Abstract
Objective
The PULsE-AI trial sought to determine the effectiveness of a screening strategy that included a machine learning risk prediction algorithm in conjunction with diagnostic testing for identification of undiagnosed atrial fibrillation (AF) in primary care. This study aimed to evaluate the cost-effectiveness of implementing the screening strategy in a real-world setting.
Methods
Data from the PULsE-AI trial – a prospective, randomized, controlled trial conducted across six general practices in England from June 2019 to February 2021 – were used to inform a cost-effectiveness analysis that included a hybrid screening decision tree and Markov AF disease progression model. Model outcomes were reported at both individual- and population-level (estimated UK population ≥30 years of age at high-risk of undiagnosed AF) and included number of patients screened, number of AF cases identified, mean total and incremental costs (screening, events, treatment), quality-adjusted-life-years (QALYs), and incremental cost-effectiveness ratio (ICER).
Results
The screening strategy was estimated to result in 45,493 new diagnoses of AF across the high-risk population in the UK (3.3 million), and an estimated additional 14,004 lifetime diagnoses compared with routine care only. Per-patient costs for high-risk individuals who underwent the screening strategy were estimated at £1,985 (vs £1,888 for individuals receiving routine care only). At a population-level, the screening strategy was associated with a cost increase of approximately £322 million and an increase of 81,000 QALYs. The screening strategy demonstrated cost-effectiveness versus routine care only at an accepted ICER threshold of £20,000 per QALY-gained, with an ICER of £3,994/QALY.
Conclusions
Compared with routine care only, it is cost-effective to target individuals at high risk of undiagnosed AF, through an AF risk prediction algorithm, who should then undergo diagnostic testing. This AF risk prediction algorithm can reduce the number of patients needed to be screened to identify undiagnosed AF, thus alleviating primary care burden.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia, and its prevalence is increasing due to many factors, including the aging populationCitation1,Citation2. Patients with AF have a near five-fold increase in risk of strokeCitation3 and the wider healthcare and societal burden associated with stroke is substantial. Of the > 100,000 strokes in the UK each year, approximately 25% are associated with AFCitation4. One-third of patients who experience a stroke live with moderate-to-severe disability 6 months after the eventCitation4, and there is a tendency for more severe and recurrent strokes in patients with AFCitation5,Citation6. Consequently, the mean health and social care costs per patient in the UK in the first year after stroke are substantial and estimated at £22,429, rising to £46,039 in the first 5 years. The total health and social care burden of stroke across England, Wales, and Northern Ireland is estimated at £3.6 billion over the first 5 years after admissionCitation7, and this economic burden is even greater when unpaid care and productivity losses are consideredCitation8. To reduce the risk of AF-related stroke, early diagnosis and optimal management of AF with oral anticoagulation is essentialCitation9. Indeed, up to two-thirds of strokes in patients with AF are avoidable with adequate anticoagulationCitation10. However, due to its often paroxysmal and asymptomatic nature, and the lack of effective screening approachesCitation11, AF frequently remains undetected, with an estimated 300,000 undiagnosed patients in the UK aloneCitation12.
To reduce the size of the undiagnosed AF population, and potentially lessen the burden of stroke on patients and the NHS, identification of these patients is crucial. However, screening strategies for AF tend to lack either diagnostic precision (e.g. pulse check) or cost-effectiveness. For example, opportunistic screening of patients aged > 65 years of age via a pulse check, as per 2020 European Society of Cardiology (ESC) Guidelines, lacks diagnostic precision and, therefore, requires approximately 70 people to be screened to identify one case of AFCitation11. In comparison, more targeted screening approaches (utilizing electrocardiogram (ECG)-based screening) can detect more cases of undiagnosed AF per individual screened, but they are resource-intensive and often not considered cost-effective at a population levelCitation13. A paucity of longer-term data on the impact and outcomes of screening programs means the number of national AF screening programs worldwide is very limitedCitation14. Therefore, there is an unmet need for both an accurate and a cost-effective screening strategy that can demonstrate longer-term value and can be implemented across a healthcare system to support earlier identification of undiagnosed AF, allowing timely anticoagulation therapy to reduce the risk of stroke and systemic embolism.
The use of augmented intelligence (AI) in cardiovascular medicine, both in diagnostic and treatment settings, is increasing. AI technology has the potential to transform current practices and overcome some of the limitations of more traditional screening approaches, potentially allowing for earlier diagnosis and more proactive management of AF. Indeed, we have recently reported on the developmentCitation15, validationCitation16, budget impactCitation17, and performanceCitation18 of a novel AF risk prediction algorithm based on machine learning (ML) techniques that quantifies individual-patient AF risk based on data routinely collected in primary care. Through the quantification of individual patient AF risk, a population at higher risk of undiagnosed AF who should be considered for diagnostic testing can be identified. The Prediction of Undiagnosed atriaL fibrillation using a machinE learning AlgorIthm (PULsE-AI) randomized controlled trial (RCT) evaluated a screening strategy that included the ML AF risk prediction algorithm combined with diagnostic testing for the identification of undiagnosed AF in a primary care setting in EnglandCitation19. This cost-effectiveness evaluation utilized data from the PULsE-AI RCT to evaluate the value of implementing this screening strategy in a real-world setting.
Methods
PULsE-AI trial overview
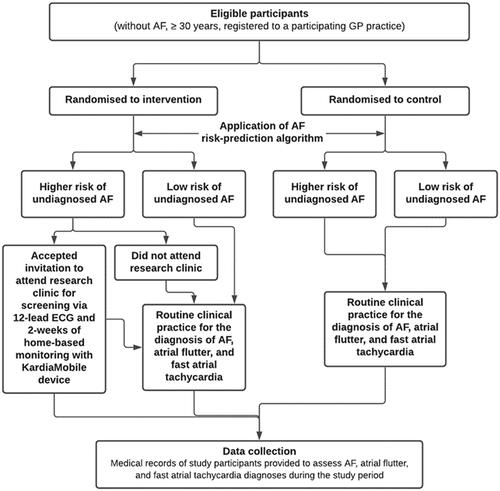
The PULsE-AI trial was a prospective, randomized, controlled trial conducted across six general practices in England, from June 2019 to February 2021, and has been described in detail elsewhereCitation18,Citation19. In summary, 23,745 eligible participants (aged ≥30 years without an AF diagnosis at randomization) were identified from practice electronic clinical records and randomized into either the intervention or control arm. In the intervention arm, 8.0% (n = 944/11,849) of participants were identified as high risk (risk score ≥7.4%) based on the AF risk prediction algorithm. Intervention arm participants at high risk of undiagnosed AF were invited to attend the research clinic for diagnostic testing. Participants who attended the research clinic underwent a 12-lead ECG and, if negative or inconclusive for AF, were provided with a portable single-lead ECG device (KardiaMobile; AliveCor Inc., CA) for 2 weeks of home-based ECG monitoring if they had access to a compatible smartphone or tablet (). The primary endpoint was the proportion of AF, atrial flutter, and fast atrial tachycardia diagnoses during the trial in high-risk participants as part of the screening strategy (intervention arm) or as part of routine care (both arms). Thirty-eight participants in the intervention arm initially identified as high risk (n = 944) were lost to follow-up. Of the 906 intervention arm participants identified as high risk and included in the final analysis, 28.1% (n = 255) accepted the invitation to attend the research clinic for diagnostic testing. In the intervention arm, 24 participants (9.4%) were diagnosed with AF or related arrhythmias during the trial, resulting in a number needed to screen (NNS) of 12. Of these, 13 were diagnosed as a direct result of the screening intervention, and the remaining 11 were diagnosed based on routine care. In total, 4.9% of control arm participants at high risk were diagnosed with AF or related arrhythmias during the trialCitation18. The PULsE-AI trial was registered on CT.gov (NCT04045639).
AF risk prediction algorithm
The AF risk prediction algorithm was developed using machine learning techniques and retrospective data from the electronic medical records of almost 3,000,000 adult participants (aged ≥30 years and without a prior history of AF) listed on the Clinical Practice Research Datalink (CPRD) GOLD database between January 2006 and December 2016Citation15. During the 11-year study period, 3.2% of the retrospective cohort were diagnosed with AF. Patient variables incorporated into the algorithm to generate a risk score for AF include: baseline patient demographics (age, sex, race, smoking status); history of antihypertensive use; type 1 or type 2 diabetes; cardiovascular comorbidities; and time-varying characteristics such as recent cardiovascular event(s), recent BMI and change in BMI, recent pulse pressure, change in SBP and DBP, and recent frequency of SBP, DBP, and BMI recordingsCitation15. Participants with an AF risk score of ≥7.4% were considered to be at high risk of undiagnosed AFCitation15,Citation16. This threshold was determined during algorithm validation and corresponds with 50% sensitivity and 90% specificity, thus representing a pragmatic approach for a screening evaluation, to minimize the rate of false negatives whilst also ensuring a manageable screening burden for primary care practitioners.
Cost-effectiveness analysis overview
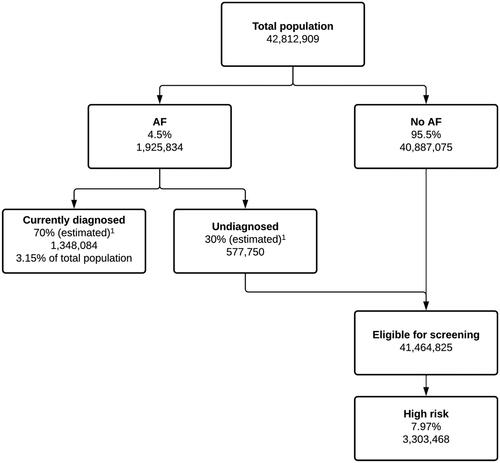
A cost-effectiveness analysis was undertaken using a hybrid screening decision tree and a Markov AF disease progression model adapted from other previously published modelsCitation20,Citation21 and informed by data from the PULsE-AI trialCitation18,Citation19. The UK NHS perspective was adopted; indirect costs incurred by participants, carers, and other agencies were not considered. Model outcomes are reported at both individual- and population-level. Population-level outcomes are based on the estimated number of individuals (≥30 years of age (to align with the population suitable for AF risk score generation by the risk prediction algorithm)) classified as high risk of undiagnosed AF in the UK (N = 3,304,468). Outcomes include number of patients screened, number of cases of AF identified, mean total and incremental costs (screening, events, treatment), quality-adjusted-life-years (QALYs), and incremental cost-effectiveness ratio (ICER). The willingness-to-pay (WTP) threshold was assumed to be £20,000/QALY and the discounting rate for benefits and costs was 3.5%Citation22. Outcomes were estimated over a patient’s lifetime (maximum of 50 years) with an upper age limit of 100 years. In addition to the base case analysis, the model contained a deterministic sensitivity analysis to demonstrate the impact of varying model inputs.
Screening decision tree
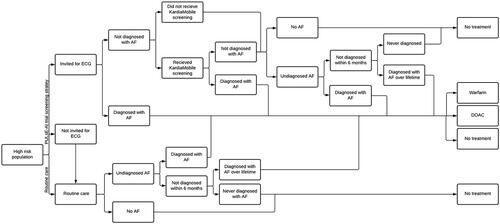
A screening decision tree was developed to identify a population aged ≥30 years and eligible for screening for AF. The total population was partitioned into individuals with “AF” or “no AF”. The “AF” cohort was further partitioned into “diagnosed” and “undiagnosed AF”. The “eligible for screening” cohort consisted of people classified as “no AF” and those with AF but undiagnosed (). Based on observations from the PULsE-AI trial, it was assumed that approximately 8.0% of individuals who were eligible for inclusion in the model were identified as high risk of undiagnosed AF by the risk prediction algorithm.
Figure 2. Screening decision tree.
Abbreviation: AF, atrial fibrillation.
1Public Health England, 2020. Atrial fibrillation estimates for local populations. https://www.gov.uk/government/publications/atrial-fibrillation-prevalence-estimates-for-local-populations.
In the screening decision tree, individuals identified as high risk of undiagnosed AF may be invited for diagnostic testing. Based on observations from the PULsE-AI trialCitation18, the model assumed that approximately 19% of high-risk patients would not be invited for screening due to being housebound, undergoing cancer treatment, receiving end-of-life care, etc. Individuals invited for diagnostic testing could either be diagnosed with AF (either by 12-lead ECG or by KardiaMobile monitoring) or not diagnosed with AF (). Individuals not diagnosed with AF as a result of the screening intervention could then go on to be diagnosed with AF during their lifetime via routine care or never diagnosed. In the PULsE-AI trial, the observed NNS in high-risk participants who received the screening intervention was 12, and the annual background incidental diagnosis rate (due to routine care) in the high-risk population who did not receive the screening intervention (i.e. all participants in the control arm and those in the intervention arm who did not attend screening) was 2.8%.
Markov AF model
A multi-state cohort-level Markov model was developed based on previously published AF screening modelsCitation20,Citation21 to estimate lifetime costs and outcomes related to the identification and treatment of AF, through the screening strategy evaluated in the PULsE-AI trial compared with routine care only. The model cycle length was set to 1 year. An overview of the model structure is provided in .
Figure 4. Markov model flow diagram.
Abbreviations: AF, atrial fibrillation; ICH, intracranial hemorrhage; MI, myocardial infarction.
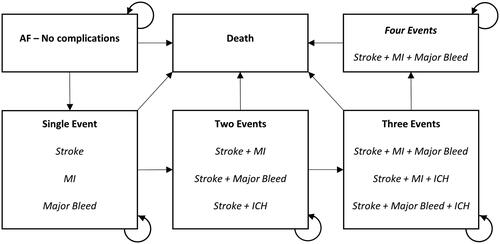
Patients diagnosed with AF entered the model in the health state of AF with no complications. During any cycle patients were at risk of stroke, myocardial infarction (MI), major bleed, or intracranial hemorrhage (ICH). If a patient experienced an event, they were assumed to stay within that health state until the next event. Patients would then progress through the model in line with published transition rates that are influenced by treatment, clinical event history, and age.
Patients with a diagnosis of AF were either assumed to be treated (with either a direct acting oral anticoagulant [DOAC] or warfarin) or remain untreated. Those without AF, or with undiagnosed AF, were assumed to receive no treatment and did not enter the model. The base probabilities of the events were adjusted using treatment-specific hazard ratios (HRs), sourced from Lopez-Lopez et al.Citation23 (Supplementary Table S1). The model did not include discontinuation, and a fixed breakdown was used to disaggregate patients on DOAC, warfarin, or no treatment.
Model inputs and parameters
The base-case analysis evaluated costs and outcomes for a population of patients at high risk of undiagnosed AF aged 30 years and over, with inputs identified from the PULsE-AI trial and previously published analysesCitation20. A summary of base-case model inputs is provided in Supplementary Table S2.
Costs
Total costs include those associated with the screening strategy, treatment costs, and event costs. All event costs and annual maintenance costs for events were sourced from Lopez-Lopez et al.Citation23. Costs associated with the screening strategy were sourced from standard UK National Institute for Health Research (NIHR) research costsCitation24 for each activity where relevant and included: invitation and reminder letters for high-risk individuals to attend screening, 12-lead ECG and cardiologist review, KardiaMobile device purchase, patient training in using the device, cardiologist review (of KardiaMobile ECGs), and device return. Each of these costs were applied as a one-off cost upon screening initiation, and it was assumed that one device would be required for every five patients identified as high risk of undiagnosed AF.
Upon entry into the Markov AF model, costs were assigned to each clinical event experienced. For each event, acute costs were applied in the cycle of incidence and ongoing annual maintenance costs were applied in each subsequent cycle. Treatment costs were applied to patients diagnosed with AF based on the following assumptions: 74.2% of patients diagnosed are assumed to receive treatmentCitation25, with 61.8% of those assumed to receive a DOACCitation26, and the remainder (39.2%) assumed to receive warfarin. Patients who had AF but who were deemed to be undiagnosed were assigned to the “no treatment” cohort. Base-case model inputs assumed that all patients prescribed DOAC therapy received apixaban, with corresponding cost and event rates based on this therapy. Apixaban was selected for the base case because it ranks highest amongst DOAC therapies for efficacy, safety, and cost-effectiveness based on current evidenceCitation23. As a simplifying assumption, discontinuation and treatment switching were not modeled, as neither are likely to affect the screening strategy employed. Screening, clinical event and treatment costs are summarized in and Supplementary Table S3.
Table 1. Overview of screening costs.
Utility
Methodology surrounding the estimation of health state utilities aligned with our previously published cost-effectiveness analysisCitation21. Patients who experienced a clinical event in the Markov AF model incurred a disutility. Health state utilities were applied multiplicatively. Therefore, if a patient experienced a stroke in any given cycle, they incurred the utility of AF with no events (0.779Citation27) multiplied by the utility of AF with a stroke (0.690Citation28) to derive a utility value of 0.541. This utility estimate was applied during the cycle of event incidence and for all subsequent cycles until there was a change in health state. An age-dependent utility adjustment was applied at the baseline health state (AF with no events). This adjusted baseline utility was multiplied with the event utilities to estimate the health state utilities used in the model. Utilities were estimated using general population age-dependent EQ-5D estimatesCitation29. Clinical event utility and disutility values are summarized in Supplementary Table S3.
Sensitivity and scenario analyses
Sensitivity and scenario analyses were undertaken to assess the impact of individual model parameters on model outcomes. Key inputs within the model were included in a univariate sensitivity analysis where base-case values were adjusted by ±20%, with exception of the background AF diagnosis rate in a high-risk population, which was varied to values of 2% and 4% from a base case of 3.15% (to better reflect the true prevalence of AFCitation30) and the discount rate which was varied between 0% and 7% (± 3.5% from base case) (Supplementary Table S4).
A range of different scenario analyses were also undertaken to assess the impact of alternative assumptions on cost-effectiveness outcomes, including changes in age, number of patients per KardiaMobile device, distribution of DOAC and warfarin therapies, and prevalence of AF. A full table of scenarios explored are described in Supplementary Table S5.
Results
Model application
Across the total eligible (≥30 years of age) UK population estimated to be at high risk of undiagnosed AF (3.3 million), implementation of the screening strategy was estimated to result in an additional 14,004 lifetime diagnoses of AF compared with routine care only. The diagnoses as a result of implementing a screening strategy would comprise 45,493 new diagnoses of AF, and a total of 204,550 AF diagnoses over the lifetimes of those at high risk; 70,739 individuals at high risk of AF are predicted to remain undiagnosed (). In comparison, routine care only was predicted to result in a total of 190,546 diagnoses of AF during the lifetimes of individuals in the population, leaving a predicted 84,743 individuals at high risk of AF undiagnosed. Total clinical events were relatively similar, at 332,752 and 333,249 in screening strategy and routine care only strategies, respectively. Comparisons between implementing the screening strategy and routine care only are presented in .
Table 2. Estimated population-level atrial fibrillation diagnoses and clinical events based on the PULsE-AI trial screening strategy compared with routine care only.
Per-patient costs in the screening strategy arm were estimated at £1,985, compared with £1,888 for individuals following routine care only (). Incremental costs per-patient over a lifetime were estimated at £98, resulting in a £322 million difference at UK population level. While differences in QALYs were minimal at a per-patient level (total QALYs of 12.57 in the screening arm compared with 12.55 in the routine care only arm), at a population level, this translated to an incremental QALY gain of 80,669. In the base case, the screening strategy demonstrated cost-effectiveness versus routine care only at an ICER threshold of £20,000 per QALY-gained, with an ICER of £3,994/QALY.
Table 3. Cost-effectiveness of the PULsE-AI trial screening strategy.
Sensitivity and scenario analysis
Sensitivity analyses within the model demonstrated that the results were robust to changes in model inputs and all results remained cost-effective at a WTP threshold of £20,000/QALY (Supplementary Table S4). Model inputs having the greatest impact on outcomes were related to treatment; lower uptake of DOAC therapy was associated with lower incremental costs but fewer incremental QALYs and a higher ICER. Varying baseline and event utilities also had an impact on outcomes. Other variables such as background diagnoses rates in routine care had minimal impact on model outcomes.
Scenario analyses also confirmed that cost-effectiveness results were robust to changes in model inputs (Supplementary Table S5). Treatment was again the greatest driver of changes in ICER, with a scenario based on 100% DOAC uptake for diagnosed patients reporting the lowest ICER, however, all scenarios based on the screening strategy remained cost-effective over routine care only. Increasing the KardiaMobile device cost to one device per patient from one device for every five patients had little impact (ICER of £4,429 compared with £3,994) on the cost-effectiveness of the screening intervention.
Discussion
The PULsE-AI trial was a multi-center RCT designed to evaluate the application of a novel screening strategy that included a ML-developed AF risk prediction algorithm in conjunction with diagnostic testing in primary care. The aim of this analysis was to evaluate the cost-effectiveness of the screening strategy evaluated in the PULsE-AI trial. At UK population level, the screening strategy was associated with additional diagnoses of AF and related arrhythmias and demonstrated cost-effectiveness and QALY gains over routine care only at established WTP thresholds.
The screening strategy evaluated in the PULsE-AI trial involved the application of the AF risk prediction algorithm to routinely collected data from the electronic medical records of eligible individuals at participating general practices to generate a risk score. Participants at high risk of undiagnosed AF were then invited for diagnostic testing, involving a 12-lead ECG and 2-weeks of home-based ECG monitoring with a KardiaMobile device. At a UK population level, the screening strategy resulted in an additional 14,004 AF diagnoses in patients who would otherwise remain undiagnosed if they never received screening. While this total is modest at a population level, the nature of the screening intervention also meant that individuals were more likely to be diagnosed earlier in the disease process than they would have been based on routine care, which likely added to the incremental changes in outcomes observed in the screening strategy arm.
Diagnosis of AF in individuals who would otherwise remain undiagnosed, and earlier diagnosis in others will benefit the wider healthcare system. AF is associated with increased risk of stroke and more severe strokeCitation3,Citation5,Citation6. Indeed, approximately 25% of all strokes occur in individuals with AF, and one fifth of these occur in individuals without a prior diagnosis of AFCitation4. Adequate anticoagulation has been shown to reduce the risk AF-related events and mortality, thereby reducing the burden of AF, and in particular stroke, on the healthcare systemCitation23,Citation31. Although the total number of events did not differ substantially between the screening strategy and routine care only, greater differences would have been predicted by the model if all patients diagnosed with AF by the screening strategy and at high risk of stroke were assumed to receive DOAC therapyCitation23. Furthermore, the screening strategy was associated with a gain, at population level, of approximately 81,000 QALYs. When adjusted for eligible population size, the estimated QALY gain based on this screening strategy is approximately 4-times greater than that observed based on national breast cancer screening in the UKCitation32.
Existing screening strategies for AF are focused around opportunistic, systematic, or targeted approaches. Cost-effectiveness analyses based on the Screening for Atrial Fibrillation in the Elderly (SAFE) study found opportunistic screening to be favorable to routine practice for the detection of AFCitation13; however, an estimated 170 individuals need to be screened to identify an additional case of AF compared with no screening/routine practiceCitation20. Current ESC guidelines recommend opportunistic screening for AF by pulse-checking or ECG rhythm strip in patients aged ≥65 years, stating this approach requires approximately 70 patients to be screened to identify one case of AFCitation11. Consequently, there is no national screening program for AF in the UK.
In the PULsE-AI trial, the first step of the screening strategy involved the identification of individuals at high risk based on routinely collected data within electronic medical records. This targeted approach resulted in a NNS of 12, where one participant was diagnosed with AF (or related arrhythmia) for every 12 participants who consented to undergo diagnostic screening. This finding is significantly lower than the approximately 70 patients required based on opportunistic screeningCitation11 and highlights the ability of the AF risk prediction algorithm to accurately identify individuals at high risk of AF. The ICER of £3,994/QALY based in this analysis is significantly lower than the National Institute for Health and Care Excellence (NICE) WTP threshold of £20,000–£30,000/QALY, indicating that a more targeted approach that involves the application of an AF risk prediction algorithm to narrow the population at high risk of AF, who should be considered for diagnostic testing, is cost-effective in the UK.
The advent of new single-lead ECG technologies such as the KardiaMobile have improved the accuracy of AF detection over pulse palpationCitation11,Citation33, and evidence indicates that single-lead ECG technologies are likely to be more cost-effective than pulse palpation followed by 12-lead ECG for the diagnosis of AF in patients presenting with signs or symptoms of the diseaseCitation34. However, NICE have concluded there is currently insufficient evidence to recommend the routine adoption of single-lead ECG assessment for single time point testing in primary careCitation35. Furthermore, these opportunistic screening approaches are likely still limited by the need for patients to visit their GP for another reason. Recent studies have evaluated the feasibility of combining opportunistic AF screening with annual influenza vaccination clinicsCitation36,Citation37, and found this approach to be economically viable. However, screening reach was again limited to those who attended the vaccination clinic and indicates there is unmet need for a screening strategy that is not solely reliant on in-person contact with healthcare professionals to identify individuals in need of further investigation for AF. This is even more pertinent now given the ongoing impact of the COVID-19 pandemic on in-person primary and secondary care consultationsCitation38, reducing the chance for opportunistic screening.
The limitations of the PULsE-AI trial have been described in detail elsewhereCitation18. The key limitation of the PULsE-AI trial was its impact by the COVID-19 pandemic. Implementation of the screening intervention was paused during the first lockdown in the UK. As a result, the trial was extended, and many assumptions used to inform study design and statistical power were incorrect, potentially impacting the primary outcome and, therefore, data to inform this cost-effectiveness analysis. Furthermore, whilst the PULsE-AI trial used a ≥7.4% risk threshold to define high risk patients, other thresholds to define the high-risk population could have been applied, each with varying sensitivities and specificities, screening burden and outcomes, and therefore cost-effectiveness. The impact of changes in this risk threshold on cost-effectiveness is unknown. In addition, the PULsE-AI trial was conducted across six general practices across the West Midlands in England and, therefore, the generalizability of the results to England more broadly and the wider UK setting is unknown. It is possible that these limitations may impact the reliability of the model inputs and therefore over- or under-estimate the results of this analysis. However, in the undertaking of various sensitivity and scenario analyses, none of the scenarios explored indicated a deviation from the main conclusion of this analysis. Lastly, while the analysis has demonstrated cost-effectiveness of the screening strategy over routine care only, the resource needed to integrate and maintain the screening strategy within primary care is unknown and therefore has not been captured within the analysis; but should be considered ahead of wider integration.
Conclusions
Overall, results from this analysis indicate that the screening strategy evaluated in the PULsE-AI trial, including an AF risk prediction algorithm in combination with diagnostic testing, is cost-effective compared with routine practice only. While there is currently no national screening program for AF in the UK, most guidelines recommend opportunistic screening for AF of patients aged ≥65 years. The screening strategy evaluated here provides an alternative effective and cost-effective approach for narrowing the population at high risk of undiagnosed AF who should undergo diagnostic testing, thus reducing the number of patients needed to be screened to identify undiagnosed AF. This analysis highlights the potential value to both patients and the healthcare system in AI-based methods to support screening for AF.
Transparency
Declaration of funding
This work was funded by Bristol Myers Squibb Pharmaceutical Ltd & Pfizer Inc. The funder of the study was involved in the study design, data interpretation, and review of the report but had no role in data collection or data analysis. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Declaration of financial/other relationships
AJC has received institutional grants and personal fees from Bayer, Boehringer Ingelheim, Bristol Myers Squibb/Pfizer Alliance and Daiichi Sankyo.
ACM has received consulting fees and a research grant from Bristol Myers Squibb/Pfizer Alliance, and consulting fees from Bayer-Healthcare and Boehringer-Ingelheim.
DAC declares fees from Oxford University Innovation, Biobeats, Bristol Myers Squibb, Sensyne Health and academic research grants from GlaxoSmithKline, AstraZeneca, Apple, RCUK, Wellcome Trust, EU Horizon2020, and NIHR.
ATC receives consulting fees from AbbVie, ACI Clinical, Alexion, Aspen, Astra Zeneca, Bayer, Boehringer-Ingelheim, Bristol Myers Squibb, Boston Scientific, CSL Behring, Daiichi-Sankyo, GlaxoSmithKline, GLG, Guidepoint Global, Johnson and Johnson, Leo Pharma, Medscape, McKinsey, Navigant, ONO, Pfizer, Portola, Sanofi, TRN; advisory board membership with Bayer, Bristol Myers Squibb, Daiichi-Sankyo, Johnson and Johnson, Pfizer, Portola, Sanofi; payments for lectures including speakers bureau services, payments for preparation of reports and payment for development of educational presentations from, Aspen, Bayer, Boehringer-Ingelheim, Bristol Myers Squibb, Daiichi, GlaxoSmithKline, Johnson and Johnson, Medscape, Pfizer and Portola. He has advised the UK Government Health Select Committee, the all-party working group on thrombosis, the Department of Health, and the NHS, on the prevention of VTE. He is also an advisor to Lifeblood: the thrombosis charity and is the founder of the European educational charity the Coalition to Prevent Venous Thromboembolism.
CA and DWD have received consulting fees from Bristol Myers Squibb Pharmaceutical Ltd & Pfizer in relation to this study.
NRH, UF, MH, KGP, BS, and SL (Steven Lister) are employees of Bristol Myers Squibb UK. MH was previously employed by Health Economics and Outcomes Research Ltd (HEOR).
JG, LG, CD, RB, and DMS are current or former employees of HEOR. HEOR were a paid consultant to Bristol Myers Squibb Pharmaceutical Ltd & Pfizer in connection with the development of this manuscript.
JR is an employee of PHASTAR. PHASTAR were a paid consultant to Bristol Myers Squibb Pharmaceutical Ltd & Pfizer in connection with the development of this manuscript.
CM and SL (Sarah Lawton) are employees of Keele University. Keele University were a paid consultant to Bristol Myers Squibb Pharmaceutical Ltd & Pfizer in connection with the development of this manuscript. CM is also funded by the National Institute for Health Research (NIHR) Applied Research Collaboration West Midlands and the NIHR School for Primary Care Research.
Author contributions
ATC was the Chief Investigator for the trial and the chair of the Trial Steering Committee (TSC). NRH, MH, KGP, BS, UF, JG, and DMS were responsible for study design, trial management, and interpretation of results. LG was responsible for day-to-day trial and site management, assisted by SL. CD wrote the first draft of the manuscript with input from DMS. RB was responsible for the modeling and cost-effectiveness analyses, with input from MH and SL. CA, DC, CM, and JR were members of the TSC, responsible for study design and trial oversight. ACM, DWD, and AJC were the trial cardiologists responsible for the review of all trial ECGs; they were also members of the TSC, responsible for study design and trial oversight. All authors revised and approved the final version of the manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (45 KB)Acknowledgements
The authors wish to thank the following principal investigators and research nurses for their significant contributions to data collection: Dr Andrew Wallis and Terri-Anne Thomson (Alcester Health Centre); Dr Manjit Kainth and Jacqueline Woodward (Primrose Lane Surgery); Dr Paul Ainsworth and Oliva Neely (Sherbourne Medical Centre); Dr Caron Morton (Station Drive Surgery); Dr Claire Jones (Spring Gardens Group Medical Practice); Dr Stefan Lachowicz and Deborah Williams (The Caxton Surgery); and Sian Jones and Jonathan Davies (National Institute for Health Research).
The authors also wish to thank Isaac Mackenzie and Aris Skandamis of Health Economics and Outcomes Research (HEOR) Ltd for their contributions to the cost-effectiveness analysis. HEOR Ltd were paid consultants to Bristol Myers Squibb Pharmaceutical Ltd & Pfizer Inc. in connection with the development of this manuscript.
Data availability statement
Deidentified individual participant data that underlie the results reported in this article and the study protocol and statistical analysis plan will be made available upon reasonable request to the corresponding author.
References
- Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129(8):837–847.
- Lip GYH, Brechin CM, Lane DA. The global burden of atrial fibrillation and stroke: a systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest. 2012;142(6):1489–1498.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the framingham study. Stroke. 1991;22(8):983–988.
- Sentinel Stroke National Audit Programme (SSNAP). Clinical Audit National Results; 2019; [cited 2021Dec 12]. Available from: https://www.strokeaudit.org/results/Clinical-audit/National-Results.aspx.
- Lamassa M, Di Carlo A, Pracucci G, et al. Characteristics, outcome, and care of stroke associated with atrial fibrillation in Europe: data from a multicenter multinational hospital-based registry (the european community stroke project). Stroke. 2001;32(2):392–398.
- Marini C, De Santis F, Sacco S, et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population-based study. Stroke. 2005;36(6):1115–1119.
- Xu X-M, Vestesson E, Paley L, et al. The economic burden of stroke care in England, Wales and Northern Ireland: using a national stroke register to estimate and report patient-level health economic outcomes in stroke. Eur Stroke J. 2018;3(1):82–91.
- Patel A, Berdunov V, Quayyum Z, et al. Estimated societal costs of stroke in the UK based on a discrete event simulation. Age Ageing. 2020;49(2):270–276.
- Cowan JC, Wu J, Hall M, et al. A 10 year study of hospitalized atrial fibrillation-related stroke in England and its association with uptake of oral anticoagulation. Eur Heart J. 2018;39(32):2975–2983.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–867.
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the european society of cardiology (ESC) developed with the special contribution of the european heart rhythm association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. Feb 1
- British Heart Foundation. Atrial fibrillation: finding the missing 300,000 2019; [cited 2020Oct 01]. Available from: https://www.bhf.org.uk/for-professionals/healthcare-professionals/blog/2019/atrial-fibrillation-finding-the-missing-300000.
- Hobbs FD, Fitzmaurice DA, Mant J, et al. A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study. iv, ix-x. Health Technol Assess. 2005;9(40):iii–i74.
- Khurshid S, Healey JS, McIntyre WF, et al. Population-based screening for atrial fibrillation. Circ Res. 2020;127(1):143–154. Jun 19
- Hill NR, Ayoubkhani D, McEwan P, et al. Predicting atrial fibrillation in primary care using machine learning. PLoS One. 2019;14(11):e0224582.
- Sekelj S, Sandler B, Johnston E, et al. Detecting undiagnosed atrial fibrillation in UK primary care: validation of a machine learning prediction algorithm in a retrospective cohort study. Eur J Prev Cardiol. 2021;28(6):598–605.
- Szymanski T, Ashton R, Sekelj S, et al. Budget impact analysis of a machine learning algorithm to predict high risk of atrial fibrillation among primary care patients. Europace. 2022;2022:euac016.
- Hill NR, Groves L, Dickerson C, et al. Identification of undiagnosed atrial fibrillation using a machine learning risk prediction algorithm and diagnostic testing (PULsE-AI) in primary care: a multi-Centre randomised controlled trial in England. Eur Heart J – Digital Health. 2022;3(2):195–204.
- Hill NR, Arden C, Beresford-Hulme L, et al. Identification of undiagnosed atrial fibrillation patients using a machine learning risk prediction algorithm and diagnostic testing (PULsE-AI): study protocol for a randomised controlled trial. Contemp Clin Trials. 2020;99:106191.
- Welton NJ, McAleenan A, Thom HH, et al. Screening strategies for atrial fibrillation: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2017;21(29):1–236.
- Hill NR, Sandler B, Mokgokong R, et al. Cost-effectiveness of targeted screening for the identification of patients with atrial fibrillation: evaluation of a machine learning risk prediction algorithm. J Med Econ. 2020;23(4):386–393.
- National Institute for Health and Care Excellence. Guide to the methods of technology appraisal.2013. Available from: https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781.
- Lopez-Lopez JA, Sterne JAC, Thom HHZ, et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network Meta-analysis, and cost effectiveness analysis. BMJ. 2017;359:j5058.
- National Institute for Health Research. Clinical Research Network Industry Costing Template (Primary Care) version October 2018. v1.32.
- NHS Digital. Quality and Outcomes Framework. 2019–20; [cited 2021 Nov 08]. Available from: https://digital.nhs.uk/data-and-information/publications/statistical/quality-and-outcomes-framework-achievement-prevalence-and-exceptions-data.
- Afzal S, Zaidi STR, Merchant HA, et al. Prescribing trends of oral anticoagulants in England over the last decade: a focus on new and old drugs and adverse events reporting. J Thromb Thrombolysis. 2021;52(2):646–653.
- Berg J, Lindgren P, Nieuwlaat R, et al. Factors determining utility measured with the EQ-5D in patients with atrial fibrillation. Qual Life Res. 2010;19(3):381–390.
- Haacke C, Althaus A, Spottke A, et al. Long-term outcome after stroke: evaluating health-related quality of life using utility measurements. Stroke. 2006;37(1):193–198.
- Self-Reported Population Health: An international perspective based on EQ-5D Szende a JB., Cabases J, editor. Dordrecht: Springer; 2014.
- Norberg J, Bäckström S, Jansson J-H, et al. Estimating the prevalence of atrial fibrillation in a general population using validated electronic health data. Clin Epidemiol. 2013;5:475–481.
- Orlowski A, Wilkins J, Ashton R, et al. Budget impacts associated with improving diagnosis and treatment of atrial fibrillation in high-risk stroke patients. J Comp Eff Res. 2020;9(4):253–262.
- Pharoah PD, Sewell B, Fitzsimmons D, et al. Cost effectiveness of the NHS breast screening programme: life table model. BMJ. 2013;346:f2618.
- Taggar JS, Coleman T, Lewis S, et al. Accuracy of methods for detecting an irregular pulse and suspected atrial fibrillation: a systematic review and meta-analysis. Eur J Prev Cardiol. 2016;23(12):1330–1338.
- Duarte R, Stainthorpe A, Greenhalgh J, et al. Lead-I ECG for detecting atrial fibrillation in patients with an irregular pulse using single time point testing: a systematic review and economic evaluation. Health Technol Assess. 2020;24(3):1–164.
- National Institute for Health and Care Excellence. Lead-I ECG devices for detecting symptomatic atrial fibrillation using single time point testing in primary care [DG35]. 2019. Available from: https://www.nice.org.uk/guidance/dg35/chapter/1-Recommendations.
- Savickas V, Stewart AJ, Rees-Roberts M, et al. Opportunistic screening for atrial fibrillation by clinical pharmacists in UK general practice during the influenza vaccination season: a cross-sectional feasibility study. PLoS Med. 2020;17(7):e1003197.
- Kaasenbrood F, Hollander M, Rutten FH, et al. Yield of screening for atrial fibrillation in primary care with a hand-held, single-lead electrocardiogram device during influenza vaccination. Europace. 2016;18(10):1514–1520.
- Murphy M, Scott LJ, Salisbury C, et al. Implementation of remote consulting in UK primary care following the COVID-19 pandemic: a mixed-methods longitudinal study. Br J Gen Pract. 2021;71(704):e166–e177.