG emissions that are equivalent to the total annual carbon footprint of 172 million individuals globallyCitation37 or close to 175 million passenger vehicles driven for one year (US Environmental Protection Agency). Our findings highlight the negative climate externalities of status quo asthma inhaler use, which are expected to increase with growth in the global asthma population and in access to guideline-recommended care. To date, MDIs are the most frequently used inhalers for treatment of respiratory conditions, despite their high carbon footprint. Higher MDI market shares likely reflect their earlier entry into the market and their lower price point relative to alternative low-emissions inhalers. Every year, close to 800 million MDIs are manufactured globally using approximately 11,500 tonnes of HFA propellantCitation38. In 2014, HFAs released from MDIs were about 3% of the GWP-weighted CO2e emissions of all HFAs globallyCitation6. Assuming the current trajectory of MDI usage remains the same, HFAs from MDIs will likely represent a growing proportion of total F-gas emissions as HFA uses in other sectors are phased out due to international agreements and increased national regulations to combat climate change.
In this analysis, we used two robust SCC estimates to derive a plausible range of values for total costs across the three scenarios. We found that across both the 10-year and 50-year time horizons, using the central SCC estimate resulted in increased costs for scenarios favoring substitution of currently available MDIs with DPIs as compared with the status quo scenario. On the other hand, using the high-impact SCC estimate resulted in cost savings for scenarios favoring substitution of MDIs with DPIs as compared with the status quo scenario over the 50-year time horizon. Our findings imply that whether a substitution scenario is cost-effective or not depends not only on the price of the device but also on the true economic cost of damages from CO2e emissions. While the SCC accounts for the societal value of climate change impacts, the three integrated assessment models used to estimate the SCC may not capture the economic effects of all possible adverse consequences of climate changeCitation34. As such, the SCC estimates used for this analysis may be underestimates of the true SCC. Given this, it is plausible that scenarios favoring substitution of MDIs with DPIs may be even more cost-effective relative to the continuation of status quo asthma inhaler use, assuming the entry of generic DPIs by year 2030, estimated from a review of patent expiration dates of current inhalers. While MDIs typically have a lower price point than DPIs, results from our analysis indicate that the environmental costs of long-term MDI use far outweigh that of DPIs. Notably, for the status quo scenario, the social cost of CO2e emissions was substantially higher than for the other two scenarios and amounted to 21% − 65% of the projected global spending on inhalers over 50 years, highlighting the negative environmental impact of currently available MDIs. However, our results show that the balance between lower environmental costs and higher inhaler costs is sensitive to the SCC. Our findings are supported by previous studies that have highlighted the high carbon footprint of MDIs and complement these studies by elucidating the long-term environmental costs of the continuation of asthma inhaler use that favors MDIs over DPIsCitation6,Citation10,Citation19. In our study, scenarios that favored substitution of currently available MDIs with DPIs were associated with improved DALYs due to GHG emissions as compared with the status quo scenario, highlighting the negative health impact of status quo asthma inhaler use. Both MDIs and DPIs are clinically recommended for the appropriate treatment of asthma, and as such, confer critical health benefits to patients. However, results from this study suggest that the benefits of MDIs for treatment of asthma should be evaluated considering the global health consequences that may ensue from their high carbon footprint. Over a 50-year time horizon, continuation of status quo asthma inhaler use is expected to contribute to global increases in GHG emissions, which can alter the global climate and result in negative consequences for human health. These potential health consequences include an increase in respiratory disease, cardiovascular disease, injuries, and premature deaths, which are driven by extreme weather events, changes in the prevalence and geographical distribution of food- and water-borne illnesses, and threats to mental healthCitation39.
In addition to cost concerns, a few studies have argued that some patients benefit medically from MDIs, arguing that some patients (e.g. the elderly) are unable to generate the inspiratory flows necessary to activate DPIsCitation40,Citation41. However, other studies have found that most DPI users are in fact capable of generating enough inspiratory flow to operate the inhaler efficientlyCitation42. Moreover, several studies have found DPIs to be equally or more efficacious as MDIs for treating asthmaCitation43–46. In contrast with MDIs, DPIs do not require priming that often leads to drug release into the atmospthereCitation47 and they are not associated with problems such as difficulty in coordinating actuation and inhalation which are common among MDI usersCitation48. Additionally, recent updates to the GINA guidelines suggest that some drug classes such as SABAs, traditionally delivered in MDI form, may lead to adverse events that warrant a switch towards combination therapies such as ICS/LABAs which are available as DPIs. Another key consideration for switching to DPIs is the patients’ perspective on making such a switch. Patient preferences regarding the choice of DPIs over MDIs is multifaceted. While a recent study suggested that asthma patients prefer pressurized inhalers over DPIsCitation49 another study by Liew and Wilkinson found that most patients prescribed inhalers are as equally concerned about the carbon footprint of an inhaler as they are about its costCitation50. This suggests that while nonconsensual switching is unadvised and some patients may benefit from the continued use of MDIs, current efforts made by bodies such as NICE and NHS to promote lower-emissions inhalers may be aligned with the preferences of a subset of the asthma population. Taken together, global policies to implement switching to low-emissions inhalers for asthma care should account for patient needs and perspectives, to enable their successful integration. In the near term, other actions to decrease the carbon footprint of asthma care include improving the disposal and recycling of used inhalersCitation51 and improving asthma control through better diagnosis and assessment to reduce SABA use and asthma-related hospital admissionsCitation16.
As this analysis consists of global projections over 10-year and 50-year time horizons, the present study has important limitations. Several assumptions were employed to make these projections. Data available from a set of 56 countries was used to impute information on asthma inhaler volumes for other countries (based on GDP per capita and asthma population size). Additionally, this analysis assumed that inhaler sale volumes corresponded to inhaler consumption, which could potentially lead to overestimates of GHG emissions and costs if more inhalers were sold than consumed (assuming proper disposal of unused inhalers). To model generic entry considerations, this analysis assumed spending on inhalers would undergo a 90% reduction over the time horizon after generic equivalents become availableCitation52. If reductions in inhaler costs are more conservative following generic entry, total costs across scenarios would be higher than observed, and scenarios favoring DPI substitution may be less cost-effective than observed in the current analysis. While robust, the SCC estimates used in this study contain some uncertainty and may not capture the economic effects of all possible adverse consequences of climate change. Therefore, total costs due to GHG emissions across scenarios may be underestimates. Additionally, this study does not report on GHG emissions associated with potential adverse events due to switching inhalers. The study models a gradual rate of shift in inhaler market shares achievable with choices made at the time of therapy initiation and other switches agreed upon by patients and providers. As such, it was assumed that prescribers will not subject patients to increased risk of hospitalization or other harm and that clinical outcomes were unaffected in scenarios substituting MDIs with DPIs. Moreover, there is not consensus regarding the clinical effects of switching between inhalers in the literature. While studies such asCitation53 found that patients switching from a pressurized MDI to a DPI experienced better asthma control assessed using the Asthma Control Test (ACT) and the use of rescue medication, other studies such as Thomas et al.Citation54 and Ekberg-Jansson et al.Citation55 suggested that switching devices, even in the case of a DPI to DPI switch as is the case in Ekberg-Jansson et al.Citation55 may lead to worse asthma control, higher use of short-acting β-agonists, and higher number of general practitioner visits and hospitalizations. Given this disparity in the findings from literature, this study does not incorporate the carbon footprint of these adverse events due to switching inhalers. Should these events occur, policies aimed at phasing out MDIs in favor of DPIs should consider additional GHG emissions that may result from worse asthma control due to device changes. Another limitation is that the present study focuses on the carbon footprint associated with the use of currently available MDIs and DPIs instead of the life cycle environmental impact of inhalers. While DPIs have a much lower global warming potential than MDIs, their environmental impact may be higher when assessed using a life cycle assessment approachCitation56. Future studies evaluating the environmental impacts and costs of switching from MDIs to DPIs that use a life cycle assessment approach are needed to elucidate on the broad environmental impacts of switching to DPIs. Additionally, the model does not incorporate technological changes that could impact the effectiveness, costs, or GHG emissions associated with asthma care. One potential change on the horizon is MDIs incorporating alternative low GWP propellants, three of which are under investigation: HFA152a, HFO1234a, and isobutaneCitation19. HFA152a and HFO1234a are in the earliest stages of development and isobutane has yet to be commercialized. As the regulatory process needed for the approval of these products involves numerous steps, it is estimated that transition to novel MDI propellants would likely take at least a decade based on the timeline for the transition from chlorofluorocarbon-based MDIsCitation19. Moreover, the costs of MDIs incorporating these alternative propellants are presently unknown. Finally, the present study does not investigate the long-term effects of more aggressive scenarios substituting currently available MDIs with DPIs. As the global climate crisis has become widely recognized, there have been multiple efforts to bring about change across countries and in specific industries. Given this, one could argue that a more rapid switch from MDIs to DPIs, for instance a 10% YOY increase in DPIs, is a more suitable aspiration for reducing global GHG emissions. However, there are several factors that must be considered when instituting more aggressive scenarios substituting MDIs with DPIs. First, there are cost considerations which may prohibit an aggressive replacement of currently available MDIs with DPIs in some regions in the near future. As highlighted in the literature and in our manuscript, across drug classes, DPIs are more expensive than MDIs. As such, cost pressures facing patients should be considered when designing more aggressive policies, to encourage patient adherence to treatment. Second, patient preference is an important driving factor for an aggressive substitution of MDIs with DPIs. To date, studies have shown disparity in patients’ opinions of different asthma inhaler devices, which in turn affects adherence and treatment successCitation46,Citation49. Finally, there are expected device changes in the horizon for MDIs, notably the manufacturing of low GWP alternatives, which are expected to enter the market by 2025Citation16. If these new devices have the same efficacy and inhalation techniques as current MDIs, it is likely that physicians will recommend them for patients instead of switching to a DPI. Taken together, while more aggressive substitution of currently available MDIs with DPIs may result in further reductions in GHG emissions, they would also lead to higher global costs compared to the status quo and the conservative scenarios explored in this study, which may make them difficult to implement in practice. Moreover, such policies should also integrate patients’ perspectives to guarantee continued treatment compliance and success.
There are several strengths to our study. Our economic projections are built upon well-established data and projections from bodies such as the WHO, the World Bank, and the UN, which are used for policy planning. The global approach used in this analysis to quantify environmental impacts and total costs across scenarios is aligned with the fact that emissions and their impacts are global. Countries that reduce emissions are still subject to the impact of emissions arising from other countries. Finally, our modeled 2% and 5% YOY increases in DPI shares and limiting substitutions to inhalers of the same drug class, result in gradual changes in MDI/DPI shares that are plausibly achievable from a clinical perspective – supposing that policies and economic arrangements were in place to support the shift.
Conclusions
Findings from this global economic analysis underscore the high carbon footprint and societal costs associated with status quo asthma inhaler use. Climate change is a present threat to human health and the environment and has both immediate and long-term consequencesCitation57. As the global asthma population grows and there is, ideally, increased access to guideline-recommended care, GHG emissions from currently available MDIs are expected to grow under status quo utilization patterns. Global efforts by environmental and health-policy decision-makers to gradually substitute currently available MDIs with DPIs for asthma control would result in substantial reductions in GHG emissions with manageable costs, or potential cost savings, depending on the realized SCC over a 50-year time horizon. Policies that support decreased use of currently available MDIs warrant global attention.
Transparency
Declaration of funding
This study was funded by Novartis Pharma AG, whose employees were involved in the study design, writing of the report, and the decision to submit the paper for publication. Novartis Pharma AG had no undue influence over the study results/findings.
Declaration of financial/other relationships
IF, JS, KKS, KN and TS are employees of Analysis Group, Inc. a consultancy that received funding from Novartis Pharma AG to conduct this study. NS was previously an employee of Analysis Group, Inc, which received financial support from Novartis Pharma AG. JM and RI are employees of and own stocks in Novartis Pharma AG. JM received editorial support (preparation, review, and finalization of the manuscript) for this manuscript from Analysis Group; Analysis Group received funding for editorial support from Novartis Pharma AG. All Analysis Group authors (IF, JS, KKS, KN, TS, and NS) provided the editorial support. AC and HK are employees of Novartis Healthcare Private Limited.
A reviewer on this manuscript has disclosed that they have performed consulting, served on advisory boards, or received travel reimbursement from Amphastar, AstraZeneca, Chiesi, Connect Biopharma, GlaxoSmithKline, Mylan, Novartis, Sunovion and Theravance. They have also conducted multicenter clinical research trials for some 40 pharmaceutical companies. The other peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
All authors contributed to the study design and conception. IF, KKS, KN, and NS conducted the analyses. IF, JS, KKS, KN, NS, and TS drafted the initial manuscript. All authors revised the manuscript. All authors approved the final manuscript as submitted.
Previous presentation
These data were previously presented at the ISPOR EUROPE 2021 Annual Congress.
Acknowledgements
No assistance in the preparation of this article is to be declared.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Global Asthma Network. The global asthma report. 2018. Available from: http://globalasthmareport.org/resources/Global_Asthma_Report_2018.pdf.
- Lee LK, Obi E, Paknis B, et al. Asthma control and disease burden in patients with asthma and allergic comorbidities. Journal of Asthma. 2018;55(2):208–219.
- National Health Society. Long term plan. 2019. Available from: https://www.longtermplan.nhs.uk/wp-content/uploads/2019/08/nhs-long-term-plan-version-1.2.pdf.
- National Institute for Health and Care Excellence. Patient decision aid: inhalers for asthma. 2020. Available from: https://www.nice.org.uk/guidance/ng80/resources/inhalers-for-asthma-patient-decision-aid-pdf-6727144573.
- Lavorini F, Corrigan CJ, Barnes PJ, et al. Retail sales of inhalation devices in European countries: so much for a global policy. Respir Med. 2011;105(7):1099–1103.
- Janson C, Henderson R, Löfdahl M, et al. Carbon footprint impact of the choice of inhalers for asthma and COPD. Thorax. 2020;75(1):82–84.
- Myrdal PB, Sheth P, Stein SW. Advances in metered dose inhaler technology: formulation development. AAPS PharmSciTech. 2014;15(2):434–455.
- Greenhouse Gas Protocol. Global warming potential values. Available from: https://www.ghgprotocol.org/sites/default/files/ghgp/Global-Warming-Potential-Values%20%28Feb%2016%202016%29_1.pdf.
- Myhre G, Shindell D, Bréon FM, et al. Anthropogenic and natural radiative forcing. In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, editors, Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge, United Kingdom and New York, United States: Cambridge University Press; 2013.
- Ortsäter G, Borgström F, Soulard S, et al. A budget impact model to estimate the environmental impact of adopting RESPIMAT® re-usable in the Nordics and Benelux. Adv Ther. 2019;36(12):3435–3445.
- Ozone Secretariat UN Environment Programme. Medical and Chemicals Technical Options Committee 2018 assessment report. 2018. Available from: https://ozone.unep.org/sites/default/files/2019-04/MCTOC-Assessment-Report-2018.pdf.
- Ozone Secretariat UN Environment Programme. Handbook for the Montreal protocol on substances that deplete the ozone layer. Nairobi (Kenya): Ozone Secretariat UN Environment Programme; 2020.
- European Environment Agency. Fluorinated greenhouse gases 2019. 2019. Available from: https://www.eea.europa.eu/publications/fluorinated-greenhouse-gases-2019.
- Environmental Protection Agency. Phasedown of hydrofluorocarbons: establishing the allowance allocation and trading program under the American Innovation and Manufacturing Act. 2021. Available from: https://www.govinfo.gov/content/pkg/FR-2021-05-19/pdf/2021-09545.pdf.
- Pritchard JN. The climate is changing for metered-dose inhalers and action is needed. Drug Des Devel Ther. 2020;14:3043–3055.
- Pernigotti D, Stonham C, Panigone S, et al. Reducing carbon footprint of inhalers: analysis of climate and clinical implications of different scenarios in five European countries. BMJ Open Resp Res. 2021;8(1):e001071.
- Sustainable Development Unit (Producer). Carbon hotspot: reducing inhalers. Available from: https://www.sduhealth.org.uk/documents/AboutUs/LTP%20comms/inhalers%20(2%20slides)%20v1.1.pptx.
- Global Initiative for Asthma. Asthma management and prevention for adults and children older than 5 years, a pocket guide for health professionals. 2019. Available from: https://ginasthma.org/wp-content/uploads/2019/04/GINA-2019-main-Pocket-Guide-wms.pdf.
- Wilkinson AJ, Braggins R, Steinbach I, et al. Costs of switching to low global warming potential inhalers. An economic and carbon footprint analysis of NHS prescription data in England. BMJ Open. 2019;9(10):e028763.
- Hänsel M, Bambach T, Wachtel H. Reduced environmental impact of the reusable respimat® soft mist™ inhaler compared with pressurised metered-dose inhalers. Adv Ther. 2019;36(9):2487–2492.
- IQVIA MIDAS Database. Global inhaler sales. 2020. Available from: https://www.iqvia.com/.
- Global Initiative for Asthma. Global strategy for asthma management and prevention. 2021. Available from: https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf.
- British Thoracic Society/Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma – a national clinical guideline. 2019. Available from: https://www.sign.ac.uk/media/1773/sign158-updated.pdf.
- European FluoroCarbons Technical Committee. European pharmaceutical company announces low GWP HFC-152A Metered Dose Inhaler (MDI). 2020. Available from https://www.fluorocarbons.org/news/european-pharmaceutical-company-announces-low-gwp-hfc-152a-metered-dose-inhaler-mdi/.
- United Nations Department of Management Strategy, Policy and Compliance. 2019. United Nations Secretariat climate action plan 2020–2030. Available from: https://www.un.org/management/sites/www.un.org.management/files/united-nations-secretariat-climate-action-plan.pdf.
- The World Bank. World Bank Country and Lending Groups. 2020. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519.
- United Nations Department of Economic and Social Affairs – Population Division (2019). 2019. World population prospects 2019, online edition. Rev. 1. Available from: https://population.un.org/wpp/.
- Global Burden of Disease Collaborative Network. Global burden of disease study 2016 (GBD 2016) reference life table. 2017. Available from: https://ghdx.healthdata.org/record/ihme-data/gbd-2016-reference-life-table.
- United Nations Department of Economic and Social Affairs – Population Division (2019). 2019. World urbanization prospects: the 2018 revision. Available from: https://www.un.org/development/desa/publications/2018-revision-of-world-urbanization-prospects.html.
- The World Bank. GDP per capita, PPP (constant 2011 international $). Available from: https://databank.worldbank.org/source/jobs/Series/NY.GDP.PCAP.PP.KD.
- World Health Organization. Updated WHO projections of mortality and causes of death, 2016–2060. Available from https://www.who.int/healthinfo/global_burden_disease/projections_method.pdf?ua=1.
- United Nations Department of Economic and Social Affairs – Economic Analysis (2020). 2021. World economic situation and prospects. Available from: https://www.un.org/development/desa/dpad/wp-content/uploads/sites/45/WESP2020_FullReport.pdf
- US Food & Drug Administration. Orange book: approved drug products with therapeutic equivalence evaluations. Available from https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm.
- U.S. Government Interagency Working Group on Social Cost of Greenhouse Gases. Technical support document: social cost of carbon, methane, and nitrous oxide – interim estimates under executive order 13990. Available from: https://www.whitehouse.gov/wp-content/uploads/2021/02/TechnicalSupportDocument_SocialCostofCarbonMethaneNitrousOxide.pdf.
- Tang L, Ii R, Tokimatsu K, et al. Development of human health damage factors related to CO 2 emissions by considering future socioeconomic scenarios. Int J Life Cycle Assess. 2018;23(12):2288–2299.
- IPCC. Summary for policy makers – emissions scenarios. 2000. Available from: https://www.ipcc.ch/site/assets/uploads/2018/03/sres-en.pdf.
- Ritchie H, Roser M. CO2 and greenhouse gas emissions. 2020. Published online at OurWorldInData.org. Available from: https://ourworldindata.org/co2-emissions.
- Wilkinson AJK, Anderson G. Sustainability in inhaled drug delivery. Pharmaceut Med. 2020;34(3):191–199.
- Prevention CFDCA. Climate effects on health. 2021. Available from: https://www.cdc.gov/climateandhealth/effects/default.htm.
- Adachi Y, Adachi Y, Itazawa T, et al. Measurement of peak inspiratory flow rates with an in-check meter to identify preschool children's ability to use dry powder inhalers; diskus and turbuhaler. J Aller Clin Immunol. 2004;113(2):S114.
- Al-Showair RA, Tarsin WY, Assi KH, et al. Can all patients with COPD use the correct inhalation flow with all inhalers and does training help? Respirat Med. 2007;101(11):2395–2401.
- Lavorini F. The challenge of delivering therapeutic aerosols to asthma patients. ISRN Allergy. 2013;2013:102418.
- Anand A, Palaksha S, P.a M. Prs63 comparison of clinical outcomes associated with different inhaler devices in asthma and COPD. Value Health. 2020;23:S360.
- Kemp L, Haughney J, Barnes N, et al. Cost-effectiveness analysis of corticosteroid inhaler devices in primary care asthma management: a real world observational study. Clinicoecon Outcomes Res. 2010;2:75–85.
- Morice AH, Peterson S, Beckman O, et al. Therapeutic comparison of a new budesonide/formoterol pMDI with budesonide pMDI and budesonide/formoterol DPI in asthma. Int J Clin Pract. 2007;61(11):1874–1883.
- Ramadan WH, Sarkis AT. Patterns of use of dry powder inhalers versus pressurized metered-dose inhalers devices in adult patients with chronic obstructive pulmonary disease or asthma: an observational comparative study. Chron Respir Dis. 2017;14(3):309–320.
- Rubin BK, Fink JB. Optimizing aerosol delivery by pressurized metered-dose inhalers. Respir Care. 2005;50(9):1191–1200.
- Azouz W, Campbell J, Stephenson J, et al. Improved metered dose inhaler technique when a coordination cap is used. J Aerosol Med Pulm Drug Deliv. 2014;27(3):193–199.
- Tervonen T, Hawken N, Hanania NA, et al. Maintenance inhaler therapy preferences of patients with asthma or chronic obstructive pulmonary disease: a discrete choice experiment. Thorax. 2020;75(9):735–743.
- Liew K, Wilkinson A. P280 How do we choose inhalers? Patient and physician perspectives on environemntal, financial and ease-of-use factors. Thorax. 2017;72:A235–A237.
- Panigone S, Sandri F, Ferri R, et al. Environmental impact of inhalers for respiratory diseases: decreasing the carbon footprint while preserving patient-tailored treatment. BMJ Open Resp Res. 2020;7(1):e000571.
- Gerald JK. Generic competition for orally inhaled respiratory medications. Two steps forward, one step back. Ann Am Thorac Soc. 2017;14(2):165–167.
- Woodcock A, Janson C, Rees J, et al. Effects of switching from a metered dose inhaler to a dry powder inhaler on climate emissions and asthma control: post-hoc analysis. Thorax. 2022. DOI:10.1136/thoraxjnl-2021-218088
- Thomas M, Price D, Chrystyn H, et al. Inhaled corticosteroids for asthma: impact of practice level device switching on asthma control. BMC Pulm Med. 2009;9:1.
- Ekberg-Jansson A, Svenningsson I, Rågdell P, et al. Budesonide inhaler device switch patterns in an asthma population in Swedish Clinical Practice (ASSURE). Int J Clin Pract. 2015;69(10):1171–1178.
- Jeswani HK, Azapagic A. Life cycle environmental impacts of inhalers. J Cleaner Prod. 2019;237:117733.
- IPCC. Climate change 2021: the physical science basis. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press; 2021.
