?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objectives
For hospitalized patients with chronic obstructive pulmonary disease (COPD), albuterol and levalbuterol can both be used as relievers to alleviate bronchoconstriction. This study aimed to evaluate levalbuterol and albuterol's cost-utility and budget impact in hospitalized patients with COPD.
Interventions
A cost-utility analysis was used to evaluate the impact on the costs of nebulized levalbuterol verse albuterol in hospitalized patients with COPD. The decision tree model was employed to estimate the incremental cost per quality-adjusted life year in the admission setting. A budget impact model was used to examine the impact of budget on levalbuterol's entry into the Chinese market from the healthcare system's perspective. One-way sensitivity and probabilistic sensitivity analyses were performed to test the uncertainty of the parameters.
Results
The cost-utility results showed that levalbuterol saved ¥495.7 ($105.1) per hospitalization, while the budget impact analysis revealed a potential saving of ¥22.3 ($6.8) million in 3 years. The sensitivity analysis indicated that the results were robust to the changes in input parameter values.
Conclusion
Levalbuterol is a cost-saving option for treating hospitalized patients with COPD in China.
PLAIN LANGUAGE SUMMARY
Chronic obstructive pulmonary disease (COPD) is a common disease in China, with an increased financial burden over the years. Nebulized albuterol is the most commonly used short-acting beta2-agonist, often regarded as the initial bronchodilator to treat hospitalized COPD patients. Its R-isomer, levalbuterol, entered the Chinese market in 2019. The new intervention always impacts the expenditure of the health system. We built a cost-utility and budget impact model to analyze the difference between albuterol and levalbuterol. The cost-utility results showed that levalbuterol saved ¥495.7 ($105.1) per hospitalization compared with albuterol, while the budget impact analysis revealed a potential saving of ¥22.3 ($6.8) million in 3 years.
Introduction
Chronic obstructive pulmonary disease (COPD) is a common disease in ChinaCitation1, with an increased financial burden over the yearsCitation2. The direct medical cost of COPD ranges from 72 to 3,565 United States dollars per capita per year in China, accounting for 33.33–118.09% of local average annual incomeCitation3,Citation4. Medicine cost was the biggest contributor (45.53%)Citation5. Considering the financial burden, cost-effective drugs should be used in COPD treatments.
Nebulized inhaled short-acting beta2-agonists (SABA) are considered an easier delivery method for sicker patients and are recommended as the initial bronchodilators to treat hospitalized patients with COPDCitation6,Citation7, and the most commonly used SABA is albuterol. Levalbuterol is the R-isomer of albuterolCitation8, which entered the Chinese market in 2019Citation9. Compared with albuterol, nebulized levalbuterol could significantly improve lung symptoms in patients with COPD and reduce the risk of adverse events and exacerbationCitation10,Citation11, resulting in 1 day less hospitalization and $553 saving per patient with COPD over the exacerbation periodCitation12. However, this pattern of results is not universalCitation11,Citation13,Citation14. These conflicting results have made it difficult for physicians to choose better nebulized SABAs for treating patients with COPD.
This study aimed to perform a cost-utility analysis (CUA) comparing nebulized levalbuterol with albuterol in hospitalized patients with COPD from a Chinese societal perspective. A budget impact analysis (BIA) was also conducted to evaluate the impact on the Chinese healthcare budget of levalbuterol for treating COPD over three years.
Methods
Cost-utility analysis
Cost-utility model, perspective, and model outcomes
The assessment of the cost-difference and health outcomes of the use of levalbuterol vs. albuterol was performed using a decision tree model from a Chinese societal perspective. The decision tree model was built based on China Guidelines for Pharmacoeconomic EvaluationsCitation15, to estimate the incremental cost per quality-adjusted life year in one admission setting. Since as need use SABAs are the initial bronchodilators to treat hospitalized patients with COPDCitation7, which don’t have an impact on the prognosis of disease status, we only considered the short-term economic differences between levalbuterol and albuterol during hospitalization. Microsoft Excel 2016 software was used to analyze the model. The model structure was presented in Supplemental Figure 1. According to guidelines and previously published studiesCitation15–17, health outcomes were presented as an additional unit of utility (QALY). QALY is widely used for comprehensively reflecting treatment effects, such as clinical efficacy, patient psychology, and living status. If the levalbuterol has a lower cost and better outcome than the albuterol, it will be strictly dominant; if the levalbuterol has both a higher cost and a better outcome than the albuterol, the incremental cost-utility ratio (ICUR) needs to be calculatedCitation15. The ICUR was associated with cost and QALY, and was calculated using the following formula. The target population was hospitalized patients with COPD.
Intervention, assumption, and time horizon
Considering the as-needed usage for nebulized SABAs with no strict determined dosage, the maximum doses were chosen according to package inserts [levalbuterol, 1.25 mg, three times a day (tid) vs. albuterol, 5 mg, four times a day (qid)]. A 1-year time horizon was adopted to capture the short-term clinical and economic outcomes in patients with COPD, and thus discounting was not appliedCitation15. The model applied the following assumptions: (A) The frequency of COPD-related hospitalization was assumed to be once a year per patient, and the treatments of levalbuterol and albuterol continued throughout the hospitalization so that the length of hospital stay was equal to the administration time. All patients were treated with the same basic and maintenance therapy. (B) No people died in the study.
Base case model parameters
To compare the differences in efficacy, safety, and economics between levalbuterol and albuterol, a structured literature review was conducted on PubMed, Cochrane Library, Medline, Embase, and Web of Science from the earliest record date to June 2021. In total, 66 studies were identified and individually screened. The final included studies were based on the following criteria:
Clinical trials
Hospitalized COPD patients
Comparing levalbuterol with albuterol, nebulized administration
The article is written in English or Chinese
Outcomes with the length of stay per hospitalization
The information obtained through the literature search was used in the cost-utility model (implemented in Microsoft Excel). The parameters used in the model are summarized in .
Table 1. Summary of cost-utility model inputs.
Clinical data
Due to the limited published evidence, the length of hospitalization was the only efficacy parameter that could be used to build the model. Clinical trials did not report other factors associated with hospitalization costs, such as comorbidity, surgery, and antibiotic use.
The mean length of hospital stay in the levalbuterol group was one day less than that in the albuterol group (5.1 vs. 6.1 days)Citation12. Adverse drug reactions (ADRs) were not considered because of varied resultsCitation10 and the low incidence of ADRs in inhaled SABAs.
Utilities
Health-related quality-of-life inputs (utilities) were applied to each health state to generate QALYs. Only two studies reported utilities for well-controlled and severe COPD states after the literature search. One study calculated the utility of 0.751 for severe COPD state based on European Quality of Life 5 Dimensions (EQ-5D)Citation19, while the other study reported the utility of 0.989 for “no symptoms” asthmaCitation18, based on a utility valuation survey with standard gamble methodology. As the result of the literature search and recommendation of COPD guidelinesCitation6,Citation7 and experts' investigation, we assumed the utility values for the “hospitalization” state and “well-controlled COPD” state were 0.751 and 0.989, respectively. Based on the clinical practice, drugs were administered right after the attack. The duration of single attacks to drug alleviation was set to 1 h, and the drug administration times were regarded as the number of attacks. According to the label inserts, the usage of levalbuterol was tid, with qid for albuterol. The QALY lost in COPD was calculated using the following formula:
Costs
The analysis considered both direct and indirect medical costs from the societal perspective. Direct medical costs comprised drug costs, nursing costs, and hospitalization costs. Drug costs were calculated based on the average wholesale prices and market shares in 2020 and are presented in Supplemental Table 2. Nursing costs included costs of nebulization (¥7 per event) and nursing (¥10 per day), while hospitalization costs included intravenous infusion (¥18 per day), oxygen therapy (¥30 per day), and ward costs (¥60 per day)Citation23. The human capital approach was used to calculate the indirect medical costsCitation15, which consisted of family members or guardians' productivity loss. Considering the existence of patient's own productivity, it was necessary to calculate the labor loss of the patient and the caregiverCitation15. The costs related to drug and hospitalization (such as intravenous infusion and oxygen therapy costs) are presented in . Some key points of price (CNY) were included USD using the average exchange rate for the year 2022Citation24.
Scenario analysis and sensitivity analysis
Three scenarios were considered. In scenario 1, considering the conflicting conclusion in levalbuterol benefits compared with albuterol, we assumed that the length of hospital stay was similar between the two SABAs. Thus, the length of stay in the two groups was 6.1 days. In scenario 2, we only considered the direct costs, such as drug, nursing, and hospitalization costs, but no indirect costs like productive costs. Moreover, scenario 3 neglected the duration differences between levalbuterol and albuterol, which resulted in the same number of daily nebulization and attacks.
One-way sensitivity analysis was performed to explore uncertainty in individual parameters. Inputs were varied to their upper and lower 95% CIs to determine which factors impacted the model most. A probabilistic sensitivity analysis (PSA) was also undertaken to assess the robustness of the ICUR to simultaneous changes in parameters, in which most parameters were input not as fixed values but as distributions. Distributions were chosen based on data from the literature or on plausible assumptions and experts' opinions when no clear evidence was available. The ICUR obtained from 1,000 iterations and stochastically sampled from the distributions were used to estimate a mean value. The cost-utility acceptability curve determined the probability of the ICUR being less than the willingness-to-pay (WTP) value. The threshold of three times annual gross domestic product (GDP) per capita (¥212,676, $64,388.6)Citation22 was applied since the WTP per QALY is not yet determined in ChinaCitation15,Citation25,Citation26. The range of input parameters in the sensitivity analysis is summarized in Supplemental Table 1.
Budget impact analysis
Budget impact model, perspective, and time horizon
Based on the conclusion from the CUA model, a companion budget impact model (BIM) was developed to address the hypothetical changes to the Chinese Healthcare Service budget considering the market expansion of levalbuterol from the healthcare system perspective. The model structure was presented in Supplemental Figure 2. The BIA for hospitalized patients was based on epidemiological data and expert investigation over a 3-year time horizon (2021–2023).
Target population, intervention, and market shares
Chinese epidemiologic and demographic data used to calculate the BIM target population are included in . The intervention was the same as the CUA.
Table 2. Summary of budget impact model inputs.
Market share data were obtained from China Hospital Pharmaceutical Audit in 2020. Current market share projections of SABAs from the years 2021 through 2023 were used for the “Before levalbuterol entry National Reimbursement Drug List (NDRL)” and then adjusted for expected change for “levalbuterol post-entry.” Annual costs associated with admission setting were estimated based on the yearly cost of each treatment and the projected market share of each treatment. The detailed market share is shown in Supplemental Tables 2 and 3.
Resource used and costs in the base case
From the healthcare system perspective, only direct medical costs were considered, for the indirect costs are usually irrelevant to decision-makers. Considering the as-needed use of SABAs, the length of drug therapy was equivalent to the length of hospital stay. Inputs related to hospitalization costs applied for the BIM were presented in .
Scenario and sensitivity analyses
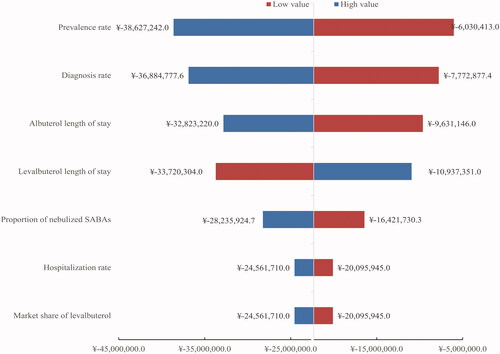
Potential arguments may lead to varied conclusions on levalbuterol benefits. Hence, a no-benefit scenario analysis was conducted with the same length of stay in two groups (6.1 days). A univariate sensitivity analysis was conducted to test the robustness of the results by modifying the following parameters: prevalence, diagnosis rate, rate of hospitalization for COPD, market share of levalbuterol, length of hospitalization, and price of levalbuterol. The range of input parameters in the sensitivity analysis is summarized in Supplemental Table 4.
Patient and public involvement
The study did not involve any direct patient and public involvement.
Results
Cost-utility analysis
In the base case, levalbuterol saved ¥495.7 ($150.1) per hospitalized patient compared with albuterol. The results are shown in . Improved cost-saving and QALY were established owing to the reduced length of hospital stay in the levalbuterol group. In the scenario analysis, each patient could save ¥37.2 ($11.3), ¥263.6 ($79.8), and ¥311.5 ($94.3) in scenario 1, scenario 2, and scenario 3, separately.
Table 3. CUA results.
Sensitivity analysis
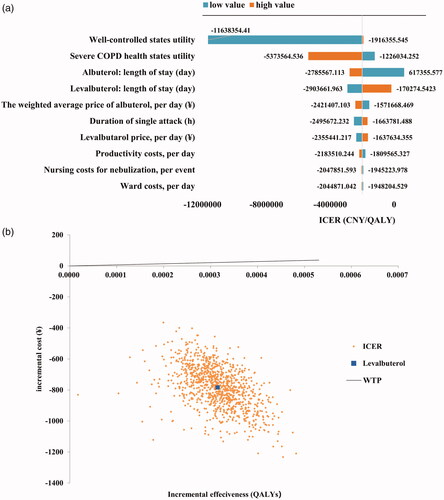
The results of the one-way sensitivity analysis are shown in a tornado diagram (). The diagram shows the effects of uncertainty on the ICUR for each parameter. The utility value of well-controlled and severe COPD states had the most significant impact on the model, while the lengths of stay in the two regimens and the weighted average price of albuterol also play crucial roles. The ICUR resulting from each PSA iteration is shown in . Points lying below the frontier represent ICUR at the conventional cost/QALY threshold of ¥212,676 ($64,388.6). No inputs changed the conclusion, which supported the robustness of the model.
Figure 1. Sensitivity analysis. (a) Tornado diagram of univariate (one-way) sensitivity analysis. (b) ICUR scatterplot for levalbuterol vs. albuterol. This graph represents the incremental cost and corresponding incremental effect for each iteration of the probabilistic sensitivity analysis. The central dot represents the base case analysis ICUR.
Budget impact analysis
From the Chinese healthcare system perspective, the target number of hospitalized patients with COPD in 2020 was estimated to be 138,132 in China. Hospitalized patients with COPD in this group were eligible for levalbuterol vs. albuterol.
Levalbuterol led to a total budget saving of ¥ 22.3 ($6.8) million after three years of entry on the market. The total budget-saving included a decrease in drug costs (¥ 0.9, $0.3 million) and hospitalization (¥ 21.4, $6.5 million).
Scenario and sensitivity analyses
A scenario analysis was conducted considering no benefits in the decreased length of stay. Despite the unconsidered benefit, no significant impact of levalbuterol entry on the Chinese healthcare budget was observed, with a slight increase in drug costs in three years (¥7,401, $2,240.7). Given the uncertainty of all the parameters for the two treatments, a sensitivity analysis of the base case was conducted using plausible ranges given in Supplemental Table 4. Prevalence rate, diagnosis rate, and length of stay in the two regimes were found to have the highest impact on the model results, while the nebulized SABAs, hospitalization rate, and market share of levalbuterol were the underlying drivers of the results in the model ().
Discussion
The results from both CUA and BIA indicated that levalbuterol could be considered a cost-effective therapy in hospitalized patients with COPD compared with albuterol. The analysis suggested that using levalbuterol resulted in lower costs and a gain in QALYs. The total medical costs were ¥495.7 ($150.1) less than albuterol, with an 0.0002483 QALY gained per patient in the levalbuterol group. Regarding the albuterol alternative, levalbuterol could save ¥22.3 ($6.8) million in hospitalized patients with COPD over three years from the Chinese healthcare system perspective.
Concerning efficacy, some investigators found that using levalbuterol resulted in fewer hospital stays and exacerbationsCitation10,Citation12 and less need for rescue treatmentsCitation10 in patients with COPD. However, the pattern of results was quite controversial, and some studies suggested opposite opinionsCitation11,Citation13,Citation32. Due to limited published evidence comparing the efficacy of levalbuterol and albuterol, the study by Truitt et al.Citation12 was the only one showing a reduction in hospital stay, which might mainly be due to clinical benefits, such as better lung function and symptoms. Our result was consistent with published studies in the US, where levalbuterol showed a significant saving of ∼$967 for patients with COPDCitation33, indicating that cost-effectiveness derived mainly from hospitalizationCitation34. The no-benefit situation was discussed in scenario 1 analysis, which was almost consistent with the base case.
The present cost-utility and budget impact models involved conservative estimations for the following reasons. First, without considering the benefits of ADRs and direct non-medical costs (such as nutrition costs), the present study might underestimate the cost-saving of levalbuterol. A randomized, multi-center, controlled trial reported that patients in the levalbuterol treatment group experienced significantly fewer β-mediated adverse effects (which might be caused by S-isomer) than patients in the albuterol treatment group (e.g. rapid heartbeat, 17.3 vs. 6.1%)Citation11. However, most were mild or moderate in severity. Considering the very low probability of severe ARDs of inhaled SABA, we assumed that levalbuterol and albuterol had the same safety profile. Second, China's rate of hospitalization due to COPD might be underestimated because of the lack of recent epidemiological studies. Zhang et al. reported that the inpatient rate would be 36.4%Citation35, while Fang et al. suggested the rate as 3.0%Citation29. This vast gap might derive from the sample size, geographic region, hospital grade, and data resources. The nationwide, cross-sectional study reported that the rate of hospitalized patients with COPD in China was 3% and was elected to build the BIM. However, this cross-sectional study was conducted in 2015. The prevalence and diagnostic rates might increase over the past seven years due to the enhancement of cognition in COPDCitation3, which might result in population underestimation.
However, this study had several limitations. First, the low number of data resources might have reduced the precision of the results. For example, the well-controlled state's utility was derived from a CUA of asthma instead of COPD conducted in ColombiaCitation18. The utility of “no symptoms” asthma might over-evaluate the utility value of a well-controlled state in COPD patients might under-evaluate the benefits of levalbuterol. However, the main symptoms of asthma and COPD were respiratory symptoms and airflow limitationCitation6,Citation36. SABAs were the standard rescue therapy for both diseases; therefore, the shared utility in the well-controlled state may be acceptable. Second, the generalizability of the results might be limited. Since the obtained outcomes have not been proven in the Chinese population, it will be necessary to explore the related parameters in China in future studies. Once obtain such a parameter, we will consider updating it. Also, the numeric parameters were not universal for all patients. We assumed that the hospitalization visit was set to be once a year in the QALYs calculation and BIM, but it would be ranged depending on individual characteristicsCitation37. Meanwhile, the reimbursement percentage varied among regions since 65% was inputted. The model could better reflect the actual cost and budget-saving in each region and facilitate region-wise decision-making when adjusted with region-specific values and real-world data.
Conclusions
Overall, the analysis was based on varying evidence and assumptions, so the results need to be considered cautiously. The study combined a CUA with BIA, and the findings support the use of levalbuterol in hospitalized patients with COPD.
Transparency
Declaration of funding
This research received no specific grant from any funding agency in public, commercial, or not-for-profit sectors.
Declaration of financial/other relationships
The authors report that there are no competing interests to declare.
Author contributions
LC and XC participated in the study's design, adapted the model, performed the economic analysis, and drafted the manuscript. CZ and XL participated in interpreting the results and model adaptation and critically revised the article. YH helped and oversaw the study, reviewed the manuscript, and acted as a guarantor for the overall content. All authors proofread and approved the final manuscript and agree to be accountable for all aspects of the work.
Acknowledgements
The authors thank Dandan Zhao (Joincare, Shenzhen, China) for editorial support during the development of this manuscript.
Supplemental Material
Download MS Word (49.9 KB)Data availability statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. The Lancet. 2018;391(10131):1706–1717.
- Jinjuvadia C, Jinjuvadia R, Mandapakala C, et al. Trends in outcomes, financial burden, and mortality for acute exacerbation of chronic obstructive pulmonary disease (COPD) in the United States from 2002 to 2010. COPD. 2017;14(1):72–79.
- Zhu B, Wang Y, Ming J, et al. Disease burden of COPD in China: a systematic review. Int J Chron Obstruct Pulmon Dis. 2018;13:1353–1364.
- Perera PN, Armstrong EP, Sherrill DL, et al. Acute exacerbations of COPD in the United States: inpatient burden and predictors of costs and mortality. COPD. 2012;9(2):131–141. Apr
- Li M, Wang F, Chen R, et al. Factors contributing to hospitalization costs for patients with COPD in China: a retrospective analysis of medical record data. COPD. 2018;13:3349–3357.
- 2022 GOLD reports – global initiative for chronic obstructive lung disease – GOLD [Internet]. Global initiative for chronic obstructive lung disease – GOLD; 2021 [cited 2021 Nov 22]. Available from: https://goldcopd.org/2022-gold-reports-2/
- Chronic obstructive pulmonary disease group of Chinese Thoracic Society, Chronic Obstructive Pulmonary Disease Committee of Chinese Association of Chest Physician. [Guidelines for the diagnosis and management of chronic obstructive pulmonary disease (revised version 2021)]. Zhonghua Jie He He Hu Xi Za Zhi. 2021;44(3):170–205.
- Boulton DW, Fawcett JP. The pharmacokinetics of levosalbutamol. Clin Pharmacokinet. 2001;40(1):23–40.
- Basic information for levosalbutamol hydrochloride nebuliser solution [Internet]. Beijing: National Medical Products Administration; 2019 [cited 2022 May 25]. Available from: https://www.nmpa.gov.cn/datasearch/search-info.html?nmpa=aWQ9MTY2NzkzJml0ZW1JZD1mZjgwODA4MTdjODMxMmM0MDE3YzliYmZjOGRlMDM2MA==
- Donohue JF, Parsey MV, Andrews C, et al. Evaluation of the efficacy and safety of levalbuterol in subjects with COPD. COPD. 2006;3(3):125–132. Aug
- Donohue JF, Hanania NA, Ciubotaru RL, et al. Comparison of levalbuterol and racemic albuterol in hospitalized patients with acute asthma or COPD: a 2-week, multicenter, randomized, open-label study. Clin Ther. 2008;30:989–1002.
- Truitt T, Witko J, Halpern M. Levalbuterol is not more cost-effective than albuterol for COPD-to the editor. Chest. 2003;124(3):1176–1178.
- Brunetti L, Poiani G, Dhanaliwala F, et al. Clinical outcomes and treatment cost comparison of levalbuterol versus albuterol in hospitalized adults with chronic obstructive pulmonary disease or asthma. Am J Health Syst Pharm. 2015;72(12):1026–1035.
- Khorfan FM, Smith P, Watt S, et al. Effects of nebulized bronchodilator therapy on heart rate and arrhythmias in critically ill adult patients. Chest. 2011;140(6):1466–1472.
- Liu G, Hu S, Wu J. [China guidelines for pharmacoeconomic evaluations (2020)]. 1st ed. Beijing: China Market Press; 2020.
- Sabatelli L, Seppälä U, Sastre J, et al. Cost-effectiveness and budget impact of routine use of fractional exhaled nitric oxide monitoring for the management of adult asthma patients in Spain. J Investig Allergol Clin Immunol. 2017;27(2):89–97.
- Briones JR, Talungchit P, Thavorncharoensap M, et al. Economic evaluation of carbetocin as prophylaxis for postpartum hemorrhage in the Philippines. BMC Health Serv Res. 2020;20(1):1–12.
- Rodríguez-Martínez CE, Sossa-Briceño MP, Castro-Rodriguez JA. Cost-utility analysis of the inhaled steroids available in a developing country for the management of pediatric patients with persistent asthma. J Asthma. 2013;50(4):410–418.
- Hertel N, Kotchie RW, Samyshkin Y, et al. Cost-effectiveness of available treatment options for patients suffering from severe COPD in the UK: a fully incremental analysis. Int J Chron Obstruct Pulmon Dis. 2012;7:183–199.
- Drug price database [Internet]. Chongqing: db.yaozh.com; 2021 [cited 2021 Jun 22]. Available from: https://db.yaozh.com/yaopinzhongbiao.
- Truitt T, Witko J, Halpern M. Levalbuterol compared to racemic albuterol: efficacy and outcomes in patients hospitalized with COPD or asthma. Chest. 2003;123(1):128–135.
- Statistical Communiqué of the People's Republic of China on the 2019 National Economic and Social Development [Internet]. Stats.gov.cn; 2020 [cited 2021 Jun 22]. Available from: http://www.stats.gov.cn/english/PressRelease/202002/t20200228_1728917.html
- Instructions for Chengdu Medical Service Projects and Prices (2016 Edition) [Internet]. Chengdu: cddrc.chengdu.gov.cn; 2016 [cited 2021 Jun 22]. Available from: http://cddrc.chengdu.gov.cn/cdfgw/uploads/0921144846hcrbkbixvxv.pdf
- CCEMG – EPPI-Centre cost converter [internet]. London: Campbell and Cochrane Economics Methods Group (CCEMG); 2019 [cited 2022 May 25]. Available from: https://eppi.ioe.ac.uk/costconversion/
- Gulácsi L, Rencz F, Péntek M, et al. Transferability of results of cost utility analyses for biologicals in inflammatory conditions for central and eastern European countries. Eur J Health Econ. 2014;15 Suppl 1:S27–S34.
- Castro Jaramillo HE, Moreno Viscaya M, Mejia AE. Cost-utility analysis of primary prophylaxis, compared with on-demand treatment, for patients with severe hemophilia type a in Colombia. Int J Technol Assess Health Care. 2016;32(5):337–347.
- Index [Internet]. Stats.gov.cn; 2011 [cited 2021 Jun 22]. Available from: http://www.stats.gov.cn/tjsj/pcsj/rkpc/6rp/indexch.htm
- Zhong N, Wang C, Yao W, et al. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med. 2007;176(8):753–760.
- Fang L, Gao P, Bao H, et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respir Med. 2018;6(6):421–430.
- Singh M, Duarte AG, Hsu E-S, et al. Trends and factors associated with nebulized therapy prescription in older adults with chronic obstructive pulmonary disease from 2008 to 2015. J Aerosol Med Pulm Drug Deliv. 2020;33(3):161–169.
- Yang F. [Cost analysis of 3849 patients with chronic obstructive pulmonary disease with acute exacerbation]. Chinese Med Rec. 2020;21(7):53–56.
- Datta D, Vitale A, Lahiri B, et al. An evaluation of nebulized levalbuterol in stable COPD. Chest. 2003;124(3):844–849.
- Ozminkowski RJ, Wang S, Long SR. The impact of nebulized levalbuterol on health care payments for elderly asthma and chronic obstructive pulmonary disease patients in medicaid plans. Dis Manage Health Outcomes. 2007;15(1):41–55.
- Quinn C. The cost effectiveness of levalbuterol versus racemic albuterol. Am J Manag Care. 2004;10(5 Suppl):S153–S157.
- Zhang Y, Lin YX. [Risk factors analysis for one-year and long-term mortality in patients hospitalized for acute exacerbation of chronic obstructive pulmonary disease]. Zhonghua Jie He He Hu Xi Za Zhi. 2019;42(12):895–900.
- Carson SA, Kallen AN. Diagnosis and management of infertility: a review. JAMA. 2021;326(1):65–76.
- Mullerova H, Maselli DJ, Locantore N, et al. Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest. 2015;147(4):999–1007.