Abstract
Aim
To evaluate the public health impact of the UK COVID-19 booster vaccination program in autumn 2021, during a period of SARS-CoV-2 Delta variant predominance.
Materials and methods
A compartmental Susceptible-Exposed-Infectious-Recovered model was used to compare age-stratified health outcomes for adult booster vaccination versus no booster vaccination in the UK over a time horizon of September–December 2021, when boosters were introduced in the UK and the SARS-CoV-2 Delta variant was predominant. Model input data were sourced from targeted literature reviews and publicly available data. Outcomes were predicted COVID-19 cases, hospitalizations, post-acute sequelae of COVID-19 (PASC) cases, deaths, and productivity losses averted, and predicted healthcare resources saved. Scenario analyses varied booster coverage, virus infectivity and severity, and time horizon parameters.
Results
Booster vaccination was estimated to have averted approximately 547,000 COVID-19 cases, 36,000 hospitalizations, 147,000 PASC cases, and 4,200 deaths in the UK between September and December 2021. It saved over 316,000 hospital bed-days and prevented the loss of approximately 16.5 million paid and unpaid patient work days. In a scenario of accelerated uptake, the booster rollout would have averted approximately 3,400 additional deaths and 25,500 additional hospitalizations versus the base case. A scenario analysis assuming four-fold greater virus infectivity and lower severity estimated that booster vaccination would have averted over 105,000 deaths and over 41,000 hospitalizations versus the base case. A scenario analysis assuming pediatric primary series vaccination prior to adult booster vaccination estimated that expanding vaccination to children aged ≥5 years would have averted approximately 51,000 additional hospitalizations and 5,400 additional deaths relative to adult booster vaccination only.
Limitations
The model did not include the wider economic burden of COVID-19, hospital capacity constraints, booster implementation costs, or non-pharmaceutical interventions.
Conclusions
Booster vaccination during Delta variant predominance reduced the health burden of SARS-CoV-2 in the UK, releasing substantial NHS capacity.
Introduction
Coronavirus disease 2019 (COVID-19), the highly contagious viral illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has had a substantial impact in the UK, with over 21 million confirmed cases of COVID-19,Citation1 1.8 million cases of post-acute sequelae of COVID-19 (PASC),Citation2 830,000 hospitalizations,Citation3 and 190,000 deathsCitation4 as of 6 May 2022. The UK National Health Service (NHS) has faced unprecedented levels of pressure from the COVID-19 pandemic,Citation5 including increased frontline costs of £4–5 billion a yearCitation6 and increased treatment burden from COVID-19 patients.Citation6
The UK response to the pandemic has included numerous public health measures,Citation7–9 including rapid rollout of a mass vaccination program against SARS-CoV-2.Citation10 Although primary series vaccination against COVID-19 has been considered effective, having averted at least 427,900 (397,600 − 494,700) UK deaths between December 2020 and December 2021,Citation11 vaccine-derived protection waned with time.Citation12–14 Furthermore, emerging SARS-CoV-2 variants had been thought to increase the likelihood of reinfection and reduce vaccine-derived protection.Citation15 The Delta variant (B.1.617.2), which was escalated to a variant of concern in the UK on 6 May 2021,Citation16 has been associated with greater transmissibility, greater risk of hospitalization,Citation17 and less protection from vaccinationCitation15,Citation18,Citation19 compared to prior variants.
The use of an additional (“booster”) dose of vaccine was considered in the UK and elsewhere as a public health strategy to maintain protection against severe outcomes of COVID-19. The UK Joint Committee on Vaccination and Immunisation advised on 14 September 2021 that regardless of the vaccine given in the primary series, a booster dose of either BNT162b2 vaccine (Pfizer-BioNTech) or mRNA-1273 vaccine (Moderna; half dose of 50 µg) be offered to the most vulnerable to protect against COVID-19 hospitalization and death.Citation20 Populations initially offered a booster included all adults aged > 50 years, those aged 16–49 years with underlying health conditions that put them at higher risk of severe COVID-19 and their adult carers, household contacts (aged ≥ 16 years) of immunosuppressed individuals, and frontline health and social care workers.Citation20 Booster recommendations were extended to those aged 40–49 years on 15 November 2021Citation21 and further extended to all individuals aged ≥ 18 years on 29 November 2021.Citation22 Initial guidance stipulated that boosters be given no earlier than six months after completion of primary vaccination,Citation20 though this interval was shortened to three months on 29 November 2021.Citation20 As of 19 January 2022, the UK booster program had administered over 36.3 million booster doses, covering 69% of the UK adult population.Citation23 As of 18 May 2022, approximately 76% of booster doses administered were BNT162b2, and 24% were mRNA-1273.Citation24
Given the lack of robust data quantifying the health impact and resource savings associated with booster vaccination in the UK, deterministic analyses using real-world data are needed. This study aimed to retrospectively estimate the public health impact of the UK booster vaccination program in its first months compared to a hypothetical alternative with only primary vaccination and for a range of scenarios.
Methods
Overview
A deterministic, compartmental dynamic transmission model (DTM) was developed and fitted to UK epidemiology data over the retrospective time horizon of 13 September to 5 December 2021, when the UK booster program was being rolled out amid a period of SARS-CoV-2 Delta variant dominance. The model included the entire UK population, based on UK Office for National Statistics (ONS) estimates from mid-2020 (Supplementary Methods),Citation25 and captured both direct (i.e. individual) and indirect (i.e. herd) transmission effects of SARS-CoV-2 in those with or without booster vaccination. Health outcomes evaluated were predicted COVID-19 cases (asymptomatic and symptomatic), general ward and intensive care unit (ICU) hospitalizations and bed-days, PASC cases, and deaths averted. Predicted healthcare resources saved were calculated from the NHS perspective, and greater societal impacts were estimated using a human capital approach, considering averted productivity loss for persons both in and outside of the workforce. Scenario analyses were used to explore how hypothetical variations in booster coverage, virus transmissibility and severity, and time horizon could change the public health impact of booster vaccination. Model calibration was performed as described in Supplementary Methods.
Dynamic transmission model
The DTM was developed from the classical Susceptible-Exposed-Infectious-Recovered (SEIR) structureCitation26 and consisted of “Susceptible,” “Vaccinated,” “Exposed,” “Infectious with symptoms,” “Infectious and asymptomatic,” and “Recovered” compartments (). The “Susceptible” compartment contained a cohort without protection against SARS-CoV-2 from vaccination-induced protection or infection-induced immunity. The model contained separate “Vaccinated” compartments for cohorts with vaccine-induced protection from one primary series dose (“V1”), two primary series doses (“V2”), or a booster dose (“VB”). Separate “Exposed” compartments were modeled based on vaccination status, and “Infectious” compartments were stratified by symptomatic versus asymptomatic infection. The “Recovered” compartment contained those who had previously experienced and recovered from COVID-19 infection. All compartments were stratified by age group, with 5-year age bands for people aged 0–9 years, 10-year age bands for those aged 10–79 years, and an age band for those ≥ 80 years.
Figure 1. Dynamic transmission model diagram. Compartments—all compartments are further stratified by age: S—susceptible. V1,2,B—vaccinated with dose 1, 2 and booster. E—Exposed, unvaccinated. EV1,2,B—exposed and vaccinated by dose 1, 2 and booster. IS, V1S, V2S, VBS—infectious with symptoms by vaccination status. IA, V1A, V2A, VBA—infectious and asymptomatic by vaccination status. R—recovered. Parameters: υ1, 2, B—coverage by vaccination status; λ—force of infection; σ1, 2, B—vaccine effectiveness against infection by vaccination status; ψ0,1, 2, B—waning rate (1/duration of immunity) by infection-induced immunity and vaccination status; ε1, 2, B—probability of displaying symptoms by vaccination status; γ—recovery rate (1/duration of symptoms); κ—rate of progression to infectious disease.
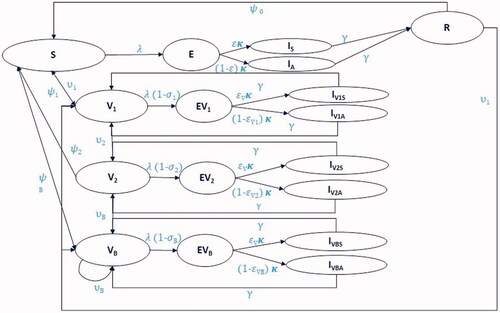
Individuals entered the model in the “Susceptible,” “V1,” “V2,” “Recovered,” “Infectious symptomatic,” or “Infectious asymptomatic” compartments. The starting “Recovered” cohort (Supplementary Table 1) was calculated based on age-stratified UK seroprevalence data for anti-N antibodies from 16 September 2021.Citation27 Anti-N antibodies were used because people with these antibodies were likely to have been infected with COVID-19 in the past.Citation28 Individuals in the “Recovered” compartment were assumed to have full protection against infection (i.e. infection-induced immunity) for an average duration of nine months after recovery from infection. Infection-induced immunity was assumed to wane at a constant rate, with individuals returning to the appropriate “Vaccinated” compartment depending on their vaccination status. For simplicity, no additional protection was conferred for infection-induced immunity achieved during or after periods of vaccine-induced protection.
Vaccination inputs
The proportion of the starting model population (Supplementary Table 1) in the “V1” and “V2” compartments was based on age-stratified UK vaccine coverage data from 13 September 2021 (Supplementary Methods). As booster vaccination had not yet begun at the start of the model time horizon, the “VB” compartment was initially empty. Overall estimates for vaccine protection against infection and hospitalization were derived from published estimates of vaccine-specific effectiveness and vaccine uptake in England ().Citation14,Citation32 Each additional vaccine dose received by an individual was assumed to confer increased protection against infection, symptomatic disease, hospitalization, and death. Breakthrough infections during periods of vaccine-induced protection were included in the model, with the probability of experiencing breakthrough infection dependent on dose-specific probabilities of vaccine effectiveness against infection.
Table 1. Vaccine effectiveness and duration inputs.
The duration of vaccine-induced protection was assumed to wane over six months at a constant rate (). To investigate the sensitivity of model results to this assumption, we assessed the impact of increasing and decreasing the duration of protection by 20% and 50%. Although data suggested that vaccine effectiveness decreased less against hospitalization compared to the vaccine effectiveness against infection,Citation33 due to constraints in the model structure, only one rate of waning for vaccine protection could be implemented. Waning was based on the rate for protection against infection, and this (shorter) duration of protection was assumed to be the same for both infection and hospitalization.
During the model time horizon, individuals could receive a maximum of one booster dose. For simplicity, individuals could not receive a first or second dose of primary vaccination during the model time horizon; individuals who had not received primary vaccination were assumed to remain unvaccinated. No national mandates for non-pharmaceutical intervention (NPI), including social distancing or face masks, were in place in the UK during the model time horizon,Citation34 so no specific NPIs were included in the model.
Exposure inputs
The “Exposed” cohorts were calculated from age-stratified and week-specific UK CoMix data based on mixing within the model population,Citation35,Citation36 in conjunction with vaccination status (Supplementary Methods).
Health inputs
Health inputs are detailed in . For the “Infected” compartment, the model was fitted to UK infection epidemiology using Public Health England (PHE) Pillar 1 (swab testing in PHE labs and NHS hospitals) and Pillar 2 (swab testing using in-person tests and home test kits) case data from 13 September to 5 December 2021. This time period was selected to correspond to the initial rollout of the UK booster vaccination program, which began on 14 September 2021, and ended before the emergence of the Omicron variant in the UK. The Delta variant had been escalated to a variant of concern in the UK on 6 May 2021Citation16 and was predominant in the UK in the period between September and December 2021. As such, all infections were assumed to be of the SARS-CoV-2 Delta variant based on the predominance of this variant during the model time horizon.
Table 2. Health inputs for model population infected with COVID-19.
Both symptomatic and asymptomatic cases were included in the model, the proportions of which were based on age groupCitation37 and vaccination statusCitation19 (; details provided in the Supplementary Methods). Asymptomatic cases were considered 3.85 times less infectious than symptomatic casesCitation43 and were assumed to never lead to hospitalization or death. For both asymptomatic and symptomatic cases, a 5-day latent (“Exposed”) period followed by a 5-day “Infectious” period was assumed based on average values from published COVID-19 models.Citation38,Citation44–48 Hospitalization was assumed to occur with a 1-week delay after becoming symptomatic. The age-stratified probabilities of ICU admission for those hospitalized were derived from Intensive Care National Audit & Research Centre data from July 2021Citation49 ( and Supplementary Methods). A proportion of the population within each age group was considered “high risk” according to prevalence of pre-existing conditions defined in Walker et al.Citation39 and were assigned a higher probability of hospitalization, ICU admission, and death. The proportion of patients with COVID-19 (including both asymptomatic and symptomatic patients) developing PASC was assumed to be 32% for those non-hospitalized and symptomatic and 51% for those hospitalized, based on data from the Belgian Health Care Knowledge Centre.Citation42
Resource use inputs
NHS healthcare resource use was calculated for all cases receiving primary care/outpatient or inpatient medical care. Length of stay (bed-days) in a general wardCitation50 or ICUCitation49 was calculated from UK data specific to COVID-19 patients ( and Supplementary Methods).
Productivity loss inputs
Productivity loss was estimated using a human capital approach,Citation51 and included the number of productive days for persons both in and outside of the paid work force. Length of COVID-19-related absence was based on the duration of isolation for diagnosed cases (10 days) plus the length of stay for hospitalized cases (Supplementary Methods).
For those in the paid work force, productive work days were calculated using UK ONS workforce participation rates.Citation52 For the entire model population, productive unpaid work days (e.g. time spent volunteering, caring for others, cleaning) were calculated using UK ONS data.Citation53 Unpaid work performed was estimated for each week based on age and weighted by gender (Supplementary Table 3).
Scenario analyses
Seven scenario analyses were conducted to explore the public health impact of booster vaccination under hypothetical variations in virus and vaccination parameters. Booster coverages used in each scenario are given in Supplementary Table 4.
Scenario 1.1. Accelerated coverage
Scenario 1.1 explored the hypothetical impact of an accelerated booster rollout. The maximum age-stratified coverage achieved in the actual UK booster program by 5 December 2021 would be reached after only four weeks of the time horizon (by 19 October 2021). Coverage was assumed to increase at a constant rate.
Scenario 1.2. Higher infectivity and lower severity
Scenario 1.2 explored the hypothetical effects of increased virus infectivity and lower virus severity. Infectivity levels were increased four-fold from those used in the base case, and the probabilities of hospitalization and mortality were reduced relative to the base case (hospitalization: 38% of base case; mortality: 60% of base case).
Scenario 1.3. Accelerated coverage, higher infectivity, and lower severity
Scenario 1.3 explored the hypothetical effects of accelerated booster coverage, higher virus infectivity, and lower virus severity. Actual age-stratified maximal booster coverage levels were assumed to have been reached within the first month of the time horizon, infectivity levels were increased four-fold from those used in the base case,Citation54,Citation55 and the probabilities of hospitalization and mortality were reduced relative to the base case (hospitalization: 38% of base case; mortality: 60% of base case).
Scenario 1.4. Lower infectivity
Scenario 1.4 explored the hypothetical effect of lower virus infectivity. Infectivity levels were decreased by 50% from those used in the base case.
Scenario 1.5. Accelerated coverage and lower infectivity
Scenario 1.5 explored the hypothetical effects of accelerated booster coverage and lower virus infectivity. Actual age-stratified maximal booster coverage levels were assumed to have been reached within the first month of the time horizon, and infectivity levels were decreased by 50% from those used in the base case.
Scenario 1.6. Primary series uptake in pediatrics
Scenario 1.6 explored a hypothetical vaccination program in which 60% of the pediatric population aged 5–19 would have been immunized with primary vaccination prior to the start of the time horizon. No additional pediatric vaccination would occur during the time horizon, and adult booster vaccination would occur according to observed booster coverage in adults.
Scenario 2. Extended time horizon
Scenario 2 explored the impact of booster vaccination in a hypothetical situation in which the Delta variant remained the dominant variant throughout the winter season of 2021–2022 and the Omicron variant (B.1.1.529) did not exist. This scenario was simulated over a 22-week time horizon from 17 September 2021 to 11 February 2022. For the first 11 weeks of the time horizon (ending 5 December 2021), booster coverage was calculated as in the base case. For the second 11 weeks of the time horizon, booster coverage for those aged 20–49 years was based on age-stratified UK booster coverage data from the UK Health Security Agency,Citation56 and booster coverage for those aged ≥ 50 years was assumed to increase up to a maximum level based on the level of primary booster uptake in that age group.Citation56 The model did not allow for a second (or subsequent) booster vaccination.
Results
Base case results
Health outcomes
Relative to a hypothetical scenario in which booster vaccination did not occur, the UK booster vaccination program was estimated to have averted approximately 547,000 infections (16% reduction; ), 36,000 hospitalizations (31% reduction), 147,000 PASC cases (20% reduction), and 4,200 deaths (27% reduction) over the model time horizon ().
Figure 2. Modelled new weekly COVID-19 cases with and without booster vaccination. Base case estimation of new weekly COVID-19 cases per 100,000 population over the model time horizon, with (green) or without (grey) booster vaccination.
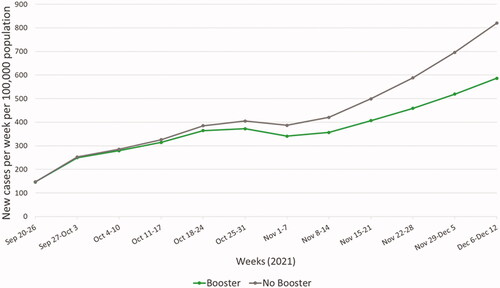
Table 3. Base case outcomes and difference with UK booster vaccination program.
Resource use from the UK National Health Service perspective
Over the model time horizon, the UK booster vaccination program was estimated to have reduced direct NHS healthcare resource use by approximately 36,000 hospitalizations (31% decrease), including over 5,800 ICU admissions (29% decrease), relative to no booster vaccination. Booster vaccination was estimated to have released over 257,000 bed-days in general wards (31% decrease), nearly 59,000 bed-days in ICUs (29% decrease), and over 228,000 outpatient appointments (17% decrease) to treat patients with other conditions ().
Averted productivity loss
The UK booster vaccination program was estimated to have averted approximately 16.5 million days of productivity loss for patients (18% decrease), including days of paid and unpaid work ().
Scenario results
Scenario 1.1. Accelerated coverage
In a scenario that assumed accelerated booster coverage within the first month of the time horizon, booster vaccination would have averted approximately 1.4 million infections (41% reduction), 62,000 hospitalizations (53% reduction), 357,000 PASC cases (50% reduction), and 7,600 deaths (49% reduction) relative to no booster vaccination. In this scenario, boosters would have reduced direct NHS healthcare resource use by approximately 434,000 general ward bed-days (53% reduction), 106,000 ICU bed-days (53% reduction), and 580,000 outpatient appointments (43% reduction) relative to no booster vaccination (; Supplementary Table 5). Accelerated coverage was estimated to result in approximately double the impact of the observed booster rollout shown in the base case for all health and resource use outcome measures ().
Table 4. Averted health outcomes and resource use savings with booster vaccination in base case and hypothetical scenarios.
Scenario 1.2. Higher infectivity and lower severity
In a scenario where infectivity levels were increased to four times higher than in the base case and the probabilities of hospitalization and mortality were reduced relative to the base case (hospitalization: 38% of base case; mortality: 60% of base case), the UK booster vaccination program would have averted approximately 11.5 million infections (10% reduction), 78,000 hospitalizations (7% reduction), 3.6 million PASC cases (12% reduction), and 110,000 deaths (20% reduction) relative to no booster vaccination (; Supplementary Table 6). The booster program would have reduced direct NHS resource use by approximately 602,000 general ward bed-days (8% decrease), 72,000 ICU bed-days (5% decrease), and 5.5 million outpatient appointments (11% decrease) relative to no booster vaccination.
Scenario 1.3. Accelerated coverage, higher infectivity, and lower severity
If both booster coverage and infectivity levels were increased and virus severity was reduced relative to the base case (hospitalization: 38% of base case; mortality: 60% of base case), booster vaccination would have averted approximately 35 million infections (31% reduction), 144,000 hospitalizations (14% reduction), 10.9. million PASC cases (36% reduction), and 228,000 deaths (42% reduction) relative to no booster vaccination (; Supplementary Table 7). Booster vaccination would have reduced direct NHS resource use by approximately 1.1 million general ward bed-days (14% decrease), 157,000 ICU bed-days (11% decrease), and 15.6 million outpatient appointments (31% decrease) relative to no booster vaccination.
Scenario 1.4. Lower infectivity
If infectivity levels were decreased, the UK booster vaccination program would have averted approximately 10,700 infections (4% reduction), 600 hospitalizations (13% reduction), 3,100 PASC cases (6% reduction), and 100 deaths (5% reduction) relative to no booster vaccination (; Supplementary Table 8). The booster program would have reduced direct NHS resource use by approximately 4,400 general ward bed-days (12% decrease), 1,000 ICU bed-days (13% decrease), and 4,700 outpatient appointments (4% decrease) relative to no booster vaccination.
Scenario 1.5. Accelerated coverage and lower infectivity
If booster coverage was accelerated and infectivity levels were decreased, booster vaccination would have averted approximately 44,000 infections (15% reduction), 1,400 hospitalizations (29% reduction), 12,000 PASC cases (23% reduction), and 270 deaths (12% reduction) relative to no booster vaccination (; Supplementary Table 9). Booster vaccination would have reduced direct NHS resource use by approximately 10,000 general ward bed-days (29% decrease), 2,600 ICU bed-days (33% decrease), and 18,300 outpatient appointments (17% decrease) relative to no booster vaccination.
Scenario 1.6. Primary series uptake in pediatrics
A hypothetical program in which 60% of the pediatric population aged 5–19 had received primary vaccination prior to the start of the time horizon and adults received booster vaccination as per the actual UK booster program would have averted approximately 1.9 million infections (64% reduction), 51,000 hospitalizations (63% reduction), 329,000 PASC cases (57% reduction), and 5,400 deaths (48% reduction) relative to adult-only booster vaccination (; Supplementary Table 10). Such a program would have reduced NHS resource use by approximately an additional 361,000 general ward bed-days (64% decrease), 88,000 ICU bed-days (62% decrease), and 705,000 outpatient appointments (63% decrease) compared to adult-only boosters.
Scenario 2. Extended time horizon
If the time horizon were extended until 11 February 2022 and Delta remained the dominant variant, booster vaccination would have averted approximately 7.7 million infections (47% reduction), 600,000 hospitalizations (65% reduction), 2.1 million PASC cases (54% reduction), and 59,000 deaths (62% reduction) relative to no booster vaccination (; Supplementary Table 11). Booster vaccination would have reduced direct NHS resource use by approximately 4.3 million general ward bed-days (65% decrease), 991,000 ICU bed-days (63% decrease), and 3.2 million outpatient appointments (48% decrease) relative to no booster vaccination.
Duration of protection
Sensitivity of the model outputs to the assumed six-month duration of protection from booster vaccination was tested by increasing and reducing the duration of protection (Supplementary Table 12). Increasing the duration of protection to nine months increased the impact of booster vaccination on the number of infections by only two percentage points (reduction 16% in the base case versus 18% with a nine-month duration of protection). Reducing the duration of protection to three months reduced the impact of booster vaccination on the number of infections by six percentage points (reduction 16% in the base case versus 10% with three-month duration of protection). Among health outcomes, the largest impact of reducing the duration of protection to three months was on hospitalizations (reduction 31% in the base case versus 15% with a three-month duration of protection). Overall this analysis demonstrates the robustness of the base case results to changes in the assumed of duration of protection, although a large reduction in the assumed duration of protection would reduce the impact of booster vaccination.
Discussion
This modeling analysis showed that booster vaccination reduced the UK’s COVID-19 burden relative to primary vaccination alone between September and December 2021—a time when the Delta variant predominated—averting approximately 547,000 infections and 4,200 deaths, and releasing over 257,000 hospital bed-days in the general ward and approximately 59,000 bed-days in the ICU. Benefit to public health was observed in the base case and in all of the hypothetical booster vaccination program scenarios analyzed. Our results have demonstrated that expanding the vaccination coverage to younger age groups who play an important role in transmission helps reduce disease burden and the success of the booster campaign is highly sensitive to the timing of its initiation in the presence of new variants.
The positive impact of boosters shown in this study is supported by similar analyses performed for the UK,Citation44,Citation57 France,Citation58 Israel,Citation59 and the US.Citation60 Barnard et al. estimated that the UK booster vaccination program was highly effective in reducing transmission and mitigating severe outcomes of COVID-19 from October 2021 to April 2022 and observed that most benefit was derived from booster uptake in those aged ≥ 50 years. Gavish et al.Citation59 retrospectively investigated the impact of observed booster rollout in Israel, whereas Sandmann et al.Citation44 in the UK, Li et al.Citation60 in the US, and Bosetti et al.Citation58 in France made predictions about the potential impact of hypothetical booster scenarios. All of the aforementioned studies concluded that booster vaccination would have a substantial health or economic benefit, and some studies predicted an additional benefit for more rapid booster rollout and an expansion of booster uptake to a broader age range.Citation58,Citation59 The present study adds value to the literature by considering outcome measures and scenarios not addressed in prior studies.
Unlike in prior models of booster vaccination, the present model included high-risk groups and considered the impact of PASC.Citation42 PASC, referred to in National Institute for Health and Care Excellence clinical guidelines as ongoing symptomatic COVID-19 (4–12 weeks after symptom onset) or post-COVID-19 syndrome (12 weeks after symptom onset), is a growing threat to public health.Citation61 As of 31 October 2021, an estimated 1.2 million people in the UK self-reported experiencing PASC, and nearly two-thirds of individuals with PASC reported that PASC symptoms had a detrimental impact on their daily activities.Citation62 Future models used for longer term public health evaluations of booster vaccination should further incorporate PASC outcomes.
This study also evaluated indirect effects of booster vaccination from a societal perspective, estimating that boosters would have averted approximately 16.5 million days of patient productivity loss. By including productive non-working days, the productivity loss estimation also considered older adults and other individuals who are not in the workforce but who participate in activities such as childcare, adult care, or volunteering.
Scenario analyses showed that extending booster coverage to more age groups and/or increasing the speed of uptake would enhance the public health benefit of boosters. Immunization of the pediatric population with 60% primary vaccination coverage among those aged 5–19 years would have substantially increased the benefit of the autumn booster vaccination program. If the virus had been both more infectious and less severe (as with the Omicron variantCitation63) the UK booster vaccination program would have still provided substantial benefit to public health, especially with faster rollout to more age groups. Notably, model estimates from the Omicron-like scenarios appear to have undervalued the benefit of boosters against hospitalization when compared against UK Health Security Agency estimates during Omicron predominance;Citation64 however, the Omicron-like scenario with increased booster coverage generated a closer approximation. Benefit in the less-infectious scenario is understandably lower. When considering the impact of boosters over a longer time horizon in the hypothetical absence of the Omicron variant, the booster campaign would have resulted in a two- to three-fold higher public health impact. This suggests that boosters may have a longer term public health benefit throughout the autumn-winter season.
A key strength of the model was its basis on UK-specific COVID-19 input data. Data specific to COVID-19 were used to estimate outpatient appointments and hospitalized bed-days in the general ward and ICU, therefore enabling more precise accounting of resource use from the NHS perspective. Another key strength was the model’s dynamic transmission structure, which enabled the complexity of SARS-CoV-2 transmissibility to be modeled more accurately than with static models. Herd effects of infection and vaccination were considered across the entire UK population, which allowed the model to better estimate the protective effects of vaccination for the community according to age-stratified population mixing matrices and to estimate transmission from those not eligible for booster vaccination (e.g. children). The use of 10-year age bands across most ages and the inclusion of a high-risk population allowed the model to better estimate the impact of boosters on severe outcomes of hospitalization, ICU admission, and death, especially for the most vulnerable. Supporting this, during the model’s calibration, the numbers of hospitalizations and deaths were reproduced with considerable accuracy to real-world data.
Model limitations include assumptions made where relevant data were lacking or simplifications were required to limit model complexity. First, the model did not account for healthcare capacity constraints, which affected care for patients with other conditionsCitation65 and may have worsened outcomes for COVID-19 patients.Citation66 Second, the model also did not account for differential receipt of booster vaccination by socio-demographic characteristics (e.g. socio-economic status) or potential impacts from NPIs (e.g. social distancing, wearing face masks) in the hypothetical comparator scenario without booster vaccination, which might affect our estimates. Notably, booster effectiveness inputs were calculated using the combined effectiveness of BNT162b2 and mRNA-1273 boosters according to their relative uptake in the UK; as such, model results cannot be attributed to a single vaccine. Third, vaccine effectiveness was assumed the same across age groups, although data suggest that effectiveness for primary series and booster vaccines differs between younger and older adults.Citation14 Moreover, the model did not incorporate reduced vaccine effectiveness or duration of protection in people who are immunocompromised or have underlying clinical conditions which appears to be a general phenomenon across COVID-19 vaccines and vaccine platformsCitation67 and therefore the estimated impact of vaccination might be affected. Additionally, despite data showing that protection against infection wanes more quickly than protection against hospitalization,Citation68 only a single rate was used in the model, and thus, the rate of protection waning against infection was applied to both infections and hospitalizations. This assumption should ensure that the results are a conservative estimate of the public health impact—the true reductions in hospitalization and death were likely greater. Fourth, the same vaccine effectiveness was assumed across all scenario analyses (e.g. by flexing the infectivity and severity of COVID-19), whereas this is expected to differ depending on COVID-19 mutation strains which might affect our estimates.Citation69 Lastly, the model results are not generalizable to different populations, SARS-CoV-2 variants, or time periods than those included in the model. Further research is needed to evaluate the impact of booster vaccination across different parameters and considering different outcome measures.
Conclusions
This public health impact analysis showed that the UK booster vaccination program produced substantial health benefits while releasing substantial NHS capacity during September to December 2021, when the Delta SARS-CoV-2 variant was predominant. Future booster vaccination programs with accelerated uptake across a wider age range could have greater impact, particularly during the predominance of more transmissible SARS-CoV-2 variants.
Transparency
Declaration of funding
This study was sponsored by Pfizer, Inc.
Declaration of financial/other relationships
DM, JA, JN, LH, MDF, CC, and JY are employees of Pfizer and may own Pfizer stock. RC and PG are employees of Evidera, which received financial support from Pfizer, Inc. in connection with the study and the development of this manuscript. Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Author contributions
All authors were involved in the conception and design of the study, contributed to data analysis and interpretation, and drafted the manuscript. All authors revised the manuscript critically for intellectual content, approved the final version of the manuscript, and agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (118.1 KB)Acknowledgements
Medical writing was provided by Jacqueline Janowich Wasserott, Jonathan Pitt, and Stephen Gilliver (Evidera) and was funded by Pfizer, Inc. Input into early model development was provided by Russell Becker, Thomas Scassellati Sforzolini, and Julie Roiz (Evidera) and was funded by Pfizer, Inc. Elena Aruffo (Evidera) provided analytical support and was funded by Pfizer, Inc.
Data availability statement
Data generated or analyzed during this study are available upon request.
References
- UK Government. Coronavirus in the UK: cases in the United Kingdom; 2022 [updated 2022 Apr 21; 2022 Apr 22]. Available from: https://coronavirus.data.gov.uk/details/cases
- Office for National Statistics (UK). Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK; 2022 May 6 [updated May 6, 2022]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/6may2022
- UK Government. Coronavirus (COVID-19) in the UK: healthcare in the United Kingdom; 2022 Apr 22. Available from: https://coronavirus.data.gov.uk/details/healthcare
- UK Government. Coronavirus (COVID-19) in the UK: deaths in United Kingdom; 2022 [updated 2022 Apr 21; 2022 Apr 22]. Available from: https://coronavirus.data.gov.uk/details/deaths
- UK National Health Service. Reducing burden and releasing capacity to manage the COVID-19 pandemic; 2021. Available from: https://www.england.nhs.uk/coronavirus/documents/reducing-burden-and-releasing-capacity-to-manage-the-c-19-pandemic/
- NHS Confederation & NHS Providers. A reckoning: the continuing cost of COVID-19. 2021 [cited 2022 April 22]. Available from: https://www.nhsconfed.org/sites/default/files/2021-09/A-reckoning-continuing-cost-of-COVID-19.pdf.
- Barber S, Brown J, Ferguson D. Coronavirus: lockdown laws. London (UK): House of Commons; 2021. Available from: https://commonslibrary.parliament.uk/research-briefings/cbp-8875/
- Brown J, Kirk-Wade E. Coronavirus: a history of ‘Lockdown laws’ in England. London (UK): House of Commons; 2021. Available from: https://commonslibrary.parliament.uk/research-briefings/cbp-9068/
- UK Government. Social distancing review: report. London (UK): UK Government; 2021. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/999413/Social-Distancing-Review-Report.pdf
- Baraniuk C. Covid-19: how the UK vaccine rollout delivered success, so far. BMJ. 2021;372:n421.
- Watson OJ, Barnsley G, Toor J, et al. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022. DOI:10.1016/S1473-3099(22)00320-6
- Public Health England. Duration of protection of Covid-19 vaccines against clinical disease. London (UK): UK Government; 2021. Available from: https://www.gov.uk/government/publications/phe-duration-of-protection-of-covid-19-vaccines-against-clinical-disease-9-september-2021
- Katikireddi SV, Cerqueira-Silva T, Vasileiou E, et al. Two-dose ChAdOx1 nCoV-19 vaccine protection against COVID-19 hospital admissions and deaths over time: a retrospective, population-based cohort study in Scotland and Brazil. Lancet. 2022;399(10319):25–35.
- Andrews N, Stowe J, Kirsebom F, et al. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med. 2022;28(4):831–837.
- Tao K, Tzou PL, Nouhin J, et al. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat Rev Genet. 2021;22(12):757–773.
- Public Health England. SARS-CoV-2 variants of concern and variants under investigation in England. London (UK): Public Health England; 2021. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/984274/Variants_of_Concern_VOC_Technical_Briefing_10_England.pdf
- Sheikh A, McMenamin J, Taylor B, et al. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461–2462.
- Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(7):585–594.
- Pouwels KB, Pritchard E, Matthews PC, et al. Impact of Delta on viral burden and vaccine effectiveness against new SARS-CoV-2 infections [pre-print]. medRxiv 2021. DOI:10.1101/2021.08.18.21262237
- JCVI. JCVI statement regarding a COVID-19 booster vaccine programme for winter 2021 to 2022 [Internet]; London (UK): UK Government; 2021 [cited 2022 April 21]. Available from: https://www.gov.uk/government/publications/jcvi-statement-september-2021-covid-19-booster-vaccine-programme-for-winter-2021-to-2022/jcvi-statement-regarding-a-covid-19-booster-vaccine-programme-for-winter-2021-to-2022
- JCVI. Update to JCVI advice on booster vaccination in adults, 2021 [Internet]. London (UK): UK Government. Available from: https://www.gov.uk/government/publications/covid-19-booster-vaccine-programme-for-winter-2021-to-2022-jcvi-statement-november-2021/update-to-jcvi-advice-on-booster-vaccination-in-adults-15-november-2021
- JCVI advice on COVID-19 booster vaccines for those aged 18 to 39 and a second dose for ages 12 to 15 [Internet]; 2021 Nov 29. Available from: https://www.gov.uk/government/news/jcvi-advice-on-covid-19-booster-vaccines-for-those-aged-18-to-39-and-a-second-dose-for-ages-12-to-15
- UK Government. Estimated COVID-19 administrative vaccine uptake of UK population aged 18 and over. London (UK): UK Government; 2022 Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1048360/vaccine-uptake-18-and-over-19-January-2022.ods
- UK Government. Coronavirus vaccine - weekly summary of Yellow Card reporting [Internet]; 2021. London (UK): UK Government. Available from: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting#yellow-card-reports
- Office for National Statistics (UK). Population estimates for the UK, England and Wales, Scotland and Northern Ireland: mid2020 [Internet]; 2021. London (UK): UK Government. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/mid2020
- Bjørnstad ON, Shea K, Krzywinski M, et al. The SEIRS model for infectious disease dynamics. Nat Methods. 2020;17(6):557–558.
- Public Health England. National flu and COVID-19 surveillance reports: 2021 to 2022 season; 2021. Available from: https://www.gov.uk/government/statistics/national-flu-and-covid-19-surveillance-reports-2021-to-2022-season
- UK Health Security Agency. Antibody testing for SARS-CoV-2. Extended information for medical professionals and researchers on using and interpreting SARS-CoV-2 antibody tests; 2022. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1066325/Extended-guidance-antibody-testing.pdf
- Pouwels KB, Pritchard E, Matthews PC, et al. Impact of Delta on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK [pre-print]; 2021.
- Andrews N, Tessier E, Stowe J, et al. Vaccine effectiveness and duration of protection of Comirnaty, Vaxzevria and Spikevax against mild and severe COVID-19 in the UK [pre-print]; medRxiv 2021. DOI:10.1101/2021.09.15.21263583
- Andrews N, Stowe J, Kirsebom F, et al. Effectiveness of BNT162b2 (Comirnaty, Pfizer-BioNTech) COVID-19 booster vaccine against COVID-19 related symptoms and hospitalisation in England: test negative case-control study [pre-print]; medRxiv 2022. DOI:10.1101/2021.11.15.21266341
- Public Health England. PHE weekly national influenza and COVID-19 report. 16 September 2021 – week 37 report (up to week 36 data). London (UK): UK Government; 2021. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1018185/Weekly_Influenza_and_COVID19_report_data_w37.xlsx
- UK Health Security Agency. COVID-19 vaccine surveillance report. Week 50. London (UK): UK Government; 2021. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1041593/Vaccine-surveillance-report-week-50.pdf
- Institute for Government Analysis. Timeline of UK Government coronavirus lockdowns and measures, March 2020 to December 2021? London (UK): Institute for Government; 2022. Available from: https://www.instituteforgovernment.org.uk/sites/default/files/timeline-coronavirus-lockdown-december-2021.pdf
- Centre for the Mathematical Modelling of Infectious Diseases (CMMID) COVID-19 Working Group, CMMID: Social contacts in the UK from the CoMix social contact survey. London (UK): UK Government; 2020.
- Jarvis CG A, Wong K, Edmunds J. Social contacts in the UK from the CoMix social contact survey. Report for survey week 89. London (UK): UK Government; 2021. Available from: https://cmmid.github.io/topics/covid19/reports/comix/Comix%20Weekly%20Report%2089.pdf
- Davies NG, Klepac P, Liu Y, et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26(8):1205–1211.
- Moore S, Hill EM, Tildesley MJ, et al. Vaccination and non-pharmaceutical interventions for COVID-19: a mathematical modelling study. Lancet Infect Dis. 2021;21(6):793–802.
- Walker JL, Grint DJ, Strongman H, et al. UK prevalence of underlying conditions which increase the risk of severe COVID-19 disease: a point prevalence study using electronic health records. BMC Public Health. 2021;21(1):484.
- Sapey E, Gallier S, Mainey C, Nightingale P, et al. Ethnicity and risk of death in patients hospitalised for COVID-19 infection in the UK: an observational cohort study in an urban catchment area. BMJ Open Resp Res. 2020;7:e000644.
- Mason KM, McHale P, Pennington A, et al. Age-adjusted associations between comorbidity and outcomes of COVID-19: a review of the evidence from the early stages of the pandemic. Front Public Health. 2021;9:584182.
- Castanares-Zapatero D, Kohn L, Dauvrin M, et al. Long COVID: Pathophysiology - epidemiology and patient needs – Synthesis. Health Services Research (HSR) Brussels: Belgian Health Care Knowledge Centre (KCE). 2021. Available from: https://www.kce.fgov.be/sites/default/files/2021-11/KCE_344C_Long_Covid_Short_report.pdf
- Sayampanathan AA, Heng CS, Pin PH, et al. Infectivity of asymptomatic versus symptomatic COVID-19. Lancet. 2021;397(10269):93–94.
- Sandmann FG, Davies NG, Vassall A, et al. The potential health and economic value of SARS-CoV-2 vaccination alongside physical distancing in the UK: a transmission model-based future scenario analysis and economic evaluation. Lancet Infect Dis. 2021;21(7):962–974.
- Jentsch PC, Anand M, Bauch CT. Prioritising COVID-19 vaccination in changing social and epidemiological landscapes: a mathematical modelling study. Lancet Infect Dis. 2021;21(8):1097–1106.
- Giordano G, Colaneri M, Di Filippo A, et al. Modeling vaccination rollouts, SARS-CoV-2 variants and the requirement for non-pharmaceutical interventions in Italy. Nat Med. 2021;27(6):993–998.
- Li YG, Zhou Y, Cao X, et al. Toward the impact of non-pharmaceutical interventions and vaccination on the COVID-19 pandemic with time-dependent SEIR model. Front Artif Intell. 2021;4:648579.
- Hogan AB, Winskill P, Watson OJ, et al. Within-country age-based prioritisation, global allocation, and public health impact of a vaccine against SARS-CoV-2: a mathematical modelling analysis. Vaccine. 2021;39(22):2995–3006.
- ICNARC. ICNARC report on COVID-19 in critical care: England, Wales and Northern Ireland. London (UK): ICNARC; 2021.
- Vekaria B, Overton C, Wiśniowski A, et al. Hospital length of stay for COVID-19 patients: Data-driven methods for forward planning. BMC Infect Dis. 2021;21:700.
- Drummond. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford, UK: Oxford University Press; 2005.
- Office for National Statistics (UK). A05 SA: Employment, unemployment and economic inactivity by age group (seasonally adjusted). In: Watson B, editor. London (UK): UK Government; 2022. Available from: https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/employmentandemployeetypes/datasets/employmentunemploymentandeconomicinactivitybyagegroupseasonallyadjusteda05sa
- Office for National Statistics (UK). Leisure time in the UK: 2015. London (UK): Office of National Statistics; 2017. Available from: https://www.ons.gov.uk/releases/leisuretimeintheuk2015
- Lyngse FP, Mortensen LH, Denwood MJ, et al. SARS-CoV-2 omicron VOC transmission in Danish households [pre-print]; 2021.
- Public Health England. SARS-CoV-2 variants of concern and variants under investigation in England. London (UK): UK Government; 2021. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/984274/Variants_of_Concern_VOC_Technical_Briefing_10_England.pdf
- UK Health Security Agency. COVID-19 vaccine weekly surveillance reports (weeks 39 to 19, 2021 to 2022); 2022 [updated May 12, 2022]. Available from: https://www.gov.uk/government/publications/covid-19-vaccine-weekly-surveillance-reports
- Barnard RC, Davies NG; Centre for Mathematical Modelling of Infectious Diseases COVID-19 working group, Jit M, Edmunds WJ. Behaviour, booster vaccines and waning immunity: modelling the medium-term dynamics of SARS-CoV-2 transmission in England in the Omicron era [pre-print]. medRxiv. 2022;2021.11.22.21266584.
- Bosetti P, Kiem T, Andronico C, et al. Impact of booster vaccination on the control of COVID-19 Delta wave in the context of waning immunity: application to France in the winter 2021/22. Euro Surveill. 2022;27(1):2101125.
- Gavish N, Yaari R, Huppert A, et al. Population-level implications of the Israeli booster campaign to curtail COVID-19 resurgence. Sci Transl Med. 2022;14(647):eabn9836.
- Li R, Liu H, Fairley CK, et al. Cost-effectiveness analysis of BNT162b2 COVID-19 booster vaccination in the United States. Int J Infect Dis. 2021;119:87–94.
- National Institute for Health and Care Excellence. COVID-19 rapid guideline: managing the long term effects of COVID-19. London (UK): National Institute for Health and Care Excellence; 2022. Available from: https://www.nice.org.uk/guidance/ng188/resources/covid19-rapid-guideline-managing-the-longterm-effects-of-covid19-pdf-51035515742
- Office for National Statistics (UK). Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK. London (UK): Office for National Statistics; 2021. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/2december2021
- UK Health Security Agency. SARS-CoV-2 variants of concern and variants under investigation in England. London (UK): UK Health Security Agency; 2021. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1043807/technical-briefing-33.pdf
- Boosters prevented over 105,000 hospitalisations, UKHSA analysis estimates [Internet]; 2022 Feb 10. Available from: https://www.gov.uk/government/news/boosters-prevented-over-105-000-hospitalisations-ukhsa-analysis-estimates#:∼:text=Vaccinations%20for%20coronavirus-,Boosters%20prevented%20over%20105%2C000%20hospitalisations%2C%20UKHSA%20analysis%20estimates,19)%20since%20mid%2DDecember
- McCabe R, Schmit N, Christen P, et al. Adapting hospital capacity to meet changing demands during the COVID-19 pandemic. BMC Med. 2020;18(1):329.
- Rocks S, Idriss O. Did hospital capacity affect mortality during the pandemic’s first wave?: the Health Foundation; 2020 [cited 2022 May 27]. Available from: https://www.health.org.uk/news-and-comment/charts-and-infographics/did-hospital-capacity-affect-mortality-during-the-pandemic
- Parker EPK, Desai S, Marti M, et al. Response to additional COVID-19 vaccine doses in people who are immunocompromised: a rapid review. Lancet Glob Health. 2022;10(3):e326–e328.
- UK Health Security Agency. COVID-19 vaccine surveillance report. Week 19. London (UK): UK Health Security Agency; 2022. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1075115/COVID-19_vaccine_surveillance_report_12_May_2022_week_19.pdf
- Hayawi K, Shahriar S, Serhani MA, et al. Vaccine versus variants (3Vs): are the COVID-19 vaccines effective against the variants? A systematic review. Vaccines. 2021;9(11):1305.
- Public Health England, PHE Weekly National Influenza and COVID-19 Report. 16 September 2021 – Week 37 report (up to week 36 data). 2021. London (UK): Public Health England. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1016273/Weekly_Influenza_and_COVID19_report_data_W36.xlsx
- Wise J. Covid-19: booster doses to be offered to 30 million people in UK. BMJ. 2021;374:n2261.
- Wise J. Covid-19: UK will offer third vaccine dose to severely immunosuppressed people. BMJ. 2021;374:n2160.
- National Health Service, Who is at high risk from coronavirus (COVID-19) [Internet]; 2022 [cited 2022 May 26]. Available from: https://www.nhs.uk/conditions/coronavirus-covid-19/people-at-higher-risk/who-is-at-high-risk-from-coronavirus/
- Iacobucci G. Covid-19: how is the UK’s vaccine booster programme faring? BMJ. 2021;375:n2702.
- National Health Service. Hospital admitted patient care activity 2019-20. NHS Digital. 2020. London (UK): National Health Service. Available from: https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity/2019-20/summary-reports---acc---days
- Office for National Statistics (UK). Coronavirus (COVID-19) infection survey, characteristics of people testing positive for COVID-19, UK; 2022 May 11 [updated 2022 May 11; 2022 May 25]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19infectionsurveycharacteristicsofpeopletestingpositiveforcovid19uk/11may2022#symptoms-profile-of-strong-positive-cases-uk
- Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232.
- UK Government. UK coronavirus dashboard; 2022 [cited 2022 July 27]. Available from: https://coronavirus.data.gov.uk/
