Abstract
Aims
To assess real-world use of emicizumab in adult people with hemophilia A (PwHA) without inhibitors including healthcare resource utilization (HCRU) and costs.
Materials and methods
Adult, male PwHA without inhibitors initiating emicizumab (index date) were identified using IBM MarketScan after 4 October 2016. Patients were required to have continuous health insurance coverage for ≥180 days prior to and ≥90 days after index date and have ≥90 days of continuous use of emicizumab. Patients were followed until treatment gap, disenrollment, or end of data. Results were reported overall and among a subgroup with prior factor VIII (FVIII) prophylaxis. Emicizumab use, concomitant FVIII treatment use, HCRU, and costs were assessed separately over baseline, the emicizumab induction period, emicizumab maintenance period, and annualized.
Results
Among the 71 emicizumab patients (FVIII prophylaxis subgroup: 52) included in the study, the mean age was 35 (subgroup: 34) years and mean follow-up was 12 (subgroup: 11.1) months. At baseline, the annualized mean total healthcare cost was $532,948 (subgroup: $645,727). After emicizumab initiation, per-patient-per-month (PPPM) HCRU was higher in the emicizumab induction period compared to the maintenance period with higher monthly FVIII fills/in-office administrations (0.37 vs 0.17), non-FVIII outpatient visits (2.23 vs 1.55), and emergency department visits (0.06 vs 0.03). The FVIII prophylaxis subgroup yielded similar HCRU trends. Hemophilia treatment costs accounted for over 95% of total healthcare costs. The annualized mean cost was $50,491 (subgroup: $61,512) for concomitant FVIII treatment and $777,171 (subgroup: $793,168) for emicizumab and concomitant FVIII treatment for the first year of emicizumab treatment.
Conclusion
This study represented experience with emicizumab after the approval for PwHA without inhibitors. The study cohort may not be representative of all PwHA taking emicizumab. The findings highlight the continued burden of treatment and healthcare cost for PwHA without inhibitors despite advances in treatment options.
Introduction
Hemophilia A (HA) is a congenital X-linked bleeding disorder characterized by a deficiency in clotting factor VIII (FVIII), affecting over 20,000, predominantly male, patients in the United States (US)Citation1. FVIII activity defines the severity of the disease, ranging from mild to severe, and the aim of care is to minimize the acute and chronic complications of bleeding. For patients with severe HA, prophylaxis with FVIII concentrate or other hemostatic agents are used to prevent spontaneous bleeds and acute bleeds are treated with additional FVIII replacementCitation2,Citation3.
Clotting factor concentrates (CFCs) have historically been the treatment of choice for people with hemophilia A (PwHA) and hemophilia B for treating and preventing bleeding events. Treatment guidelines recommend CFC prophylaxis as the standard of care for individuals with severe HACitation3. The development of non-factor replacement therapies such as emicizumab provides an alternative prophylactic treatment approach. Emicizumab was initially approved for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients with HA with FVIII inhibitors in November 2017Citation4 and subsequently approved for prophylaxis in patients without inhibitors in October 2018Citation5. Emicizumab is a subcutaneous injection administered weekly, every 2 weeks, or every 4 weeks.
Although real-world utilization of FVIII prophylaxis has been well describedCitation6–9, the description of real-world utilization patterns of emicizumab has been limited. Currently, the real-world healthcare resource utilization (HCRU) and costs associated with emicizumab have primarily described: (1) mixed populations of patients with and without inhibitors and/or including both children and adultsCitation10–17, and (2) from the experience of a single centerCitation12,Citation13,Citation17, or the long-term outcomes of the emicizumab clinical trial populationCitation18,Citation19.
With the advent of novel therapeutics, such as gene therapy, understanding of HCRU and costs with emicizumab use in a population of patients who may be eligible for these interventions is needed. The aim of this study is to describe the utilization of emicizumab, concomitant FVIII treatment, and overall HCRU and costs among adult, male PwHA without inhibitors, including those switching from FVIII prophylaxis, in real-world practice.
Methods
Study design and data sources
This retrospective cohort study utilized data from the IBM MarketScan (“MarketScan”) databases of de-identified, US administrative claims from December 2015 through December 2020. The data available for study included the Commercial Claims and Encounters (CCAE) Database component of MarketScan, with 243,931 covered lives of US active employees, early retirees, COBRA recipients, and their dependents who are insured by commercial plans. The database includes information such as demographics, plan enrollment, hospitalizations, emergency department and outpatient visits, and pharmacy fills including payer-paid and patient-paid cost components for these services. This data is widely used for healthcare resource utilization and cost studies due to the large sample size, inclusion of fully adjudicated cost information (including for mail order and specialty pharmacies), and is used in other, recently published literature on PwHACitation20,Citation21.
Study population
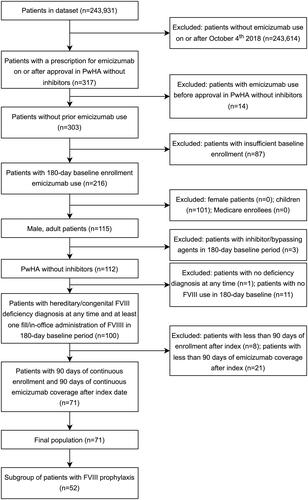
Patients were included in the study based on a claim (pharmacy fill or in-office administration) for emicizumab between 4 October 2018 and 1 October 2020, corresponding to the date of indication approval by Food and Drug Administration (FDA) for PwHA without inhibitors. New users of emicizumab were identified and followed until they were censored due to a treatment gap, disenrollment, or the end of data (last date for which information was available in the dataset; 31 December 2020). The date of each patient’s first emicizumab claim (pharmacy fill or in-office administration) during this period was recorded as their index date. The study population was additionally required to have a diagnosis of a hereditary/congenital FVIII deficiency (ICD-10-CM code of D66 in any position) between 31 December 2015 and 31 December 2020, be male aged between 18 and 65 years of age at index date, have ≥1 medical or pharmacy claim for FVIII in 180 days preceding the index date, and have continuous enrollment in a health plan for 180 days prior to the index date and 90 days after the index date. Patients were excluded from the study if there were any emicizumab claims (pharmacy fill or in-office administration) prior to 4 October 2018, had a pharmacy fill or in-office administration suggestive of FVIII inhibitors (i.e. bypassing medications) in the 180 days prior to and including the index date, or had a > 30-day treatment gap in emicizumab in the 90 days after the index date.
Gaps in emicizumab treatment were defined based on the expected medication coverage of each pharmacy fill or in-office administration. Coverage of emicizumab pharmacy fills was defined based on reported days supply; see Supplementary Appendix 1 for further details on coverage for in-office administrations of emicizumab.
Study variables and outcomes
Patients were described regarding demographics at index including age, region, and date of emicizumab initiation. HCRU and cost variables including emicizumab and FVIII use, HCRU and healthcare costs by site of care (inpatient, outpatient, emergency department, and pharmacy), and health status outcomes including arthropathy, pain, and pain management (definitions contained in Supplementary Appendix 1) were described during the baseline (180 days prior to index date) and follow-up (index date and after) periods. All patient outcomes were measured over the follow-up period until a gap in treatment greater than 30 days, disenrollment from their health plan, or end of data (31 December 2020) and reported for baseline, the induction period (month 1), the maintenance period (month 2 through end of follow-up), and total follow-up (index date through end of follow-up).
Statistical analysis
Descriptive statistics were summarized using mean, standard deviation (SD), median, and interquartile range (IQR) for continuous variables and patient counts and percentages for categorical variables. All claims occurring on a single day were assumed to have occurred at the same inpatient, outpatient, emergency department, or HA treatment encounter (i.e. one visit/fill/in-office administration).
Baseline HCRU and costs prior to emicizumab initiation were annualized by multiplying the HCRU/total cost observed in the 6-month baseline period by two. HCRU/costs were calculated as per-patient-per-month (PPPM).
During follow-up, costs were annualized separately for the first year of treatment and projected for the subsequent years of treatments (i.e. years 2+). Annualized costs during the first year of treatment were calculated using the following formula to account for cost differences expected during the induction period: induction period costs + (11 * maintenance period costs). Annualized costs expected during subsequent years of treatment were calculated removing the induction period costs: 12 * maintenance period costs. Costs were adjusted to 2020 dollars using the medical component of the consumer price index (CPI [Series ID: CUUR0000SAM, not seasonally adjusted, US Bureau of Labor Statistics]). All analyses were performed using the Aetion Evidence Platform, version R4.40.
Subgroup analysis
A subgroup of patients who received FVIII prophylaxis during the 180-day baseline period was identified based on FVIII dispensings aligned to an algorithm developed in a prior publicationCitation21. Specifically, patients who met any of the following three criteria during the baseline period were considered as receiving prior FVIII prophylaxis: (1) patients who received three or more FVIII dispenses outside of inpatient and emergency department settings, (2) those with gaps in FVIII coverage of 60 days or fewer, or (3) those who had FVIII coverage for at least 135 daysCitation21.
Results
Study sample and baseline characteristics
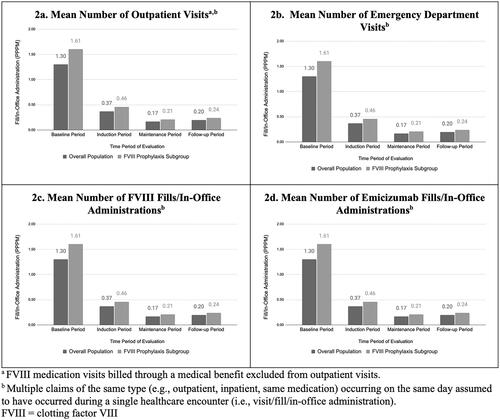
A total of 317 patients with emicizumab treatment were identified in the database at the time of the analysis. After the application of all inclusion and exclusion criteria, 71 male patients were included in the overall population, with a subset of 52 male patients included in the FVIII prophylaxis subgroup (). Index dates varied over time with greater than 30% of the overall population (40% of the FVIII prophylaxis subgroup) having an index date in 2020 and 17% (23% of the FVIII prophylaxis subgroup) having an index date in the third quarter of 2020, which was the latest time period that would allow for at least 3 months of follow-up (). The patients identified were primarily from the South (52%), with 18% from the North Central, 10% from the Northeast, and 20% from the West regions of the US (). During the 180-day baseline period, the mean number of outpatient visitsFootnotei was 1.03 (subgroup: 1.14) (), and the mean number of emergency department visits was 0.03 (subgroup: 0.04) (). Annualized mean total healthcare cost during baseline was $532,948 for the overall study population, while the FVIII prophylaxis subgroup had an annualized mean total healthcare cost of $645,727 (). The average follow-up time for the overall population in the study was 12.0 months, and 11.1 months of follow-up in the FVIII prophylaxis subgroup. 15.5% of patients were censored due to disenrollment from their health insurance, while 22.5% of patients were censored due to an emicizumab treatment gap before the end of data.
Table 1. Baseline characteristics of male hemophilia A patients without inhibitors on emicizumab for the overall population and FVIII prophylaxis subgroup.
Healthcare resource utilization
All-cause healthcare utilization
During the induction period, patients had an average of 2.23 outpatient visitsFootnoteii and 0.06 emergency department visits PPPM (), while during the maintenance period, patients had an average of 1.55 outpatient visitsFootnoteiii and 0.03 emergency department visits PPPM (). However, less than a quarter of patients experienced emergency department visits during the follow-up period (16 patients; 22.5%). Only one patient experienced an inpatient visit (not related to bleeding). PPPM HCRU rates were similar in the FVIII prophylaxis subgroup, with an average of 2.37 outpatient visitsFootnoteiv and 0.06 emergency department visits in the induction period, and 1.64 outpatient visitsFootnotev and 0.02 emergency department visits in the maintenance period (). Twelve patients (23.1%) in the FVIII prophylaxis subgroup had an emergency department visit during the follow-up period.
Hemophilia A medication use
The majority of the patients (68% of the overall population; 73% of the FVIII prophylaxis subgroup) received at least one FVIII fill/in-office administration during the follow-up period (Supplementary Table S3). The mean number of PPPM FVIII fills/in-office administrations was 0.20 over all follow-up, with a higher rate of fills/in-office administrations occurring during the induction period (0.37) than during the maintenance period (0.17) in the overall population (), with higher rates in the FVIII prophylaxis subgroup (0.24, 0.46, and 0.21 PPPM, respectively). PPPM FVIII fills/in-office administrations were highest during the baseline period (1.30) prior to emicizumab initiation, as expected since this is assumed to be their primary method of hemophilia treatment before initiation of emicizumab. The mean PPPM number of emicizumab fills/in-office administrations in the induction period was approximately 1.77 (subgroup: 1.81) and dropped to 1.04 (subgroup: 1.05) in the maintenance period ().
Arthropathy and pain management
Before emicizumab initiation, patients in the overall study population had a mean of 0.30 arthropathy associated visits PPPM and 0.10 pain visits PPPM, along with 0.24 opioid and 0.09 non-opioid pain management drug fills/in-office administrations PPPM. Baseline rates of arthropathy visits and pain management (visits/medications) were similar in the FVIII prophylaxis subgroup (). Rates of arthropathy visits and pain management (visits/medications) were similar during the follow-up period as to baseline for both the overall population and subgroup (Supplementary Table S2).
Costs
The annualized mean cost of FVIII treatment for the first year after emicizumab was $50,491, and the annualized mean cost for emicizumab and FVIII treatment was $777,171 within the overall population. The annualized mean total healthcare cost, including inpatient, outpatient, and pharmacy costs, for the first year of treatment in the overall population was $808,617. The majority of total healthcare costs were driven by treatment costs (). The corresponding cost outcomes for the FVIII prophylaxis subgroup follow the same trends but were higher than those in the overall population, with $61,512 for FVIII treatment, $793,168 for emicizumab and FVIII treatment, and $814,592 for total healthcare cost (). Annualized mean costs expected for subsequent years of treatment were lower due to the increased costs during the induction period, which only occurs at initiation (); $40,444 for FVIII treatment, $683,067 for emicizumab and FVIII treatment, and $711,660 for total healthcare cost. FVIII-related treatment costs were lower after patients initiated emicizumab across both the overall population and the FVIII prophylaxis subgroup ( and ).
Table 2. Costs of male hemophilia A patients without inhibitors after starting use of emicizumab for the overall population and FVIII prophylaxis subgroup.
Discussion
This study, to our knowledge, is the first to describe HCRU and costs among a national sample of commercially-insured adults with hemophilia A without inhibitors initiating emicizumab in the real world in the US. Our study highlighted continued use of concomitant FVIII replacement therapies and high total treatment cost in the first year of treatment.
The approval of emicizumab represented a major advancement in hemophilia treatment. Our study showed a steady increase in emicizumab prescriptions throughout 2019. In early 2020, the lower number of new patients starting emicizumab may be attributed to the wide-spread impact of the COVID-19 pandemic. By the third quarter of 2020, an increased number of emicizumab prescriptions were observed, which coincided with a gradually subsiding pandemic. In addition, less than 25% of patients were censored due to a treatment gap with emicizumab, suggesting that patients are fairly adherent on filling emicizumab within the first year based on the granularity of data in claims.
Prior to emicizumab use, most patient encounters occurred in the outpatient setting or in the emergency department, with no inpatient visits recorded for this study population during the 6-month baseline period. This suggests that bleeding events could be managed outside of the hospital.
During the follow-up period, medication use related to HA was higher during the induction period relative to the maintenance period. This trend was observed separately for both emicizumab and FVIII use. The initial higher number of emicizumab claims is likely related to the administration of loading doses for emicizumab, which requires four weekly injections. The initial increase in FVIII fills/in-office administrations during the induction period could be due, in part, to physicians prescribing FVIII to have on-hand to be used as-needed due to the risk of breakthrough bleeding occurring during the loading phase of emicizumab when therapeutic levels have not been reached, as opposed to an increase in bleeding during this period. Similar to the pattern observed with medication usage, outpatient visitsFootnotevi were higher during the induction period than during the subsequent months of follow-up. This is likely attributed to, in part, in-office administration of emicizumab during the induction period (which requires four weekly injections) and ongoing patient education on self-injection of emicizumab and on the difference between FVIII replacement products and emicizumab.
During the follow-up period, all inpatient (N = 1) and emergency department visits observed were not related to hemophilia or a bleeding event (e.g. claims were related to respiratory conditions, flu, and alcohol dependence). However, it is possible that the low level of inpatient and emergency department utilization may be attributable in part to the COVID-19 pandemic in the latter portion of the study period, as other studies of similar patients on FVIII prophylaxis from pre-pandemic times reported 7.1% of patients experiencing an inpatient visit over a 12-month periodCitation21. More broadly, other researchers have shown that, during the COVID-19 pandemic, healthcare resource use was considerably lower than in prior years across therapeutic areasCitation22.
The FVIII prophylaxis subgroup demonstrated similar data patterns to those observed in the overall study population, as expected since the subgroup comprised approximately 75% of the overall population. However, estimates of treatment cost and utilization were higher in the subgroup as would be expected as the subgroup likely reflects patients with a bleeding phenotype warranting a prophylaxis regimen. A slight increase in the mean number of fills/in-office administrations PPPM indicates that the FVIII prophylaxis subgroup was more likely to use FVIII during the follow-up period than those in the overall population.
FVIII costs dropped once patients began emicizumab, however, annualized total HA-related treatment costs were 1.5-times greater during the first year after emicizumab initiation (including the induction period). Estimated annual cost in subsequent years of treatment was lower than the first year, as expected, but still higher than before initiation of emicizumab.
It is important to note the variability of costs in this relatively small cohort of patients. As commonly observed in costs analyses, this study’s results suggest a non-normal distribution of data. Non-emicizumab healthcare costs show a distinct left-skewed distribution (Supplementary Table S4), with the median value being lower than the mean value, indicating the existence of outlier patients who accrued extremely high medical and FVIII treatment costs during this time period. However, less variability was observed for costs of emicizumab, suggesting more consistent utilization and cost among patients and across practices.
This study builds on previously published work on treatment, overall HCRU, and cost for adults with hemophilia A receiving prophylaxis with FVIII replacement therapiesCitation19,Citation21,Citation23. Using similar databases, this study focuses on patients receiving emicizumab, a newer treatment option. Previous studies have shown substantial total healthcare costs associated with FVIII prophylaxis, ranging from $650,065 to $759,661, 96% of which is attributable to FVIII costsCitation24. Emicizumab is the first approved non-factor therapy for HA and has been shown to be an effective prophylactic bleeding control treatmentCitation24–26. Overall, we did not observe a reduction in healthcare resource utilization and total healthcare costs in our study after initiation of emicizumab. The emergence of several gene therapies for hemophilia raises the question of whether or not these novel treatments, once approved, could reduce utilization and associated treatment cost while maintaining adequate protective FVIII activity level and reduce treatment burden. Healthcare resource and cost evaluation studies, similar to this research, for these novel treatments will be crucial in understanding the long-term cost-benefit analysis of gene therapy versus continual prophylaxis treatment with replacement or non-replacement therapies.
This study represents experience for a relatively small sample of PwHA without inhibitors initiating (or switching to) emicizumab shortly after the approval of emicizumab in this indication in October 2018. Due to data availability, the study cohort had limited duration of follow-up. Due to the small sample size and inability to identify certain clinical parameters that may impact cost outside of treatment choice (blood clotting factor level, major bleeds), this analysis did not perform any statistical adjustment for possible confounders. Areas of future research include replicating the study with a larger sample size, with non-commercial payers, and in an ex-US population.
This study included several quarters of data that overlapped with the COVID-19 pandemic, which may have led to an underestimation of HCRU and cost. The distribution of claims was examined and while a reduction in claims was seen in the first two quarters of 2020, claim rates appeared to return to their pre-pandemic levels starting in the third quarter of 2020.
While claims data are valuable for the efficient and effective examination of health care outcomes, treatment patterns, HCRU, and costs, all claims databases have certain inherent limitations. There may be some misclassification bias as the presence of a diagnosis code on a medical claim is not always positive for the presence of disease because the diagnosis code may be incorrectly coded or included as rule-out criteria rather than actual disease. Similarly, claims data only capture medications that were dispensed to patients, but does not provide information on whether the patient took the medication as intended. This is particularly relevant for FVIII treatment where patients may fill a new prescription, but not use the FVIII until it is needed. In addition, over-the-counter medication use or medications administered during hospitalizations are not captured. Additionally, the dataset used and population identified only included patients enrolled in a commercial health plan captured in the MarketScan database. While MarketScan is considered to be generalizable to other US patients enrolled in similar insurance plans, these results may not be applicable for uninsured patients or those enrolled in plans not represented including Medicare and MedicaidCitation20.
Furthermore, there is no diagnosis code for identifying severe hemophilia. Therefore, an operational definition based on previous work leveraging days covered by FVIII treatment to differentiate between prophylactic and on-demand treatment was used to identify a subgroup that is believed to be treated with a prophylactic regimen, and therefore, more likely to have severe hemophiliaCitation21. Other algorithms were considered to identify these patientsCitation23,Citation27, however limitations in the dosing information captured across the MarketScan database prevented consistent application of alternative algorithms across dispensing methods (pharmacy vs. in-office administration).
Conclusions
In a cohort of 71 male PwHA without inhibitor treatment, healthcare utilization and costs remained high in the first year after emicizumab initiation. These results illustrate that, despite treatment with emicizumab, HA patients still require FVIII use beyond the induction period and high overall HA-related treatment cost. Improved disease management strategies, care coordination, and new treatment options are needed to reduce healthcare system burden.
Transparency
Declaration of funding
This study was sponsored by BioMarin Pharmaceuticals, Inc.
Declaration of financial/other interests
LC has no financial disclosures. AE, PP, and JT are employees and options holders in Aetion, Inc, which received funding in connection with this study and preparation of this manuscript. EC, DH, and HM are employees and stockholders in BioMarin Pharmaceuticals, Inc which contributed funding in connection with this study and the preparation of this manuscript. CLK has received honoraria for advisory board participation from Biomarin-Genzyme, Sanofi, Takeda, Spark Therapeutics, Genentech, and Pfizer. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Author contributions
All authors contributed to the conception, study design, protocol development, data analysis, interpretation, and drafting and revising of the manuscript.
Acknowledgements
None reported.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Previous presentations
Data from this study were previously presented in poster and abstract format at the 2022 ISPOR annual meeting.
Compliance with ethics guidelines
This was a secondary analysis of data and was performed using deidentified data without access to personal identifying information. The MarketScan data are Health Insurance Portability and Accountability Act of 1996 compliant. Institutional review board approval was not required as this study only used deidentified patient records and did not involve the collection or use of individually identifiable data.
Supplemental Material
Download MS Word (40.3 KB)Notes
i FVIII medication visits billed through a medical benefit excluded from outpatient visits.
ii Ibid.
iii Ibid.
iv Ibid.
v Ibid.
vi Ibid.
References
- Hemophilia A. Genetic and Rare Diseases Information Center (GARD) – an NCATS Program [Internet]. [cited 2021 Jun 17]. Available from: https://rarediseases.info.nih.gov/diseases/6591/hemophilia-a
- Hemophilia A. [Internet]. National Hemophilia Foundation. [cited 2021 Jun 17]. Available from: https://www.hemophilia.org/bleeding-disorders-a-z/types/hemophilia-a.
- Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia. 3rd ed. Haemophilia. 2020;26:1–158.
- Research C for DE and FDA approves emicizumab-kxwh for prevention and reduction of bleeding in patients with hemophilia A with factor VIII inhibitors. FDA [Internet]. 2019. [cited 2021 Oct 13]; Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-emicizumab-kxwh-prevention-and-reduction-bleeding-patients-hemophilia-factor-viii.
- Research C for DE and FDA approves emicizumab-kxwh for hemophilia A with or without factor VIII inhibitors. FDA [Internet]. 2019. [cited 2021 Oct 13]; Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-emicizumab-kxwh-hemophilia-or-without-factor-viii-inhibitors.
- Croteau SE, Cheng D, Cohen AJ, et al. Regional variation and cost implications of prescribed extended half-life factor concentrates among U.S. Haemophilia Treatment Centres for patients with moderate and severe haemophilia. Haemophilia. 2019;25(4):668–675.
- Zhou Z-Y, Koerper MA, Johnson KA, et al. Burden of illness: direct and indirect costs among persons with hemophilia a in the United States. J Med Econ. 2015;18(6):457–465.
- Shrestha A, Eldar-Lissai A, Hou N, et al. Real-world resource use and costs of haemophilia A-related bleeding. Haemophilia. 2017;23(4):e267–e275.
- McCary I, Guelcher C, Kuhn J, et al. Real-world use of emicizumab in patients with haemophilia A: bleeding outcomes and surgical procedures. Haemophilia. 2020;26(4):631–636.
- Barg AA, Budnik I, Avishai E, et al. Emicizumab prophylaxis: prospective longitudinal real-world follow-up and monitoring. Haemophilia. 2021;27(3):383–391.
- Levy-Mendelovich S, Brutman-Barazani T, Budnik I, et al. Real-World data on bleeding patterns of hemophilia a patients treated with emicizumab. JCM. 2021;10(19):4303.
- Vagrecha A, Stanco J, Ulus D, et al. Real-world experience using emicizumab prophylaxis for hemophilia a: single-center experience. Blood. 2020;136(Supplement 1):36–36.
- Mahajerin A, Khairnar R, Meyer CS, et al. Real-world persistence with and adherence to emicizumab prophylaxis in persons with hemophilia a: a secondary claims database analysis. Blood. 2020;136(Supplement 1):13–13.
- Samelson-Jones BJ, Guelcher C, Kuhn J, et al. Real-world cost estimates of initiating emicizumab in US patients with haemophilia A. Haemophilia. 2021;27(4):591–598.
- Misgav M, Brutman-Barazani T, Budnik I, et al. Emicizumab prophylaxis in haemophilia patients older than 50 years with cardiovascular risk factors: real-world data. Haemophilia. 2021;27(2):253–260.
- Warren BB, Chan A, Manco‐Johnson M, et al. Emicizumab initiation and bleeding outcomes in people with hemophilia a with and without inhibitors: a single‐center report. Res Pract Thromb Haemost. 2021;5(5):e12571.
- Callaghan MU, Negrier C, Paz-Priel I, et al. Long-term outcomes with emicizumab prophylaxis for hemophilia a with or without FVIII inhibitors from the HAVEN 1-4 studies. Blood. 2021;137(16):2231–2242.
- Skinner MW, Négrier C, Paz-Priel I, et al. The effect of emicizumab prophylaxis on long-term, self-reported physical health in persons with haemophilia a without factor VIII inhibitors in the HAVEN 3 and HAVEN 4 studies. Haemophilia. 2021;27(5):854–865.
- Croteau SE, Cook K, Sheikh L, et al. Health care resource utilization and costs among adult patients with hemophilia A on factor VIII prophylaxis: an administrative claims analysis. J Manag Care Spec Pharm. 2021;27(3):316–326.
- IBM MarketScan Research Databases for Life Sciences Researchers. 2021.
- Thornburg CD, Adamski K, Cook K, et al. Health care costs and resource utilization among commercially insured adult patients with hemophilia a managed with FVIII prophylaxis in the United States. J Manag Care Spec Pharm. 2022;28(4):449–460.
- Moynihan R, Sanders S, Michaleff ZA, et al. Impact of COVID-19 pandemic on utilisation of healthcare services: a systematic review. BMJ Open. 2021;11(3):e045343.
- Caplan EO, Patel AM, DeClue RW, et al. Real-world treatment, clinical outcomes and healthcare resource utilization among persons with hemophilia A by age. J Comp Eff Res. 2021;10(15):1121–1131.
- Lyons J, Desai V, Xu Y, et al. Development and validation of an algorithm for identifying patients with hemophilia a in an administrative claims database. Value Health. 2018;21(9):1098–1103.
- Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia a with inhibitors. N Engl J Med. 2017;377(9):809–818.
- Young G, Liesner R, Chang T, et al. A multicenter, open-label phase 3 study of emicizumab prophylaxis in children with hemophilia a with inhibitors. Blood. 2019;134(24):2127–2138.
- Pipe SW, Shima M, Lehle M, et al. Efficacy, safety, and pharmacokinetics of emicizumab prophylaxis given every 4 weeks in people with haemophilia A (HAVEN 4): a multicentre, open-label, non-randomised phase 3 study. Lancet Haematol. 2019;6(6):e295–e305.