Abstract
Aims
The Canadian province of Ontario provides full coverage for its residents (pop.14.8 M) for hospital-based diagnostic testing. Historical governance of the healthcare system and a legacy scheme of health technology assessment (HTA) and financing has led to a suboptimal approach of adopting advanced diagnostic technology (i.e. protein expression, cytogenetic, and molecular/genetic) for guiding therapeutic decisions. The aim of this research is to explore systemic barriers and provide guidance to improve patient and care provider experiences by reducing delays and inequity of access to testing, while benefitting laboratory innovators and maximizing system efficiency.
Materials and methods
A mixed-methods approach including literature review, semi-structured interviews, and a multi-stakeholder forum involving patient representatives (n = 1), laboratory leaders (n = 6), physicians (n = 5), Ministry personnel (n = 4), administrators (n = 3), extra-provincial experts, and researchers (n = 7), as well as pharmaceutical (n = 5) and diagnostic companies (n = 2). The forum considered evidence of good practices in adoption, implementation, and financing laboratory services and identified barriers as well as feasible options for improving advanced diagnostic testing in Ontario.
Results
Overarching challenges identified included: barriers to define what is needed; need for a clear approach to adoption; and the need for more oversight and coordination. Recommendations to address these included a shift to an anticipatory system of test adoption, creating a fit-for-purpose system of health technology management that consolidates existing evaluation processes, and modernizing the governance and financing of testing so that it is managed at a care-delivery level.
Conclusions
The proposals for change in Ontario highlight the role that HTA, governance, and financing of health technology play along the continuum of a health technology life cycle within a healthcare system where decision-making is highly decentralized. Resource availability and capacity were not a concern – instead, solutions require higher levels of coordination and system integration along with innovative approaches to HTA.
Introduction
Ontario is the largest of Canada’s 13 provinces and territories by population (approx. 14.8 millionCitation1, with the vast majority of the province’s inhabitants located in its southernmost regions. Residents are provided coverage for hospital and physician services through public health insuranceCitation2. Additional services (e.g. such as provision of outpatient pharmaceuticals, non-surgical dentistry) are available through a mix of private insurance or provincially-led programs.
Most hospitals in Ontario, as in the rest of Canada, are independent, not-for-profit entities, and receive an annual funding envelope from the province, supported in part by federal government contributions to the province; the federal government stipulates hospitals must provide medically necessary diagnostic testing and interpretation to Ontario resident in-patients and out-patients with no charge or deductible to patients. Outpatient testing for ambulatory patients is typically delivered by private, for-profit laboratory providers financed through large provincial tendering arrangements and based on a fee-for-service model. Their remit is ambulatory care, and although these providers do have the ability to deliver more complex testing, they are typically not incented to do so.
While this legacy scheme of financing has supported a basic laboratory function in each province, it has also led to a concentration of capacity for delivering advanced diagnostics (i.e. protein expression [e.g. immunohistochemical, proteomic]; cytogenetic [e.g. karyotyping, fluorescent in-situ hybridization, microarray] and molecular [e.g. quantitative polymerase chain reaction (PCR), sequencing]) in larger specialized tertiary care center laboratories. These centers have also relied on public and private research or specialized healthcare funding directed at academic institutions, along with donations benefiting larger hospitals to fund the development of these tests.
The end result is that highly specialized laboratories in Ontario and the rest of Canada are owned and operated independently of government (with the exception of public health laboratories) and rely on multiple sources of funding, beyond their respective Ministries of Health. With a demand for more advanced testing, provincial Ministries have either provided additional incentives to these centers or contracted services to out-of-province (or country) providers, when capacity within a province to deliver highly specialized testing is limited. While coordinating this service function is much less of a challenge in provinces with small volumes or limited numbers of centers, Ontario is faced with a unique challenge of coordinating multiple centers with unique catchment areas and financing and service delivery requirements. This decentralized model of healthcare delivery was presumably adopted to ensure more appropriate service delivery at a local levelCitation3–5, but has also made the planning and oversight of provincial needs for providing advanced diagnostic testing all the more challenging.
Demand for access to advanced testing has increased in lockstep with the understanding of the genetic basis of disease. Despite a need for system-wide coordination to manage the demand for innovation in testing, and several calls for reform in past decadesCitation6–8, by July 2020, Ontario still lacked an overarching system to facilitate planning and introduction of innovative genetic tests and approaches to testing. This has resulted in considerable delays and inequity of access to testing by geographic region, which affects patients, physicians and innovators of both new health technology and processes of care ().
To guide necessary change within the province of Ontario, we explored barriers to optimal testing and options for change through a mixed-methods approach that included literature review, semi-structured interview and a deliberative forum held with key stakeholders in Ontario. This report provides a description of the specific challenges identified, as well as the evidentiary basis and identifies key proposals for change. It also provides insights for those who seek to integrate health technology assessment as a means to manage these advanced diagnostic technologies. This analysis is intended to both provide lessons learned for other jurisdictions with similar challenges, as well as potential options for the appropriate management of these health technologies.
Methods and materials
The key steps taken to identify barriers to delivery and develop key proposals followed a mixed methods approach. First, a narrative literature review was conducted based on a purposive sample of commercially published and grey literature. Searches were performed by a medical librarian specialist and relevant information was identified by a single reviewer (DH), and a background document was prepared and reviewed by several experts. Semi-structured interviews (30–60 min) with patient representatives (n = 1), laboratory leaders (n = 6), physicians (n = 5), Ministry personnel (n = 4), administrators (n = 3), extra-provincial experts, and researchers (n = 7), as well as representatives from pharmaceutical (n = 5) and diagnostic (n = 2) companies were then undertaken to discuss good practices and challenges within the current system. Finally, background material was presented at two, 3.5-hour virtual meetings (in July 2020) where additional feedback was provided and preferred options for change were discussed. The deliberative forum was mandated to: (a) identify the most feasible and credible options for improving the organization and delivery of diagnostic testing in a way that is more predictable, streamlined, and provides best value for money while maintaining or improving high quality cancer care within the Ontario context; (b) recommend preferred options for immediate and long-term change that Ontario should prioritize to accomplish this goal. Further consultation with experts was then conducted to clarify missing information that could provide additional context and key barriers to optimal use, and proposals for change were drafted and reviewed by stakeholders and experts leading to this final report.
“Optimal” care from testing was defined as a state of health technology use that would most satisfy the Ontario “quadruple aim” of reducing per capita costs while improving population health outcomes, patient and caregiver experiences, and provider experiencesCitation9,Citation10. The potential barriers to the delivery of optimal care were first described qualitatively by an informal identification of concepts by two researchers (DH and TS) and sent to roundtable participants for validation (member checking). They are categorized according to technology life cycle domainCitation11, i.e. (1) health needs assessment (e.g. early dialogue and horizon scanning); (2) health impact assessment (e.g. preliminary assessment); (3) health technology assessment (e.g. validation and proficiency testing); and (4) health technology management (e.g. financing and monitoring).
The findings of this research are reported as follows: first, the conceptual themes and rationale based on participant interviews are provided; next, the overarching recommendations of the forum (as per the mandate) are discussed; lastly, a more in-depth exploration of implications for health technology assessment (and management) is described.
Results
Themes related to underlying challenges (as of July 2020) identified by stakeholders are identified and described in .
Table 1. The (negative) impact of the current approach to test adoption and funding on Ontario’s "quadruple aim".
Table 2. Themes from semi-structured interviews with stakeholders.
Barriers to defining what is needed
Horizon scanning
Stakeholders perceived consideration of new tests in Ontario as highly reactive and without systematic consideration of what is needed or a clear pathway for adoption. To date, horizon scanning has consisted of informal surveys of hereditary disease specialists and pharmaceutical companies; there was no dedicated horizon scanning effort to inform current and future testing needs beyond these specialty areas.
The role of research
A related challenge was the perceived need to offer innovative testing to patients participating in research intended to further knowledge about prognosis or the genetic basis of disease, efficacy of future therapies, or innovations in the organization and delivery of testing itself. Paradoxically, much research is funded through Ontario government programs, intended to promote translational research and innovation, motivated by strategic investment in genomic and cancer researchCitation12, and with a stated goal of increasing privately-funded clinical trial activityCitation13. Despite these attempts to motivate research as a means to improve Ontario’s knowledge economy (and create a learning healthcare system), research and innovation activity is still structured and financed as a parallel activity to healthcare delivery. This results in limited access to future innovation, being less attractive to private sector innovators, and dependent on where or by whom a patient is treated.
Need for a clear approach to adoption
Entry point for innovation
Similarly, clinical specialists, laboratory leaders, innovators, and patients were often aware of tests and testing modalities they felt required adoption, but where there was no obvious entry point into the healthcare system. While specialized laboratories can apply for a license after validation (see “No specific funding for test development”), a license is not linked to government funding. Instead, for unfunded tests, the applicant must explain how the test will be funded; this means without a clear funding pathway, laboratories may not be able to deliver the test within the context of routine care. In contrast, other large Canadian provinces (Quebec, Alberta, British Columbia)Citation14 in Canada have all adopted consolidated approaches (single entry points for consideration, consideration of tests not linked to drugs, a single evaluation process, and funds available for immediate financing) in an attempt to coordinate the reimbursement and delivery of testing across their provinces.
Prioritization driven by commercialization
Beyond testing for hereditary disease, much adoption of advanced testing has been driven by companion diagnostics in oncologyCitation15. Participants felt this limited, reactive approach to considering new tests or testing approaches meant much valuable innovation could be overlooked (e.g. researcher-driven innovation or process-driven innovation within the cancer care community). Many stakeholders felt this narrow approach to test adoption would become problematic; the benefits of testing are anticipated to go beyond simply identifying recipients of a companion drug. Instead, new modalities in testing (such as point-of-care testing, liquid-based biopsy, multigene assay panels or comprehensive genomic profiling) may aid in multiple decisions (e.g. decisions to not use old drugs) and may provide benefits beyond single drugs (e.g. sparing tissue or biopsy recovery beds). These “beyond-one-drug” benefits can lead to improved experiences within the healthcare system for care providers and patients, or to overall improved efficiency of testing within the healthcare system.
Lack of a consistent evaluative framework
No clear approach to test adoption has led to various committee structures, each with a unique evaluative approach (see Box 1) that have grown organically out of a number of initiatives and levels of administration and governance. There was a perception that without a consistent framework that considers the needs and impact of an entire community of practice (e.g. patients, surgery, radiology, oncology, pathology), public and private innovators of testing lack a clear signal regarding what evidence is needed. It was felt this hinders the development and introduction of needed and valuable innovation. Some informants indicated that many of these processes were not timely and lacked engagement with stakeholders and a tangible link to decision-making – conventional metrics of a principled HTA processCitation16.
A related challenge was the perception by those interviewed that meaningful metrics of value may be missing from some evaluative approaches. For example, laboratory leaders suggested the quality of care related to laboratory testing was largely judged by turnaround time, which is of interest to clinicians and patients; while they acknowledged there is economic waste and negative consequences to patients when biomarkers are not readable to an oncologist at first consultation, they also indicated a need to consider other metrics such as the ability for patients to receive a biopsy or to receive other tests of equal or greater importanceCitation17. This speaks to the need for an explicit evaluative process that addresses care pathways and needs across a care continuum and community of practice – not a single viewpoint but valuable from multiple stakeholder perspectivesCitation18.
Formal assessment of test validity, clinical utility, and cost is currently considered by the Ontario Genetics Advisory Committee (OGAC) supported by Ontario’s health technology assessment (HTA) program, which was “created to strategically plan an integrated approach … to advise … on the clinical utility, validity, and value for money of new and existing genetic and genomic tests in Ontario to support [the Ontario Health Technology Assessment Committee (OHTAC)’s] role in making recommendations.” However, this process of assessment and the use of OHTAC/OGAC was thought by forum participants to not be fit for purpose for a number of reasons. First and foremost, the length of time to conduct reviews and provide recommendations (12+ months) is not keeping in pace with the rate of change of technology. This delay is in part because the Ontario HTA process employs a priority setting and scoping process, and needs to establish relationships with care specialists and others who provide voluntary contributions for each new reviewCitation19.
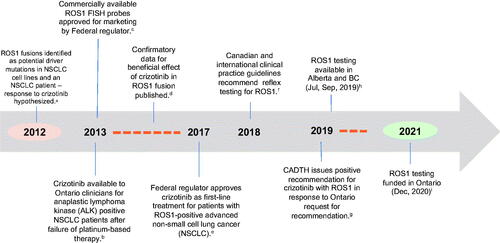
Not all new tests are considered by the Ontario HTA program. provides an illustrative example of the adoption and financing of testing for ROS1 fusion mutations. Despite the availability of commercialized fluorescence in-situ hybridization probes from 2013 a targeted therapy in 2017, and Canadian consensus guidance in 2018Citation20, the test was never evaluated in Ontario. A positive recommendation for ROS1 targeted therapy was issued by CADTH in 2019, however funding for the test was not implemented until December 2020, almost 1.5 years after funding in other provinces.
Figure 1. Adoption pathway for ROS1 fusion testing in Ontario. Abbreviations. NSCLC, non-small cell lung cancer; FISH, fluorescence in situ hybridization; aBergethon et al.Citation63; bSee CADTHCitation64; cAbbott Visis probesCitation65; dShaw et al.Citation66 and Wu et al.Citation67; eSee press releaseCitation68; fSee Melosky et al.Citation20 andCitation69; gCADTHCitation70; h,ipersonal communicationCitation71.
No specific funding for test development
A final challenge identified within this theme was the lack of funding for the development of new biomarker tests. In Canada, laboratory developed tests (LDTs) are used more frequently as an alternative to commercially developed tests, largely because of the decreased cost per test and the flexibility to make use of existing platforms within any given labCitation21–23. Both commercially available tests and LDTs require resources and time to develop, optimize, and implementCitation24. Most experts suggested costs were equivalent to 6 months of operational costs related to testing and required several months to a year to develop. The speed of implementation, however, is highly dependent on the capacity within individual laboratories, further creating an issue of inequity of access. To develop tests, laboratories not only require human resources, equipment and procedures that will not interfere with the use of tests during routine care, they also need access to validation protocols and a set of previously confirmed tissue samples to ensure test performance is reliable and valid compared to a gold standard or other commercial test.
As no specific funding is provided by the Ministry to laboratories to develop or validate new tests, most LDTs are financed through baseline operational funding, private sector grants (i.e. notably pharmaceutical companies who are interested in tests being available when drugs requiring the test are listed), or public sector translational research funds. This means different cancer centers and laboratories will have different capacities to develop tests, based on their connection to private and public research funding. It also creates further pressure to prioritize tests based on commercial interests and availability of funding.
Need for more oversight and coordination
Lack of standardization of analytic aspects of testing
New molecular biomarkers approved for clinical use and funded through MOH are generally approved without extensive consideration of the analytic parameters needed to ensure effective testing. Unlike FDA approvals for devices, which specify not only the metrics but the platform to be used for approved assays, the approval process in Ontario stops short of specifying the method or the analytic measurements needed to appropriately assess the biomarker in question. Laboratories wishing to offer the testing rely on internal processes to implement and then clinically validate assays – the platform used is generally dictated by what might be available in a given lab, and there are no mechanisms to incent harmonization between platforms that might be used in different labs. While all laboratories engage in external quality assessment schemes to mitigate this issue to some degree, this approach nevertheless leads to differences in important analytic metrics, such as sensitivity, specificity, or limits of detection.
Lack of service coordination
A further issue identified was the lack of coordination among testing centers. Informants felt complex testing over a province as geographically large as Ontario (a province almost 3 times as large as Germany) required an enhanced level of coordination to ensure consistent levels of service. Instead, individual hospitals are provided financial incentives to compete, rather than collaborate with other centers. This had the unintended consequence of oncologists creating demand for tests within their institutions upon hearing the test was being performed in other institutions, which in turn put pressure on their institutional laboratories to create a “bridging solution” until the test could be offered locally or, at least, regionally. These bridging solutions involved work-intensive human-based processes of test ordering, shipment of samples, and transcription of test results into medical records that are perceived as unsafe, and inefficient and adding further stress to a system with scarce resources.
Need for a coordinated financing approach
The lack of a coordinated approach to implementation was layered on top of a lack of a coordinated approach to financing. For example, no mechanism is in place to ensure funds are released for tests at the same time as companion drugs. This is made more difficult by laboratories having multiple sources of financing for the delivery of tests. Additionally, the cost and time associated with the interpretation of test results is often not included in new budgets allocated for testing. The constant addition of biomarker tests has a direct impact in the workload of healthcare providers involved in its interpretation, quality control, and reporting. However, public funding of positions (e.g. pathologists) in the province are based on volume, not complexity. The immediate result is adaptation to the added work, but chronically it leads to burnout, workplace tension, and potentially unsafe practicesCitation25.
Need for a nimble financing approach
The current method of financing new tests relies on a historical approach to financing medical technology which considers yearly budget cycles and weighs test adoption versus other healthcare priorities. Decentralized capital purchasing of underlying medical equipment (e.g. sequencers) by individual hospitals also does not lend itself to an overarching provincial strategy for capital planning. As budgets for testing are set annually, informants indicated the time to approval of any test is highly variable, creating uncertainty for producers of targeted therapies. Experts also expressed concern that innovation in testing is evolving more rapidly than the speed of financing and requires an open approach based on anticipated growth that is nimbler, and similar to formulary management, rather than more drawn out approaches that have been used to fund large capital equipment purchases or new medical procedures.
Ongoing monitoring and financial oversight
The lack of system-wide coordination and managed entry for new tests also creates challenges for system planners to understand broader patterns of expenditure and the relative value of testing across the health care system. In situations with targeted funding provided for individual tests, experts noted that the prices paid were largely volume-based without reassessment, and did not consider changes in approach to testing type or patient type.
Recommendations and current and future developments
The high-level options developed for consideration by the provincial Ministry in July 2020 suggest systematic changes toward a more pro-active, fit-for-purpose, and responsive system of test adoption and management (Box 2). A full report can be found onlineCitation26 and in Supplementary Appendix S1. Since this time, some changes in the approach to testing in Ontario have emerged despite the overwhelming priority of managing its response to the pandemic. In 2021, funding was provided to allow for more comprehensive testing in select somatic and hereditary oncologic conditionsCitation27. Recommendations for hereditary cancer testing were developed in September 2021Citation28 and a province-wide genetics advisory committee (separate from the Ontario Genetics Advisory Committee) has been established. Along with this, additional changes to fees that recognize multiple biomarker testing have been employed. However, a detailed, publicly available description of these new processes for the onboarding and ongoing management of testing is still in development.
The issues in Ontario are not unique to the region nor has there been a lack of awareness of these issues. Concerns about laboratory stewardship have led to multiple independent audits, commissions, reviews, and recommendationsCitation6–8,Citation29. In 2017, Ontario’s Cancer program formed two working groups to develop recommendations for improving genetic services across Ontario, specifically in the areas of clinical cancer genetic services and hereditary cancer testingCitation30.
These issues identified are also not unique to advanced testing technologies. There are many examples where the nature and associated costs of a technology are disruptive (i.e. requiring changes to care paradigm that create unique benefits and risk of increased spend), including biologicals and expensive drugs for rare diseases, medical imaging (e.g. magnetic resonance imaging, computed tomography [CT], single-photon emission CT and radioisotope services), cardiac technologies (e.g. cardiac synchronization, implantable defibrillators, and catheterization facilities), arthroplasty, interventional cardiology technologies (e.g. stents), insulin pumps, population-based screening (e.g. breast and colorectal), and newborn care technologiesCitation31,Citation32. These technologies also required healthcare stewards to take further steps to manage technology entry (through rigorous assessment and revisiting reimbursement mechanisms) to prevent inappropriate levels of diffusion and unmanageable expenditure growth.
However advanced testing, including genomic profiling and sequencing, could have technologic implications beyond other high-impact technologiesCitation33. In particular, genome-based information not only facilitates individual patient decisions and health outcomes, it also fosters opportunities for monitoring, knowledge production, and innovation. Cancer is a genetically-driven disease of high public priority, and genome-based testing is a direct means of developing innovations to reduce the burden of cancer.
Some countries have recognized these additional informational benefits of establishing molecular testing capacity. In the UK, for example, NHS England has established a genomic medicine service based on a private–public sector partnershipCitation34. In a similar vein, the Scottish Government has established a Scottish Precision Medicine Ecosystem to support operational delivery of genome-based medicineCitation35. Germany has similarly announced its initiative to set up a nationwide platform for medical genome sequencing – genomDE – to enable increased clinical application of genome sequencingCitation36. Australia has also recently announced a smaller-scale ($AUS 185 M) private–public sector partnership to enable a broader aspect to comprehensive genomic profiling for cancer patientsCitation37.
Advanced diagnostic testing and implications for HTA
Our findings highlight the unique challenges of adopting and managing complex health technologies requiring significant upfront investmentCitation38 within a healthcare system where decision-making is highly decentralizedCitation39. They also emphasize the role that the governance and financing of health technology play in achieving appropriate technology utilizationCitation40. While devolving the decisions to adopt technology to frontline teams is a recognized strategy for enabling technology diffusionCitation41, it may also foster inequity across care centers.
There are also several important implications for the role and function of HTA. First and foremost, HTA frameworks need to acknowledge the role of testing in knowledge production and benefits beyond healthcare decisions. Given many HTA processes are beholden to health systems and decision-makers, this may not be easy to adopt. The strict emphasis on the healthcare implications of health technology is a departure from the original intent of technology assessment, which emphasized consideration of all relevant societal impactsCitation42.
A second consideration is the challenge of using traditional review-based (i.e. static) methods in the realm of advanced diagnostics, where technology is constantly evolving and the value of any test is highly context-specific (i.e. dynamic). Firstly, the duration of a literature review-based process can result in delays for patients and physicians that may be perceived as lengthy. As an example, an Ontario HTA recommendation of the use of genome-wide sequencing for unexplained developmental disabilities or multiple congenital anomalies took almost a year and a half (300 business days) to completeCitation43. Recommendations relied on evidence identified almost a year before committee deliberation.
Advanced testing and HTA – possible solutions
While there are clearly challenges with traditional approaches, it by no means suggests a diminished role for HTA. Rather, a fit-for-purpose HTA process for advanced diagnostics that considers the use of one or more of the following design features should be considered:
Upstream/headroom analysis
Instead of relying on a reactive, de novo analysis of benefits and/or costs of every new test, generic economic impact models could be developed beforehand, and populated with real-world or other therapy-specific contextual information that has been defined ahead of timeCitation24,Citation44. Using this approach, HTA bodies need only to ask what performance or contextual characteristics might be required to produce a given benefit or lead to a meaningful shift in costs, rather than whether an intervention is cost-effectiveCitation45.
Multigene assay/expanded coverage framework
Given the rapid pace of innovation, a one-test-at-a-time (i.e. single technology assessment) approach to the assessment of new technology entries, common to drug-based HTA, will no longer make sense as very frequent updates (i.e. implementation and communication) to existing care pathways will be too disruptive or costlyCitation46. An anticipatory approach could involve early assessment that categorizes genes amenable to a multigene assay approach as established (well-studied), newly recognized (less-studied), or emerging (not well-understood)Citation47. Multigene assay approaches have been shown to be cost neutral or cost saving for both aiding diagnosis of hereditary conditions and aiding therapeutic decisionsCitation48. They may also have additional benefits, such as reducing risk of tissue exhaustionCitation49. Further analysis could be done to test this assumption and ensure costs from a multigene assay approach versus a single-gene testing approach are not excessive. The feasibility of this approach requires the insurer to blur distinctions between “medically necessary” and “experimental/investigational” technology and a coverage framework that recognizes the cost-effectiveness of a blended approach.
Community of practice and engagement
As costs of testing are highly dependent on care pathwaysCitation24 and other contextual information, it highlights the need to establish an assessment function where evaluators work closely with a community of practice who can provide necessary information to make optimal decisionsCitation15,Citation50. This includes clinicians, surgeons, and radiologists who must consider parallel processes (staging, consults, testing) to establish testing requirements (tumor and sample characteristics such as tumor cellularity and heterogeneity); required turnaround times; bioinformatic requirements; testing strategy (technical limitations of test, panel size); and feasibility of adoption including technical staffing, human resource impact, and infrastructure requirementsCitation43. It may also include real-world information such as the population prevalence of the variants. A review of HTA in Australia revealed that information about the health condition, population prevalence, and care pathways were considerable challenges for assessing genomic testsCitation51. This implication for HTA also means the results of an evaluation more closely linked to a given care context (such as hospital-based HTA) may be easier to implement than high-level HTA processes further removed or attempting to inform non-centralized (i.e. pluralistic) healthcare systems. Stakeholder engagement throughout the HTA function will also facilitate necessary communication regarding what tests are available and how tests are adopted and managed.
Rapid review
HTA bodies will likely still need to rely on the use of reviews. Rapid reviews, a “type of knowledge synthesis in which components of the systematic review process are simplified or omitted to produce information in a short period of time” are one approach to complement real world data so that information about a test can be synthesized quicklyCitation52,Citation53. Rapid reviews of any test could also be married to a “living review” approach, which provides updates on priority technologies as evidence emergesCitation54.
Use of real-world evidence
Evaluation of testing will benefit from HTA that relies on the use of real-world data to understand benefits. This may involve assessment after initial decisions to cover testing via headroom analysisCitation55,Citation56. Real-world evidence may be particularly useful for gauging the benefits or potential benefits of testing not amenable to multigene assay panels such as the use of liquid-based comprehensive genomic profiling, or altering the sequence of testing in a given care pathway.
Real-world evidence also bridges investigational and mainstream delivery of healthcare for newly-recognized tests or testing approaches. In Canada, translational research initiatives such as the Alberta Diagnostics Ecosystem Platform for Translation (ADEPT)Citation57 are attempting to accelerate innovations in testing that may be of more immediate benefit to patients and care providers. Ontario has also begun to do this through strategic initiatives within its Ontario Institute for Cancer ResearchCitation58.
Discussion
Our exploration of the barriers related to the optimal delivery of an advanced diagnostic service in Ontario revealed a number of challenges along the continuum of a health technology life cycle. None of these challenges relate strictly to resource availability – instead they underscore the important roles of care organization, governance, financing, and HTA that allow for potentially disruptive and costly technologies to be appropriately adopted and managed. Needs identified included a forward looking, systematic, and open process for assessing what tests are needed, a nimble and coordinated process of assessing test performance and implementation, and a finance model that can quickly release funds for valuable tests.
The challenges and potential solutions outlined here should not be interpreted as a technological imperative; none of the recommendations advocate for more widespread use of genome-based testing, but rather for an efficient process to manage the lifecycle of these technologies. While some may be concerned about unconstrained expenditure growth from the more ready adoption of new technology, we believe a more consolidated process introduces opportunities to constrain expenditure growth, as technology, including where and how it is used, and the prices paid for it are more comprehensively managed. On one level, the increase in the number of new tests and testing approaches in coming years can be thought of as similar to the rapid increase in the number of new and expensive drugs 30 years ago, that led to more sophisticated approaches to formulary managementCitation59.
The recommendations from the Ontario Forum suggest a consolidation and reallocation of current processes to create a minimal (or reduced) impact on Ontario’s current operational spend. As proposed, cost-add may arise from additional efforts to conduct formal horizon scanning processes and improve communication, deliberation, and administration of the process. Any additional costs due to increased testing are assumed to be managed from within the new process.
While these recommended solutions are highly specific to Ontario, they are based on recognized challenges that may be generalizable to other jurisdictions. Some challenges may be more difficult to reconcile in Ontario and elsewhere. Full integration of investigational and mainstream care, for example, can be difficult in any jurisdiction and may require changes in governance and the legal basis of healthcare. In Ontario, like many jurisdictions worldwide, for example, “experimental” services are not considered insurable, by lawCitation60.
Some challenges also hold lessons for HTA worldwide when applied to diagnostic technology. This includes the need for standard evaluative frameworksCitation37 and the need to consider the clinical context in which care will be delivered. The various care and referral pathways, clinical specialists involved, feasibility of obtaining samples for testing, timing, volume, disease prevalence, analytic performance, education, reporting, and other factors greatly influence the value of testing. Ultimately, testing is a complex intervention that relies on a number of interrelated components and human resourcesCitation38. This suggests that, unlike the evaluation of drugs, which is much less context dependent, evaluation of new tests requires a strong level of engagement with local providers.
One limitation of generalizing study findings to other jurisdictions is that there may be additional barriers to the optimal use of testing not addressed, as Ontario already had some necessary (or facilitating) conditions in place. This includes requirements for data infrastructure, testing equipment, health and information technology, human resources, and standardized clinical care pathways. All of these could be considered necessary conditions for the optimal use of advanced testing but were not considered significant barriers in Ontario.
Another limitation of this study is it represents a snapshot of Ontario up to July 2020. As already described, Ontario has made improvements in horizon scanning efforts, the adoption of standards around minimum required testing in inherited and somatic cancers, integration of genome-wide sequencing activities, and revisiting price formulas. Many of these changes have been facilitated by reduced decentralization and further coordination of the healthcare system, as in the background, Ontario has also adopted a single healthcare organization approach. However, the pace of change of these efforts has also been hindered by attention to COVID-19. Ironically, the pandemic has highlighted the necessity of testing, and the need for a coordinated testing function and domestic supply.
Also not described in detail is the need to consider the role of education and how it is evolving, particularly educating healthcare professionals about the intended outcomes and the context of where care is delivered when system-wide changes are employed. Genetic testing is often embedded in the complex work of interprofessional teams that are physically distributed. For instance, the members of a cancer care team usually meet once a week at multidisciplinary cancer conferences, the only point of contact of the community of practice. Changes to advanced diagnostic technology assessment and management, including implementation of new tests and changes to workflow, require outcomes-based interprofessional training at the intersection of continuing professional development, knowledge transfer, and quality improvementCitation61.
This complexity means that new teaching modalities, such as workplace-based assessment and in-situ simulation, will require consideration along with the many medical facets of testing that include “coordination of care, tissue procurement and handover, requisition and report design, clear workflow within and between services, automatic information exchange between electronic health systems, and improved communication, with fast feedback loops between health care practitioners.”Citation62 The daily practice of genetic testing in this rapidly evolving field would also benefit from standardized orders and decision-support systems, with constant monitoring of quality indicators to measure processes and outputs. The amount of data and the speed of change will also require fluency in emerging areas of education, including big data, machine learning, artificial intelligence, cybersecurity, collective competence, and adaptive expertise.
Transparency
Declaration of funding
Sponsors of the study include: Amgen Canada, Inc.; AstraZeneca Canada Inc.; Eli Lilly Canada, Inc.; Hoffmann-La Roche Canada, Inc. (Pharmaceuticals Division); Hoffmann-La Roche Canada, Inc. (Diagnostics Division); Janssen Canada Inc.; Pfizer Canada Inc.; Thermo Fisher Scientific (Canada). All sponsors contributed equally. None of the sponsors played a role in providing drafting, revising or approving the content of this research.
Declaration of financial/other interests
Grant/research
HF received grants from Astra Zeneca, Pfizer, Roche, Amgen, EMD Serono, Bayer, Eli Lilly, Canexia Health, Delphi Diagnostics; in kind support from Thermo Fisher, Illumina, Archer, Canexia Health.
TS received grants from Roche.
MMG received grants from AstraZeneca, Merck; consulting fees from Roche, AstraZeneca, and a consortium of life science companies (Amgen Canada, Inc.; AstraZeneca Canada Inc.; Eli Lilly. Canada, Inc.; Hoffmann-La Roche Canada, Inc. (Pharmaceuticals Division); Hoffmann-La Roche Canada, Inc. (Diagnostics Division); Janssen Canada Inc.; Pfizer Canada Inc.; Thermo Fisher Scientific (Canada)).
RJ received grants from Amgen, Astellas, AstraZeneca, Bold Therapeutics, BMS, Fusion Pharmaceuticals, Jannsen, Macrogenics, Merck.
BS received grants from Eli Lilly, Pfizer, Thermo Fisher, Roche, Amgen.
SK received grants from Roche.
TLS received research grants from Astra Zeneca.
Consultant/advisor
DH has received consulting fees from a consortium of life science companies (Amgen Canada, Inc.; AstraZeneca Canada Inc.; Eli Lilly Canada, Inc.; Hoffmann-La Roche Canada, Inc. (Pharmaceuticals Division); Hoffmann-La Roche Canada, Inc. (Diagnostics Division); Janssen Canada Inc.; Pfizer Canada Inc.; Thermo Fisher Scientific (Canada).
HF has received honoraria from Astra Zeneca, Pfizer, Roche, EMD Serono, Bayer, Norvartis.
MG has participated on a Data Safety Monitoring Board or Advisory Board for Bayer and AstraZeneca.
RJ has received consulting fees from BMS, Merck Pfizer; participation on advisory board or honoraria from Amgen, AstraZeneca, Bayer, BMS, EMD.
Serono, Fusion Pharmaceuticals, Jazz Pharmaceuticals, Janssen Pharmaceuticals, Lilly, merck, Novartis, Pfizer, Roche, Sanofi and Takeda.
BS has been part of a data safety monitoring board/advisory board for Astra Zeneca, Amgen, Pfizer, Roche, Merck, Eli Lilly and Novartis.
TLS has received advisory board honoraria from Janssen, Bayer, Pfizer, Merck, Astra Zeneca, Amgen, Astellas.
Speakers bureau
MG received payment or honoraria for speaking from Roche and AstraZeneca.
SK has been part of the Roche speaker’s bureau.
Stock shareholder
DH owns stocks in J&J.
MG owns stock or stock options with Abcellera Biologics Inc, CRISPR Therapeutics AG, and Nkarta Inc.
Other
TS is a consultant to the Federal government.
HF is Chair of Ontario/Health Cancer Care Ontario’s Molecular Oncology Testing Advisory Committee, and Member of the Provincial Genetics Advisory Committee for the Ontario Ministry of Health.
BS has received support for travel/attending meetings from Biocartis and Roche.
TLS has received consultancy fees from Health Canada for her work as a consultant for in vitro diagnostic device reviews.
PJ has no conflicts to declare.
Reviewer disclosures
The Editor in Chief and Deputy Editor in Chief helped with adjudicating the final decision on this paper. The peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
DH is the submitting and corresponding author. All other authors (TS, HEF, MMG, RJ, BSS, SK, TLS, and PJ) contributed to the conception of this paper and informed the recommendations. DH led the drafting and editing of the article and all authors were involved in re-drafting and revising the article critically for important intellectual content. All authors approved the final version of the article. DH is the guarantor of this work. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Box 1. HTA programs/committees affecting testing decisions in Ontario.
Ontario has a well-established history of using HTA processes for technology adoption decisions, and has already created several HTA processes that address new genetic tests. As of July 2020, HTA processes (programs/committees) used to guide decision include:
Provincial-level
Cancer program-level (Cancer Care Ontario)
Program in Evidence-Based Care / Tumor Site Groups produce evidence-based guidelines / care pathways and resources in partnership with clinical experts in all major cancer disease sites
Healthcare System-level (Ontario Health)
Health Quality Ontario / Ontario Genetics Advisory Committee advises the Minister of Health via Ontario Health (Quality), on which new and existing genetic and genomic services constitute optimal care.
Ministry of Health-level
Ontario Public Drug Programs / The Ontario Steering Committee for Cancer Drugs (OSCCD) was created in 2013 to enhance and support the Executive Officer of Ontario Public Drug Programs (OPDP) on: provincial cancer drug funding policies and decisions; program evaluation and drug-specific studies; enhancements to cancer drug programs or initiatives in Ontario for drugs that do not fall under pCODR’s purview.
Newborn Screening Ontario / The Newborn Screening Ontario Advisory Council (NSO-AC) was created to “provide policy and knowledge translation advice for NSO programs including changes to the NSO testing panel, technologies, screening protocols, disclosure of adventitious information, consent, storage and access to residual samples, alignment with other relevant screening systems, etc.”Citation28.
National-level
The pan-Canadian Oncology Drug Review (pCODR, now called CADTH Reimbursement Review process) / pCODR Expert Review Committee provides recommendations to the Ontario Ministry-led Public Drug Programs (and other participating Canadian public insurance plans) regarding whether to reimburse new targeted drugs and under what conditions (e.g. testing).
Box 2. Proposals to change Ontario’s system of managing advanced diagnostic technologyCitation48.
Shift to a proactive system of test adoption
Adopt a systematic approach involving single-entry approach supported by horizon scanning
Establish a rapid approach for preliminary assessment
Create a fit-for-purpose system of health technology management
Consolidate evaluation process into a single, fit-for- purpose process
Capture value and opportunities in: Selling genetic testing services and innovations; advanced therapeutics; and meeting other domestic requirements (e.g. pandemic preparedness)
Modernize governance towards a system that is more responsive to patient needs
The Ministry must move away from its administrative role and become a steward over a genetic program
Provide envelope funding for a genetic testing program that is managed at a care-delivery level.
Supplemental Material
Download PDF (7.5 MB)Acknowledgements
The authors would like to acknowledge other key informants who were interviewed or attended the July 2020 deliberative forum and whose insights informed the recommendations in this report. Recommendations described in this report should not be construed as being endorsed by every informant. Names of informants are listed in the Supplementary Material and include: patients representatives – Christina Sit (Lung Cancer Canada); laboratory leaders – Suzanne Kamel-Reid, Michael Kadour; physicians – Kevin Imrie, Eric Winquist, Parneet Cheema; Ministry of Health- Neeta Sarta, Cynthia Ho, Maricon Sanelli, Renee Mahalanobis; health system leaders – Aaron Pollett, Elaine Meertens, Garth Matheson; research experts – Christine Williams, Nicole Mittmann, Philip Gordon, Shariha Bhimani, Francois Sanschagrin, Stephen Yip, Irfan Dhalla.
References
- Government of Canada SC. Population estimates on July 1st, by age and sex. 2021; [cited 2022 Jan 17]. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501.
- Our Story | Ontario Health. 2022; [cited 2022 Jan 10]. Available from: https://www.ontariohealth.ca/our-story.
- Sumah AM, Baatiema L, Abimbola S. The impacts of decentralisation on health-related equity: a systematic review of the evidence. Health Policy. 2016;120(10):1183–1192.
- Abimbola S, Baatiema L, Bigdeli M. The impacts of decentralization on health system equity, efficiency and resilience: a realist synthesis of the evidence. Health Policy Plan. 2019;34(8):605–617.
- Ramos MC, Barreto JOM, Shimizu HE, et al. Regionalization for health improvement: a systematic review. PLoS One. 2020;15(12):e0244078.
- Molecular Oncology Task Force. Ensuring access to high quality molecular oncology laboratory testing and clinical cancer genetic services in Ontario- Report of the molecular oncology task force. Cancer Care Ontario. 2008; Available from: https://collections.ola.org/mon/23001/289823.pdf
- Cancer Care Ontario. Recommendations report for Ontario’s clinical genetic services. Cancer Care Ontario. 2018; Available from: https://www.cancercareontario.ca/sites/ccocancercare/files/assets/ClinicalGeneticServicesRecommendationReport.pdf
- Office of the Auditor General of Ontario. Laboratory services in the health sector. Office of the Auditor General of Ontario. 2017; Available from: https://www.auditor.on.ca/en/content/annualreports/arreports/en17/v1_307en17.pdf
- A healthy Ontario: Building a sustainable health care system: Chapter 2: The vision for health care in Ontario | Ontario.ca. 2022; [cited 2022 Jan 10]. Available from: https://www.ontario.ca/document/healthy-ontario-building-sustainable-health-care-system/chapter-2-vision-health-care-ontario.
- Sikka R, Morath JM, Leape L. The quadruple aim: care, health, cost and meaning in work. BMJ qual saf. BMJ Publishing Group Ltd. 2015;24:608–610.
- Gutiérrez-Ibarluzea I, Chiumente M, Dauben H-P. The life cycle of health technologies. Challenges and ways forward. Front Pharmacol. 2017;8(14):14.
- Cancer Research Investment in Canada, 2018. 2018;8.
- Strategic Priorities – Mission & Priorities [Internet]. Clinical Trials Ontario. 2022; [cited 2022 Jan 10]. Available from: https://www.ctontario.ca/who-we-are-clinical-trials-ontario/mission-and-priorities-strategic-priorities/.
- INESSS [Internet]. INESSS. 2022; [cited 2022 Jan 10]. Available from: http://www.inesss.qc.ca/.
- Mateo J, Steuten L, Aftimos P, et al. Delivering precision oncology to patients with cancer. Nat Med. 2022;28(4):658–665.
- Drummond MF, Schwartz JS, Jönsson B, et al. Key principles for the improved conduct of health technology assessments for resource allocation decisions. Int J Technol Assess Health Care. 2008;24(3):244–258. discussion 362-368.
- Stewart DJ, Maziak DE, Moore SM, et al. The need for speed in advanced non‐small cell lung cancer: a population kinetics assessment. Cancer Med. 2021;10(24):9040–9046.
- Augustovski F, Alfie V, Alcaraz A, et al. A value framework for the assessment of diagnostic technologies: a proposal based on a targeted systematic review and a multistakeholder deliberative process in Latin America. Value Health. 2021;24(4):486–496.
- hta-methods-and-process-guide-en.pdf. 2022; [cited 2022 Jan 17]. Available from: https://www.hqontario.ca/Portals/0/documents/evidence/reports/hta-methods-and-process-guide-en.pdf.
- Melosky B, Blais N, Cheema P, et al. Standardizing biomarker testing for Canadian patients with advanced lung cancer. Curr Oncol. 2018;25(1):73–82.
- Cheung CC, Smith AC, Albadine R, et al. Canadian ROS proto-oncogene 1 study (CROS) for multi-institutional implementation of ROS1 testing in non-small cell lung cancer. Lung Cancer. 2021;160:127–135.
- Torlakovic E, Albadine R, Bigras G, et al. Canadian multicenter project on standardization of programmed Death-Ligand 1 immunohistochemistry 22C3 Laboratory-Developed tests for pembrolizumab therapy in NSCLC. J Thorac Oncol. 2020;15(8):1328–1337.
- Cutz J-C, Craddock KJ, Torlakovic E, et al. Canadian anaplastic lymphoma kinase study: a model for multicenter standardization and optimization of ALK testing in lung cancer. J Thorac Oncol. 2014;9(9):1255–1263.
- Sabatini LM, Mathews C, Ptak D, et al. Genomic sequencing procedure microcosting analysis and health economic Cost-Impact analysis: a report of the association for molecular pathology. J Mol Diagn. 2016;18(3):319–328.
- Hede K. Breast cancer testing scandal shines spotlight on black box of clinical laboratory testing. J Natl Cancer Inst. 2008;100(12):836–844.
- Husereau D, Sullivan T, Jacobs P. Towards Optimizing the Delivery of Diagnostic Testing in Cancer Care: A proposal to change genetic testing in Ontario. 2021. Available from: https://www.dropbox.com/s/2qtion2qo0auo45/White%20paper_FINAL_CONFIDENTIALV2.pdf?dl=0.
- Comprehensive-Cancer-Testing-Announcement-Wave-1.pdf. 2022; [cited 2022 Jan 10]. Available from: https://survivornet.ca/wp-content/uploads/2021/06/Comprehensive-Cancer-Testing-Announcement-Wave-1.pdf.
- Ontario Health. 2021. Hereditary Cancer Testing Eligibility Criteria: Version 2. Ontario health; 2021; [cited 2022 Mar 1]. Available from: https://www.google.com/url?sa=t&source=web&rct=j&url=https://www.cancercareontario.ca/en/file/62671/download%3Ftoken%3D5usSZa-S&ved=2ahUKEwiistXYr6X2AhUej4kEHYUTAjkQFnoECA0QAQ&usg=AOvVaw1ukQ6iYUlawwCZ8KORFg1a.
- Sullivan T, Gordon P, Minto S. Laboratory Services Expert Panel. 2015; Available from: http://www.health.gov.on.ca/en/common/ministry/publications/reports/lab_services/labservices.pdf.
- ClinicalGeneticServicesRecommendationReport.pdf. 2022; [cited 2022 Jan 10]. Available from: https://www.cancercareontario.ca/sites/ccocancercare/files/assets/ClinicalGeneticServicesRecommendationReport.pdf.
- Sorenson C, Drummond M, Bhuiyan KB. Medical technology as a key driver of rising health expenditure: disentangling the relationship. Clinicoecon Outcomes Res. 2013;5:223–234.
- Pammolli F, Riccaboni M, Oglialoro C, et al. Medical devices competitiveness and impact on public health expenditure. Germany: University Library of Munich; 2005. Report No.: 16021. Available from: http://ideas.repec.org/p/pra/mprapa/16021.html
- Wurcel V, Cicchetti A, Garrison L, et al. The value of diagnostic information in personalised healthcare: a comprehensive concept to facilitate bringing this technology into healthcare systems. Public Health Genomics. 2019;22(1–2):8–15.
- Snape K, Wedderburn S, Barwell J. The new genomic medicine service and implications for patients. Clin Med. 2019;19(4):273–277.
- Scotland PM. About Precision Medicine Scotland Innovation Centre. Precision Medicine Scotland. 2012; [cited 2022 Jan 10]. Available from: https://www.precisionmedicinescotland.com/about-pms/.
- Die deutsche Genom-Initiative – genomDE. 2022. [cited 2022 May 10]. Available from: https://www.bundesgesundheitsministerium.de/themen/gesundheitswesen/personalisierte-medizin/genomde-de.html.
- $185 million investment to fast-track treatments for rare and ‘untreatable’ cancers. Garvan Institute of Medical Research. 2022; [cited 2022 May 3]; Available from: https://www.garvan.org.au/news-events/news/185-million-investment-to-fast-track-treatments-for-rare-and-2018untreatable2019-cancers.
- Hogervorst MA, Vreman RA, Mantel-Teeuwisse AK, et al. Reported challenges in health technology assessment of complex health technologies. Value in Health. 2021; [cited 2022 Feb 8]. Available from: https://www.sciencedirect.com/science/article/pii/S1098301521031879
- Challenges of Health Technology Assessment (HTA) in Pluralistic Healthcare Systems: Report of an ISPOR HTA Council Working Group. Value in Health (Elsevier Science). [in press].
- Ozdemir V, Husereau D, Hyland S, et al. Personalized medicine beyond genomics: New technologies, global health diplomacy and anticipatory governance. Curr Pharmacogenomics Person Med. 2009;7(4):225–230.
- Greenhalgh T, Robert G, Macfarlane F, et al. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q. 2004;82(4):581–629.
- Wong J. The history of technology assessment and comparative effectiveness research for drugs and medical devices and the role of the federal government. Biotechnol Law Rep. 2014;33(6):221–248.
- Ontario Health (Quality). Genome-Wide sequencing for unexplained developmental disabilities or multiple congenital anomalies: a health technology assessment. Ont Health Technol Assess Ser. 2020;20:1–178.
- Pollard S, Weymann D, Chan B, et al. Defining a core data set for the economic evaluation of precision oncology. Value Health. 2022;2022:S1098-3015(22)00055-9.
- Girling A, Lilford R, Cole A, et al. Headroom approach to device development: current and future directions. Int J Technol Assess Health Care. 2015;31(5):331–338.
- Fenwick E, Claxton K, Sculpher M. The value of implementation and the value of information: Combined and uneven development. Med decis making. 2008. 28:21–32.
- Trosman JR, Weldon CB, Gradishar WJ, et al. From the past to the present: Insurer coverage frameworks for Next-Generation tumor sequencing. Value Health. 2018;21(9):1062–1068.
- Pruneri G, De Braud F, Sapino A, et al. Next-Generation sequencing in clinical practice: is it a cost-saving alternative to a single-gene testing approach? Pharmacoecon Open. 2021;5(2):285–298.
- Yip S, Christofides A, Banerji S, et al. A Canadian guideline on the use of next-generation sequencing in oncology. Current Oncology. 2019;26(2):241–254.
- Baltussen R, Jansen M, Oortwijn W. Evidence-informed deliberative processes for legitimate health benefit package design – Part I: Conceptual framework. Int J Health Policy Manag. 2021;2021:158.
- Norris S, Belcher A, Howard K, et al. Evaluating genetic and genomic tests for heritable conditions in Australia: lessons learnt from health technology assessments. J Community Genet. 2021;2021:2.
- Tricco AC, Antony J, Zarin W, et al. A scoping review of rapid review methods. BMC Med. BioMed Central. 2015;13:1–15.
- Speckemeier C, Niemann A, Wasem J, et al. Methodological guidance for rapid reviews in healthcare: a scoping review. Res Synthe Methods. 2022;13(4):394–404.
- Elliott JH, Turner T, Clavisi O, et al. Living systematic reviews: an emerging opportunity to narrow the Evidence-Practice gap. PLoS Med. 2014;11(2):e1001603.
- Ramsey SD, Sullivan SD. A new model for reimbursing genome-based cancer care. Oncologist. 2014;19(1):1–4.
- Kirwin E, Round J, Bond K, et al. A conceptual framework for life-cycle health technology assessment. Value in Health [Internet]. 2022; [cited 2022 May 10]; Available from: https://www.sciencedirect.com/science/article/pii/S1098301522000018.
- Sweetman K. U of A precision health and entrepreneurship platforms get a boost to accelerate innovation. 2022; [cited 2022 Feb 8]. Available from: https://www.ualberta.ca/folio/2021/05/u-of-a-precision-health-and-entrepreneurship-platforms-get-a-boost-to-accelerate-innovation.html.
- OICR – Maximizing the benefits of cancer research for Ontarians. Ontario Institute for Cancer Research. 2022; [cited 2022 Feb 25]. Available from: https://oicr.on.ca/.
- Goldberg RB. Managing the pharmacy benefit: the formulary system. Academy of Managed Care Pharmacy. 2020;26:341–349.
- Law Document English View [Internet]. Ontario.ca. 2014.;[cited 2022 Feb 8]. Available from: https://www.ontario.ca/laws/view.
- Kitto S. Opening up the CPD imagination. J Contin Educ Health Prof. 2019;39(3):159–160.
- Cheema PK, Gomes M, Banerji S, et al. Consensus recommendations for optimizing biomarker testing to identify and treat advanced EGFR-mutated non-small-cell lung cancer. Curr Oncol. 2020;27(6):321–329.
- Bergethon K, Shaw AT, Ignatius Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. JCO. 2012;30(8):863–870.
- Provincial Funding Summary – Crizotinib (Xalkori) for Non-Small Cell Lung Cancer, first-line (pCODR10054). [cited 2022 Feb 25]. Available from: https://www.cadth.ca/sites/default/files/pcodr/pcodr-provfund_xalkorire-nsclc.pdf
- Government of Canada HC. Medical Devices Active Licence Listing. 2012 [cited 2022 Feb 25]. Available from: https://health-products.canada.ca/mdall-limh/information.do?deviceId_idInstrument=590957&deviceName_nomInstrument=VYSIS+LSI+ROS1+%28TEL%29+SPECTRUMORANGE+PROBE&lang=eng&licenceId=91493
- Shaw AT, Ou SHI, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371(21):1963–1971.
- Wu YL, Yang JCH, Kim DW, et al. Phase II study of crizotinib in East Asian patients with ROS1-positive advanced non-small-cell lung cancer. J Clin Oncol. 2018;36(14):1405–1411.
- Inc PC. XALKORI® Approved by health Canada for the treatment of patients with ROS1-positive locally advanced or metastatic non-small cell lung cancer. [cited 2022 Feb 25]. Available from: https://www.newswire.ca/news-releases/xalkori-approved-by-health-canada-for-the-treatment-of-patients-with-ros1-positive-locally-advanced-or-metastatic-non-small-cell-lung-cancer-657435843.html
- Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the study of lung cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med. 2018;142(3):321–346.
- pCODR Expert Review Committee (pERC). Final Recommendation. [cited 2022 Feb 25]. Available from: https://www.cadth.ca/sites/default/files/pcodr/Reviews2019/10151CrizotinibNSCLC_fnRec_EC_approvedbyChair_Post_23May2019_final_cleaned.pdf
- Cancer Care Ontario. Comprehensive cancer testing at diagnosis as of February 1, 2022. [cited 2022 Aug 2]. Available from: https://www.cancercareontario.ca/sites/ccocancercare/files/assets/ComprehensiveCancerTestingIndications.pdf
