Abstract
Objectives
Antiproliferative therapies based on paclitaxel have been developed to extend the durability of endovascular interventions for lower-extremity atherosclerotic peripheral artery disease, resulting in improved primary vessel patency and fewer target lesion revascularizations. This study evaluated the cost-effectiveness of the sustained-release, paclitaxel-eluting Eluvia stent (Boston Scientific, Marlborough, MA) versus the paclitaxel-coated Zilver PTX stent (Cook Medical, Bloomington, IN) for endovascular intervention in the superficial femoral or proximal popliteal artery.
Design
A microsimulation model was constructed from a United States Medicare perspective with a 24-month time horizon. Patients entering the model were assigned to initial endovascular intervention with either Eluvia or Zilver PTX. Each month patients were exposed to the risks of primary vessel patency loss, target lesion revascularization, amputation, and death. Clinical input parameters were taken from a randomized trial (IMPERIAL) comparing the two interventions at 24-months follow-up. Cost parameters were obtained from analyses of Medicare administrative and claims data. Cost-effectiveness analysis entailed sampling a complete set of clinical and cost parameters from their respective distributions, and then running cohorts of 10,000 patients through each intervention arm of the model. One-way and probabilistic sensitivity analyses were performed.
Results
In the base case microsimulation, at 24 months, the modeled target lesion revascularization was 11.6% for Eluvia and 19.0% for Zilver PTX, and the mean total direct costs were $20,010 and $21,356, respectively (Eluvia average savings=$1,346). In probabilistic sensitivity analyses, Eluvia was cost-effective in 87.8% of all simulations at a willingness-to-pay threshold of $10,000 per target lesion revascularization prevented. Eluvia was more effective and less costly (dominant) than Zilver PTX in 73.6% of simulations.
Conclusions
In this comparison of a paclitaxel-eluting to a paclitaxel-coated stent for endovascular femoropopliteal intervention, Eluvia was more effective and less costly (dominant) than Zilver PTX from a US Medicare perspective. These findings should be considered when formulating reimbursement policy and clinical practice guidelines.
PLAIN LANGUAGE SUMMARY
Paclitaxel is a drug used in the treatment of peripheral artery disease (PAD) to help maintain primary vessel patency and reduce the need for revascularization procedures. This study evaluated the cost-effectiveness of the paclitaxel-eluting Eluvia stent (Boston Scientific, Marlborough, MA) versus the paclitaxel-coated Zilver PTX stent (Cook Medical, Bloomington, IN) in Medicare patients with PAD. Cost-effectiveness is defined as the degree to which a particular treatment option is effective relative to its costs. Therefore, this study compared both the effectiveness, in terms of target lesion revascularization rates, and the costs of Eluvia versus Zilver PTX over 24 months.
A microsimulation model was developed from a United States Medicare perspective with a 24-month time horizon. Simulated patients entered the model and were assigned to receive either Eluvia or Zilver PTX. Monthly, patients were exposed to the risks of primary vessel patency loss, target lesion revascularization (TLR), amputation, and death. These risks were taken from a randomized controlled trial that compared Eluvia and Zilver PTX over 24 months. Patients also accrued costs over time. The costs used in the model were obtained from Medicare administrative and claims data analyses.
In health economics, a treatment is considered to be the dominant treatment option if it is both more effective and less costly than the alternative treatment. In this case, Eluvia was found to be dominant over Zilver PTX because it was associated with lower TLR rates and lower costs. These findings should be considered when formulating reimbursement policy and clinical practice guidelines.
Introduction
The prevalence of peripheral artery disease (PAD), which includes intermittent claudication (IC) and critical limb ischemia (CLI), is increasing [Citation1–3] due to population aging and rising numbers of patients with underlying risk factors such as diabetes. In the United States Medicare population, which accounts for 71% of all PAD inpatient admissions [Citation4] and 75% of all admissions for CLI [Citation5], the prevalence of PAD increased from approximately 8.2% in 1999 [Citation1] to 13.5% in 2012 [Citation3], suggesting that of the 60 million current Medicare enrollees [Citation6], more than 8.1 million are being treated for this disease. The cost of PAD has also risen substantially over a similar period, with current estimates ranging from $212 billion to $389 billion—higher than diabetes, coronary disease, or cancer [Citation4].
Medical therapy for PAD consists of supervised exercise, lifestyle modification, and pharmacotherapy customized to individual risk factors [Citation7]. Endovascular revascularization, which includes balloon dilation (angioplasty), stenting, and atherectomy, is recommended for patients with lifestyle-limiting claudication who have an inadequate response to medical and/or exercise therapy alone [Citation7]. Randomized trials have shown that adding endovascular revascularization with stenting to one or more components of medical therapy significantly improves both functional status and quality of life in IC patients [Citation8,Citation9]. Therefore, endovascular revascularization is preferentially offered as initial therapy for both IC and CLI, resulting in lower hospital length of stay, fewer amputations, and decreased procedural morbidity and mortality compared to surgical intervention [Citation10,Citation11].
Balloons and stents coated with paclitaxel have been developed to extend the durability of femoral-popliteal interventions in IC, and evidence from randomized trials, as summarized in a recent systematic review and meta-analysis [Citation4], demonstrates that drug-coated interventions reduce the need for target lesion revascularization (TLR), improve primary patency compared to non-coated interventions, and improve quality of life and functional outcomes. Two important trials demonstrate these advantages in femoral-popliteal intervention, the first comparing Zilver PTX (Cook Corporation, Bloomington, IN, USA), a polymer-free, paclitaxel-coated stent, to both plain old balloon angioplasty (POBA) and bare metal stenting (BMS), and showed a significant reduction in the need for TLR and significant improvement in long-term patency [Citation12]. And the second, IMPERIAL, compared a sustained-release, polymer-coated, paclitaxel-eluting stent (Eluvia: Boston Scientific, Marlborough, MA, USA) to Zilver PTX for endovascular femoropopliteal intervention [Citation10]. Eluvia delivers paclitaxel over a longer duration than Zilver PTX, which targets the observed peak of restenosis in the femoral artery at 10–12 months [Citation13]. Although IMPERIAL was designed as a non-inferiority trial, in a pre-specified superiority analysis through 12 months of follow-up, Eluvia resulted in statistically significantly higher primary patency and statistically significantly lower TLR compared with Zilver PTX [Citation10]. Recently published findings from IMPERIAL show that Eluvia resulted in significantly lower clinically driven TLR, and that its primary patency rate was sustained, through 24 months of follow-up [Citation14,Citation15].
Not surprisingly perhaps, given the clinical and economic burden of PAD, and the large body of evidence indicating that enhancements to standard angioplasty including drug-coated devices improve the outcomes of endovascular intervention, several recent studies have compared the cost-effectiveness of the various treatment options [Citation4,Citation16–23]. Most cost-effectiveness analyses have been based on simulation models that account for important clinical events, such as loss of primary vessel patency and target lesion revascularization and assign costs to those events when they occur [Citation4,Citation16,Citation17,Citation19–23]. Findings from recent analyses [Citation16,Citation17] indicate that the cost-effectiveness of drug-coated devices, including Zilver PTX, compares favorably with POBA and bail-out BMS, which was assumed in these analyses to represent the existing standard of care. In one analysis conducted from a United Kingdom National Health Service perspective, Katsanos et al. [Citation17] concluded that widespread adoption of drug-coated technologies would add meaningful clinical benefit at a reasonable cost.
Evidence from the IMPERIAL clinical trial indicates Eluvia adds meaningful clinical benefit relative to Zilver PTX [Citation10,Citation14,Citation15], which, in turn, compares favorably to non-coated devices [Citation16,Citation17]. However, the cost-effectiveness of Eluvia has not been assessed. Therefore, the objective of this study was to compare the cost-effectiveness of Eluvia to Zilver PTX for endovascular femoropopliteal intervention from a United States Medicare payer perspective, using a simulation model that incorporated clinical outcome data from the IMPERIAL randomized trial [Citation10] and real-world treatment costs from analyses of Medicare administrative and claims data.
Methods
Study design
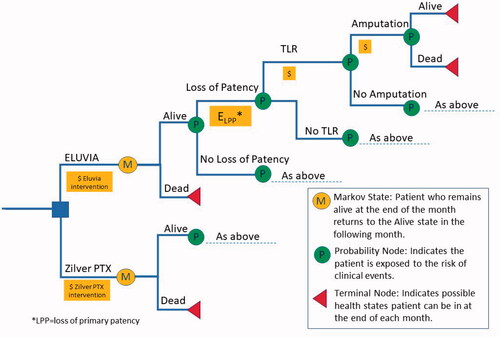
A state-transition, decision-analytic (microsimulation) model () was constructed to simulate outcomes and costs, from a US Medicare perspective, of Eluvia compared to Zilver PTX for endovascular femoropopliteal intervention. Simulated cohorts of 10,000 patients with baseline demographic and clinical characteristics reflecting those enrolled in the IMPERIAL trial [Citation10,Citation14,Citation15] entered the model one at a time and were assigned to endovascular intervention with either Eluvia or Zilver PTX. Each patient was followed monthly for primary outcomes reported in the IMPERIAL trial [Citation10,Citation14,Citation15], consisting of primary patency, clinically driven TLR, major amputation of the target limb, and death from any cause. Patients were followed for 24 months or until death, whichever came first. Medicare costs were assigned to the initial endovascular intervention, TLR, and amputation, each of which could occur only once per patient during the simulation. In addition, outcomes, e.g. TLR and primary vessel patency at 24 months, which was defined in IMPERIAL [Citation10,Citation14,Citation15] as a binary endpoint based on a duplex ultrasound peak systolic velocity ratio of 2.4 or lower in the absence of clinically driven TLR or bypass of the target lesion, were recorded for each patient. TLR at 24 months was also the measure of effectiveness in the cost-effectiveness analysis. Cost-effectiveness was evaluated using a willingness-to-pay threshold of $10,000 per TLR avoided, which was conservative relative to another used to evaluate the cost-effectiveness of superficial femoral endovascular interventions in the UK and Germany [Citation16]. The model was developed in TreeAge Pro 2019 (Williamstown, MA, USA).
Clinical inputs
Outcomes probabilities for the model () were obtained from the IMPERIAL trial as previously reported in the literature [Citation10,Citation14,Citation15]. Each 2-year event probability was converted to a monthly rate assuming that all rates were constant over time. This was considered a reasonable assumption based on inspection of the published Kaplan-Meier curves for primary vessel patency and TLR [Citation10]. Probabilities of clinical events were assumed to be independent, e.g. the probability of amputation did not depend on whether a patient had previously experienced loss of primary vessel patency.
Table 1. Input parameters.
It was assumed that each event could occur only once per patient. Specifically, once an event occurred in an individual patient, the baseline probability of that event for that patient was set to zero for all subsequent cycles of the model. In instances where either baseline event probabilities vary by patient characteristics, or event probabilities change during follow-up - as was the case here ‒ microsimulation is the recommended approach to modelling in TreeAge [Citation24,Citation25]. In this instance, tracker variables were constructed to record the first occurrence or each clinical event, and those trackers were then used in logic statements at the Boolean event nodes to “switch off” the probability of a second event once the first had occurred. The decision to allow only one of each type of event per patient may be conservative since experiencing one event likely would raise the probability of other subsequent events. However, this was dictated by the fact that the clinical event data were reported predominately through published Kaplan–Meier curves.
Cost inputs
The expected cost to Medicare of the initial endovascular intervention was obtained from Medicare administrative and claims data (). First, all hospital outpatient claims for femoral/popliteal percutaneous transluminal angioplasty plus coated stent (Healthcare Common Procedure Coding System [HCPCS] codes 37226 + C1874), without the concomitant diagnosis of stent restenosis (International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] diagnosis code T82.856) were obtained from the 2017 Medicare Outpatient Prospective Payment System ‘OPPS’ file. Second, all hospital inpatient claims for insertion of a drug-eluting stent into the femoral or popliteal artery (ICD-10-CM procedure codes: see Supplemental Table 1 for a list of the codes), without the concomitant diagnosis of stent restenosis were obtained from the 2017 Medicare Provider Analysis and Review ‘MEDPAR’ file. Third, a weighted average Medicare amount paid for all procedures across both sites (hospital inpatient and hospital outpatient) of service was calculated, with weighting based on the distribution of inpatient and outpatient procedures from a separate analysis of the 2017 Physician/Supplier Procedure Summary data. Finally, the Medicare paid amount for the physician component of endovascular intervention ($547 in both the hospital inpatient and outpatient settings) was added to the weighted average amount Medicare paid to the facility. Presently, there is no difference in Medicare reimbursement for drug-eluting versus drug-coated stents. Therefore, the cost of the initial intervention was assumed to be the same for Eluvia and Zilver PTX.
The expected cost to Medicare of TLR was estimated as follows. Medicare claims and administrative data were used to identify 571 patients who (A) underwent their first observed femoropopliteal artery revascularization with stenting (index procedure) in 2016–2017, and (B) subsequently underwent their first reintervention procedure >90 days after the index procedure. Reintervention procedures, consisting of angioplasty with or without atherectomy and/or stenting, thrombectomy, thrombolysis, or bypass, were identified using Current Procedural Terminology codes in the claims. Patients were followed from 90 days before, to up to 90 days after, their first reintervention (observation period). The observation period was divided into 30-day intervals such that the reintervention procedure occurred on the first day of the fourth interval. The first two intervals were used to establish a patient’s baseline total cost prior to reintervention. A within-patient “difference analysis” was conducted in which the average cost in the first two intervals was subtracted from the costs in each of the following four intervals. The cost of TLR was assumed to be the same in both treatment arms of the model. The cost of major amputation of the target limb was obtained from the literature () [Citation26]. The cost of these events also was assumed to be the same for Eluvia and Zilver PTX.
Other costs, such as those related to routine evaluation and management of PAD or of other conditions were not included in the model. However, since Eluvia patients had slightly lower mortality than Zilver PTX, and since patients were exposed to the risk of TLR and amputation throughout the follow-up period, the model did account for additional costs of reintervention in Eluvia patients due to slightly longer survival. All costs were inflated to January 2022 using the medical care component of the Consumer Price Index [Citation27]. Costs associated with events that occurred during the simulation were discounted at 3% per year [Citation28].
Participant consent and IRB approval
Since this study did not involve human participants, neither institutional review board approval nor participant consent was obtained.
Time horizon
The time horizon for the model was two years, which is the current extent of the published findings from the IMPERIAL clinical trial [Citation10,Citation14,Citation15].
Statistical methods
Base case microsimulation
Base case microsimulation consisted of first setting each probability and cost to its baseline value () and then running cohorts of 10,000 patients through each intervention arm of the model. Therefore, each patient was assigned the same clinical and cost parameters. However, cost trajectories varied among the patients as some experienced one or more clinical event (with attendant costs). Consequently, the base case microsimulation produced two sets (one for each intervention) of 10,000 cost results and TLR status (yes/no) results from which the mean cost, probability of remaining TLR-free at two years, and 95% confidence intervals for each were calculated and reported.
Probabilistic sensitivity analysis
Probabilistic sensitivity analysis (PSA) [Citation29] entailed first sampling a complete set of baseline clinical and cost inputs from their respective statistical distributions, and then running cohorts of 10,000 patients through each endovascular intervention arm of the model to obtain the average cumulative cost per patient and the predicted probability of TLR at two years. Clinical inputs were assumed to have beta distributions. Standard deviations for these distributions were estimated using the event probabilities and sample sizes from the IMPERIAL trial [Citation10]. Cost inputs were assumed to have gamma distributions, with standard deviations equal to their means. In certain circumstances it may be appropriate ‒ by invoking the central limit theorem ‒ to select the cost parameters from a normal distribution. However, here this approach would have resulted in negative cost parameters, and since the analyses were conducted from a Medicare insurer perspective, would have equated to an unrealistic scenario in which providers would have paid the insurer (Medicare) when they performed TLRs or amputations.
The process of sampling a set of clinical and cost inputs and then running cohorts through the model was repeated 1,000 times (simulations). For each of the simulations, the mean cost and probability of remaining TLR-free at two years were calculated, as well as the differences between the two groups.
In addition, the results of each simulation were classified as (A) Eluvia less costly and more effective (dominated Zilver PTX), (B) Eluvia less costly and less effective, (C) Eluvia more costly and more effective (potentially cost-effective depending on the willingness-to-pay threshold for avoiding TLR, i.e. $10,000, which was conservative relative to another used to evaluate the cost-effectiveness of superficial femoral endovascular interventions in the UK and Germany [Citation29], or (D) Eluvia more costly and less effective (dominated by Zilver PTX). Results also were classified using willingness-to-pay thresholds of $5,000 and $15,000.
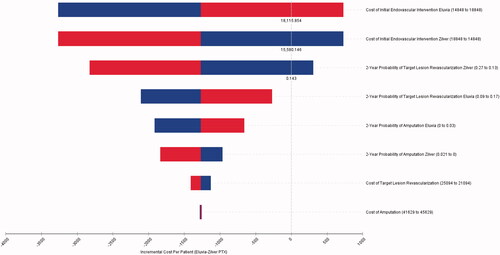
One-way sensitivity analysis also was performed, in which values of individual baseline clinical and cost parameters were varied to examine their impact on the predicted cost difference between the two interventions. The values of the parameters used in the one-way sensitivity analyses are included in the Figure reporting the findings (see Results below).
Results
Base case microsimulation
At 24 months, the modelled mean predicted proportion of patients undergoing TLR was 11.6% for Eluvia and 19.0% for Zilver PTX (difference = 7.4%; ), which very closely matched the result reported in IMPERIAL. The mean predicted cumulative cost to Medicare per patient was $20,010 for Eluvia and $21,356 for Zilver PTX (difference= −$1,346; ). Therefore, Eluvia ‘dominated’ Zilver PTX because it was less costly and more effective when the mean results were considered. The predicted proportions who survived to two years were 92.9% and 91.7% for Eluvia and Zilver PTX respectively, again closely matching the trial results. Thus, the model accurately reflected the clinical data from which it was built.
Table 2. Base Case Microsimulation.
Probabilistic sensitivity analysis
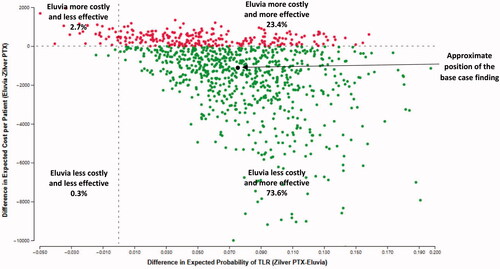
In the PSA Eluvia was both less costly and more effective than Zilver PTX (Eluvia dominated Zilver PTX) in 73.6% of the 1,000 simulations performed (). At a willingness-to-pay threshold of $10,000 per TLR avoided, Eluvia was cost-effective (either dominant or had a cost-effectiveness ratio ≤$10,000 per TLR avoided) in 87.8% of all simulations. At a willingness-to-pay threshold of $5,000 and $15,000 per TLR avoided, Eluvia was cost-effective in 84.1% and 90.7% of all simulations, respectively.
Figure 2. Each dot represents one simulation of the 1,000 performed in the probabilistic sensitivity analysis in which cohorts of 10,000 patients entered each treatment arm of the model one at a time and were exposed to the risks of clinical events and costs for up to two years after the initial procedure. The coordinates of each dot are the difference in cost (Eluvia-Zilver PTX: y-axis) divided by the difference in TLR (Zilver PTX-Eluvia: x-axis).
One-Way sensitivity analysis
Results of the one-way sensitivity analysis () show that the difference in cost between Eluvia and Zilver PTX was most sensitive to the cost of the initial endovascular intervention itself, followed by the probability of TLR, and then the probability of amputation. Changing the cost of TLR or amputation had very little impact on the difference in cost between the two interventions.
Discussion
A microsimulation model was constructed to estimate the cost-effectiveness of the sustained-release, paclitaxel-eluting Eluvia stent compared to the paclitaxel-coated Zilver PTX stent for endovascular femoropopliteal intervention. Simulation modelling is an established approach for evaluating the cost-effectiveness of alternative endovascular interventions, and the model constructed for this evaluation was similar in structure, processes, inputs, and outputs to those previously reported in the literature [Citation4,Citation16,Citation17]. Probabilistic sensitivity analyses, widely considered to be the gold standard for estimating cost-effectiveness [Citation24], as well as one-way sensitivity analyses, were performed to examine the impact of uncertainty in the baseline clinical and cost inputs on the findings.
In cost-effectiveness analysis, when one intervention is both less costly and more effective than another, it is ‘dominant’ over, or ‘dominates,’ the other. In this study, the base case PSA showed Eluvia was dominant over Zilver PTX in a substantial majority (73.6%) of the 1,000 PSA simulation trials performed. The average cost difference between the two interventions was -$1,346, indicating that, on average, Eluvia is expected to result in cost savings to Medicare during the first two years after endovascular intervention. As expected, based on the primary efficacy endpoint reported in the IMPERIAL clinical trial [Citation10,Citation14,Citation15], the findings also indicate that an additional 7.4% of Eluvia patients would be expected to avoid TLR at two years.
When one intervention is more effective but costlier than another, it may be cost-effective. However, in these instances, the difference in cost between the two divided by the difference in effectiveness (cost-effectiveness ratio) is typically compared to a willingness-to-pay threshold to establish cost-effectiveness. This study used a threshold of $10,000 per TLR avoided to establish the cost-effectiveness of Eluvia. This threshold is conservative relative to those used in countries such as the UK [Citation29], where cost-effectiveness criteria are incorporated into national technology assessments. The PSA results show Eluvia was cost-effective in an additional 14.2% of the 1,000 PSA simulations. Therefore, in most of the PSA trials (87.8%) Eluvia either dominated or was cost-effective relative to Zilver PTX.
Strengths of the model include that the clinical inputs were obtained from a randomized head-to-head trial of the two interventions, and that the cost inputs were obtained from analyses of Medicare administrative and claims data. This study was based on the IMPERIAL clinical data set from largely IC patients (by study protocol), which was connected to both inpatient and outpatient Medicare cost data for IC and CLI interventions. As such, conclusions from this analysis are most relevant for the IC population studied, but may not necessarily be transferrable to all CLI patients or those with more complex femoral-popliteal disease, both of which were excluded from the IMPERIAL protocol.
There are also several limitations. First, the model did not include other endovascular femoropopliteal interventions such as POBA with bail-out BMS, primary BMS, or drug-coated balloons, and so-called combined procedures with debulking devices, since presently there are no randomized clinical trial data with 24 months of follow-up comparing Eluvia to these interventions. Therefore, our cost-effectiveness findings are primarily applicable to instances in which the decision to use a drug-coated or drug-eluting stent for endovascular femoropopliteal intervention is the critical question.
Second, the generalizability of these results is linked to the clinical trial population studied. Patients in randomized trials tend, on average, to have fewer comorbidities and less progressive disease than those in a real-world setting because of the inclusion and exclusion criteria in these trials which seek to eliminate confounders. Clinical event rates, and the costs of managing those events, notably TLR, could differ in non-trial patients such as older patients with more advanced disease and comorbid conditions. Specifically, a more clinically effective device likely would be more cost-effective in populations with higher underlying rates of restenosis, other things being equal. In this study, results from the one-way sensitivity analyses indicate that the base case findings were particularly sensitive to changes in the rate and cost of TLR. Real-world evidence on the rate and cost of reintervention in patients with more advanced disease would provide further insight on the expected cost-effectiveness of Eluvia in routine clinical practice, and how it might differ from the current trial-based findings. Also, the results might not be generalizable to a provider perspective were the average sales price to differ between the two types of stents. However, this information is not available except through proprietary sources.
Third, patients were followed for only two years, which is the extent of the clinical data presently published from IMPERIAL [Citation10,Citation14,Citation15]. Extending the time-horizon of the model would have required making significant assumptions about the long-term risks of the clinical events, as well as the relative risks of those events in the Eluvia versus the Zilver PTX arm of the model. Therefore, this was not included among the sensitivity analyses. Fourth, primary vessel patency at 24 months, obtained from the IMPERIAL clinical trial [Citation10,Citation14,Citation15], was based in part on imaging criteria and may not accurately reflect the clinical burden of vessel restenosis in routine clinical practice. Finally, costs of routine evaluation and management of PAD not directly related to TLR or amputation, and costs of other conditions e.g. diabetes, were not included in the model. Since two-year mortality was slightly lower in the Eluvia compared with the Zilver PTX patients, accounting for these costs may have slightly reduced the difference in total costs between the two groups.
Conclusions
The findings indicate that in the first two years after the endovascular femoropopliteal intervention, Eluvia was more effective and less costly than Zilver PTX, making Eluvia the dominant treatment strategy. These findings will be useful to consider when formulating policy and practice guidelines.
Transparency
Declaration of funding
This study was supported by Boston Scientific.
Declaration of financial/other relationships
WAG serves as an advisor to Boston Scientific. RIG, AMM and MRJ are employees of, and shareholders in, Boston Scientific. At the time of writing, SLA was an employee of, and shareholder in, Boston Scientific. PWME and RLA serve as consultants to Boston Scientific. SM-H serves as a consultant to, and has received honoraria and travel grants from, Boston Scientific.
Author contributions
Conceptualization and design (WAG, RIG, PWME, SLA, AMM, MRJ, RLA, SM-H); analysis and interpretation of the data (WAG, RIG, PWME, SLA, AMM, MRJ, RLA, SM-H); drafting of paper or revising critically for intellectual content (WAG, RIG, PWME, SLA, AMM, MRJ, RLA, SM-H); final approval of the version to be published (WAG, RIG, PWME, SLA, AMM, MRJ, RLA, SM-H). All authors agree to be accountable for all aspects of the work.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (12.3 KB)Acknowledgements
None stated.
Data availability statement
The data that support the findings of this study are available upon reasonable request.
References
- Jaff MR, Cahill KE, Yu AP, et al. Clinical outcomes and medical care costs among medicare beneficiaries receiving therapy for peripheral arterial disease. Ann Vasc Surg. 2010;24(5):577–587.
- Baser O, Verpillat P, Gabriel S, et al. Prevalence, incidence, and outcomes of critical limb ischemia in the US medicare population. Vasc Dis Manag. 2013;10(2):26–36.
- Kalbaugh CA, Kucharska-Newton A, Wruck L, et al. Peripheral artery disease prevalence and incidence estimated from both outpatient and inpatient settings among medicare fee-for-service beneficiaries in the atherosclerosis risk in communities (ARIC) study. JAHA. 2017;6(5):e003796.
- Sridharan ND, Boitet A, Smith K, et al. Cost-effectiveness analysis of drug-coated therapies in the superficial femoral artery. J Vasc Surg. 2018;67(1):343–352.
- Mustapha JA, Katzen BT, Neville RF, et al. Determinants of long-term outcomes and costs in the management of critical limb ischemia: a population-based cohort study. JAHA. 2018;7(16):e009724.
- Henry J. Kaiser Family Foundation. Total number of Medicare beneficiaries. Available at https://www.kff.org/medicare/state-indicator/total-medicare-beneficiaries/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed May 17, 2019.
- Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2017;69(11):e71–e126.
- Fakhry F, Spronk S, van der Laan L, et al. Endovascular revascularization and supervised exercise for peripheral artery disease and intermittent claudication: a randomized clinical trial. JAMA. 2015;314(18):1936–1944.
- Murphy TP, Cutlip DE, Regensteiner JG, et al. Supervised exercise, stent revascularization, or medical therapy for claudication due to aortoiliac peripheral artery disease: the CLEVER study. J Am College Cardiol. 2015;65(10):999–1009.
- Gray WA, Keirse K, Soga Y, on behalf of the IMPERIAL investigators, et al. A polymer-coated, paclitaxel-eluting stent (eluvia) versus a polymer-free, paclitaxel-coated stent (zilver PTX) for endovascular femoropopliteal intervention (IMPERIAL): a randomised, non-inferiority trial. Lancet. 2018;392(10157):1541–1551.
- Egorova NN, Guillerme S, Gelijns A, et al. An analysis of the outcomes of a decade of experience with lower extremity revascularization including limb salvage, lengths of stay, and safety. J Vasc Surg. 2010;51(4):878–885.
- Dake MD, Ansel GM, Jaff MR, et al. Durable clinical effectiveness with paclitaxel-eluting stents in the femoropopliteal artery: 5-year results of the zilver PTX randomized trial. Circulation. 2016;133(15):1472–1483.
- Iida O, Uematsu M, Soga Y, et al. Timing of the restenosis following nitinol stenting in the superficial femoral artery and the factors associated with early and late restenoses. Catheter Cardiovasc Interv. 2011;78(4):611–617.
- Iida O. Two-year outcomes from the IMPERIAL randomized trial of eluvia and zilver PTX. VIVA 2019. Nov 5, 2019, Las Vegas, NV, USA.
- Gray W. 2-year outcomes from the IMPERIAL randomized study of eluvia and zilver PTX. LINC 2020. Jan 28, 2020; Leipzig, Germany. https://linc2020.cncptdlx.com/media/1630_William_Gray_28_01_2020_Room_1_-_Main_Arena_1_v1.pdf
- Kearns BC, Thomas SM. Cost-effectiveness of superficial femoral artery endovascular interventions in the UK and Germany: a modelling study. BMJ Open. 2017;7(1):e013460.
- Katsanos K, Geisler BP, Garner AM, et al. Economic analysis of endovascular drug-eluting treatments for femoropopliteal artery disease in the UK. BMJ Open. 2016;6(5):e011245.
- Salisbury AC, Li H, Vilain KR, et al. Cost-effectiveness of endovascular femoropopliteal intervention using drug-coated Balloons Versus Standard Percutaneous Transluminal Angioplasty: Results From the IN.PACT SFA II Trial. JACC Cardiovasc Interv. 2016;9(22):2343–2352.
- Simpson EL, Kearns B, Stevenson MD, et al. Enhancements to angioplasty for peripheral arterial occlusive disease: Systematic review, cost-effectiveness assessment and expected value of information analysis. Health Technol Assess. 2014;18(10):1–252.
- Pietzsch JB, Geisler BP, Garner AM, et al. Economic analysis of endovascular interventions for femoropopliteal arterial disease: a systematic review and budget impact model for the United States and Germany. Catheter Cardiovasc Interv. 2014;84(4):546–554.
- Kearns BC, Michaels JA, Stevenson MD, et al. Cost-effectiveness analysis of enhancements to angioplasty for infrainguinal arterial disease. Br J Surg. 2013;100(9):1180–1188.
- Diehm N, Schneider H. Cost-effectiveness analysis of paclitaxel-coated balloons for endovascular therapy of femoropopliteal arterial obstructions. J Endovasc Ther. 2013;20(6):819–825.
- De Cock E, Sapoval M, Pierre J, et al. A budget impact model for paclitaxel-eluting stent in femoropopliteal disease in France. Cardiovasc Intervent Radiol. 2013;36(2):362–370.
- https://treeage.zendesk.com/hc/en-us/articles/222718328-Simulations-in-TreeAge-Pro-Patient-Simulation-PSA-More.
- https://treeage.zendesk.com/hc/en-us/community/posts/115008171528-Understanding-Simulations-in-TreeAge-Pro.
- Barshes NR, Flores E, Belkin M, et al. The accuracy and cost-effectiveness of strategies used to identify peripheral artery disease among patients with diabetic foot ulcers. J Vasc Surg. 2016;64(6):1682–1690.
- CPI Adjustment Table (hrsa.gov.)
- Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second panel on Cost-Effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103.
- Claxton K, Sculpher M, McCabe C, et al. Probabilistic sensitivity analysis for NICE technology assessment: not an optional extra. Health Econ. 2005;14(4):339–347.