Abstract
Background and objective
A cost-minimization model was developed to compare recombinant factor VIII Fc (rFVIIIFc) and emicizumab as prophylaxis for hemophilia A without inhibitors.
Methods
The model was based on 100 patients from the healthcare payer perspective in the UK, France, Italy, Spain, and Germany (5-year time horizon). Costs included: drug acquisition; emicizumab wastage by bodyweight (manufacturer’s dosing recommendations); and additional FVIII for breakthrough bleeds. Scenario analyses (UK only): reduced emicizumab dosing frequency; and emicizumab maximum wastage.
Results
Total incremental 5-year savings for rFVIIIFc rather than emicizumab use range from €89,320,131 to €149,990,408 in adolescents/adults (≥12 years) and €173,417,486 to €253,240,465 in children (<12 years). Emicizumab wastage accounts for 6% of its total cost in adolescents/adults and 26% in children. Reducing the emicizumab dosing frequency reduces the incremental cost savings with rFVIIIFc, but these remain substantial (adolescents/adults, >€92 million; children >€32 million). Maximum emicizumab wastage increases by 86% and 106%, respectively, increasing the incremental cost savings with rFVIIIFc to €125,352,125 and €105,872,727, respectively.
Conclusion
Based on cost-minimization modeling, rFVIIIFc use for hemophilia A prophylaxis in patients without inhibitors is associated with substantial cost savings in Europe, reflecting not only higher acquisition costs of emicizumab, but also other costs including wastage related to available vial sizes.
Introduction
Congenital hemophilia A is a bleeding disorder caused by deficiency of factor VIII (FVIII)Citation1. Worldwide, hemophilia occurs in 1 in 5,000 live male birthsCitation2, but its prevalence varies geographically, being highest in high-income countriesCitation3. In Europe, approximately 400 boys with hemophilia are born each yearCitation4. The condition is associated with spontaneous and traumatic bleeding episodes in joints and muscles, which can lead to synovitis, chronic hemophilic arthropathy, and pseudotumorsCitation5. These, in turn, cause pain, reduced functioning, and impaired health-related quality of lifeCitation6–8. It has also been shown that people with hemophilia have an increased mortality risk versus the general populationCitation9,Citation10. For example, in an analysis of data collected in Sweden between 1969 and 2008, the hazard ratio for all-cause mortality for patients with hemophilia was 2.2 (p <0.001)Citation10.
Prophylaxis with FVIII replacement therapy is the standard of care for patients to prevent bleeds in patients with hemophilia ACitation11. These include a recombinant factor VIII Fc fusion protein (rFVIIIFc; efmoroctocog alfa), which consists of a single molecule of rFVIII fused to the Fc domain of immunoglobulin G1Citation12. In Europe, it is the only extended half-life rFVIII product approved for the treatment and prophylaxis of bleeding in patients with hemophilia A of all ages (other products are approved for patients aged >12 years only)Citation13. In phase 3 and long-term extension studies in children, adolescents, and adults with severe hemophilia A, a low annualized bleed rate (ABR) was maintained for up to 4 years with extended-interval prophylactic dosingCitation14–16. In a recently conducted matching-adjusted indirect comparison of individualized prophylaxis with rFVIIIFc or prophylaxis with pegylated rFVIII (BAY 94-9027) according to their approved dosing regimens, rFVIIIFc provided a statistically significant lower ABRCitation17. Furthermore, in a cost–utility analysis evaluating life-long prophylaxis with rFVIIIFc versus rFVIII products in patients with severe hemophilia A in Sweden, rFVIIIFc was cost-effective, generating greater quality of life and reduced costsCitation18. Similar results were obtained in the cost-effectiveness analysis of lifelong prophylaxis with rFVIIIFc or rFVIII products from an Italian healthcare perspectiveCitation19.
Emicizumab is a bispecific monoclonal antibody, which mimics the function of activated FVIII by bridging activated factor IX and factor X to induce coagulation at the site of bleedingCitation20,Citation21. In Europe, it is approved for routine prophylaxis of bleeding episodes in patients with hemophilia A with FVIII inhibitors and in patients with severe hemophilia A without inhibitorsCitation22. The recommended dose is 3 mg/kg subcutaneously once weekly (Q1W) for the first 4 weeks (loading dose), followed by maintenance doses of 1.5 mg/kg Q1W, 3 mg/kg every 2 weeks (Q2W), or 6 mg/kg every 4 weeks (Q4W)Citation22.
To date, there are no direct comparative studies of rFVIIIFc and emicizumab and a lack of data on their relative economic impact. In a matching-adjusted indirect comparison using data from phase 3 trials, individualized prophylaxis with rFVIIIFc was more efficacious than emicizumab administered Q4W for the proportion of patients with zero bleeds, whilst its efficacy in terms of mean ABR was similar to that of emicizumab administered Q1W, Q2W, and Q4W, with trends in favor of rFVIIIFcCitation23. There are several factors that differentiate rFVIIIFc and emicizumab and impact on the direct costs of treatment, including possible emicizumab wastage as a result of vial size availability relative to the weight-based dosing scheduleCitation24 and the need for additional FVIII to treat bleeds (or prior to physical exercise) in emicizumab-treated patientsCitation25,Citation26. Cost-minimization modeling provides a simple analysis with few assumptions for drugs with equivalent/similar efficacy; therefore, the present study used this approach to evaluate the economic impact of rFVIIIFcCitation27. One of the assumptions underlying cost-minimization analyses is that the products being evaluated have equivalent efficacy and safety, which has been shown previously in the matching-adjusted indirect comparisonCitation23.
The aim of the current analysis was, therefore, to use a cost-minimization model to compare rFVIIIFc with emicizumab when used as prophylactic treatment for patients with hemophilia A without inhibitors in Europe.
Materials and methods
Targeted literature review
A targeted literature review was conducted to identify resources and costs associated with rFVIIIFc and emicizumab treatment; the focus was to identify publications on resource use, hospitalization (including length of stay), and healthcare costs associated with the treatment of people with hemophilia A. A search was performed using PubMed on 5 December 2019 (see Supplementary Table S1 for the search strategy). The websites of hemophilia associations and the grey literature (e.g. reports on burden associated with hemophilia A) were also evaluated (Supplementary Table S2).
Overall, 610 abstracts were screened and 591 were excluded, mainly because they were non-clinical or related to studies with populations, outcomes, or study designs that were not relevant. The full text of the remaining 19 publications was evaluatedCitation7,Citation18,Citation19,Citation28–43. These included literature reviews (n = 3), cost-utility or cost-effectiveness analyses (n = 8), burden of disease reports (n = 6), and budget impact analyses (n = 2), most of which concerned the USA and Italy, although Portugal, India, Sweden, Thailand, Colombia, Mexico, Iran and Europe were also covered. A small number of publications considered costs according to age (child vs adult) or hemophilia severity (mild, moderate, severe). Four of the 19 publications concerned rFVIIIFc (n = 3) or emicizumab (n = 1; Supplementary Table S3). The most common direct costs considered were those associated with prophylactic treatment, treatment of bleeding episodes, hospitalization, and medical visits.
Cost-minimization model
The model was based on a population of 100 people with hemophilia A without inhibitors who were treated with prophylaxis. Children were defined as those aged ≤12 years, and adults and adolescents as those aged >12 years. The time horizon of the model was 5 years (with Year 1 set as 2020) and the analysis was from a healthcare payer perspective in the UK, France, Italy, Spain, and Germany.
The cost of prophylaxis treatment with rFVIIIFc or emicizumab was based on each product’s list price. The rFVIIIFc doses used in the model were 85.4 IU/kg/week for adolescents and adults and 88.11 IU/kg/week for children (based on data from the phase 3 A-LONG [NCT01181128]Citation14 and Kids A-LONG [NCT01458106]Citation16 studies). For emicizumab, a loading dose of 3 mg/kg per week was administered for 4 weeks (as recommended in the Summary of Product CharacteristicsCitation22) followed by maintenance treatment with 1.5 mg/kg per week (based on that used in the HAVEN 3 study [NCT02847637]Citation25) The bodyweights of adolescents/adults and children were based on data from the A-LONGCitation14 and Kids A-LONG studiesCitation16, respectively.
Other costs included in the model were those associated with emicizumab wastage according to bodyweight, based on the manufacturer’s recommendations on the most appropriate number of injections using the various available vial strengthsCitation24; cost of additional FVIII for treatment of bleeds (additional rFVIIIFc in those treated with rFVIIIFc, and octocog alfa for those treated with emicizumab); and cost of wasted on-demand FVIII due to expired shelf life (both products). For the emicizumab group, it was assumed that patients would have a single dose of octocog alfa available at home to treat breakthrough bleeds and it would be wasted if not used within 2 years (the shelf life)Citation44. Bleeding rates were based on the ABR for rFVIIIFc and emicizumab, plus a proportion of patients without bleeds for emicizumab. These model inputs were selected based on parameters commonly considered in the literature and factors that differentiate rFVIIIFc and emicizumab. Further information on the data sources used to quantify these inputs are summarized in . The manufacturer’s recommendations regarding the appropriate number of injections are designed to minimize emicizumab wastage (see Supplementary Figure S1).
Table 1. Model and scenario analyses inputs.
Assumptions of the model were: the population size was constant in consecutive years of the analysis; rFVIIIFc and emicizumab have the same market share; there was no switching between treatments or discontinuation of prophylactic treatment; and all emicizumab-treated patients received the loading dose at the start of therapy.
Scenario analyses
Scenario analyses were conducted to evaluate the effect of the following on incremental costs (from a UK perspective): reduced dosing frequency for emicizumab (3 mg Q2W and 6 mg Q4W); emicizumab wastage according to patients’ bodyweight evaluated based on potential maximum wastage rather than the manufacturer’s recommendations (which are designed to minimize wastage; see Supplementary Figure S1); maximal emicizumab wastage according to bodyweight (set at 81 kg for adolescents/adults and 41 kg for children); up-titration of emicizumab dose (8% of those treated, based on data from the HAVEN 3 studyCitation25).
Results
Base-case analysis
In adolescents and adults, total 5-year costs across the five countries ranged from €89,320,131 to €149,990,408 for rFVIIIFc and €173,417,486 to €253,240,465 for emicizumab; most of the costs were accounted for by the cost of administered drug (97% for rFVIIIFc and 93% for emicizumab; ). Total incremental 5-year savings associated with using rFVIIIFc rather than emicizumab ranged from €84,097,355 to €103,250,057.
Figure 1. Five-year costs associated with using rFVIIIFc and emicizumab for prophylactic treatment of 100 people with hemophilia A (base-case analysis: recommended wastage; emicizumab 1.5 mg/kg once weekly). (a) Adolescents/adults (≥12 years). (b) Children (<12 years). Abbreviation: rFVIIIFc, recombinant factor VIII Fc.
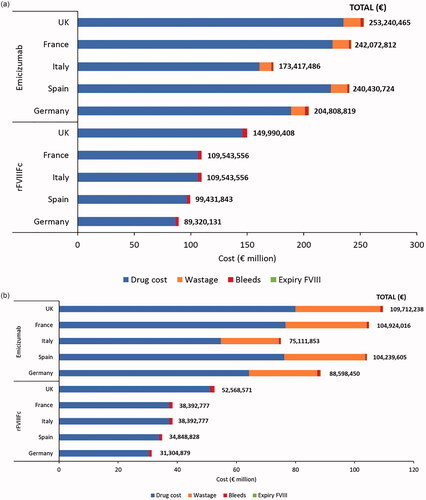
In children, total 5-year costs across the five countries ranged from €31,304,879 to €52,568,571 for rFVIIIFc and €75,111,853 to €109,712,238 for emicizumab (); as in adults and adolescents, most of the costs were accounted for by the drug acquisition costs (97% for rFVIIIFc and 73% for emicizumab), but in emicizumab-treated patients, the contribution of wastage was higher in children (26%) than in adolescents and adults (6%). Total incremental 5-year savings associated with using rFVIIIFc rather than emicizumab ranged from €43,806,974 to €57,143,667.
Scenario analyses
Total and incremental costs for the scenario analyses are summarized in . Reducing the dosing frequency of emicizumab reduced its costs (), but the incremental cost savings associated with using rFVIIIFc remained. In adolescents/adults, cost savings with rFVIIIFc were €94,168,828 versus emicizumab Q2W and €92,520,460 versus emicizumab Q4W; corresponding savings in children were €38,832,812 and €32,481,446, respectively.
Figure 2. Scenario analyses (UK): 5-year costs associated with using rFVIIIFc and emicizumab for prophylactic treatment of 100 people with hemophilia A, according to (a) Reduced dosing frequency for emicizumab, (b) Emicizumab potential maximum wastage, and (c) Maximal emicizumab wastage according to bodyweight. (a) Base-case dosing interval for emicizumab (1.5 mg every week) included for comparative purposes. (b) Elocta dose set at 88.11 IU/kg/week. Abbreviations: QW, once weekly; Q2W, every 2 weeks; Q4W, every 4 weeks; rFVIIIFc, Recombinant factor VIII Fc.
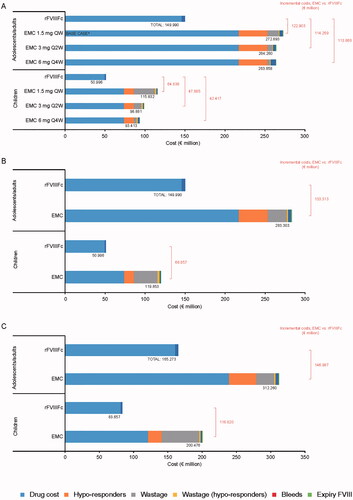
When emicizumab wastage was based on potential maximum wastage rather than the manufacturer’s recommendations (which are designed to minimize wastage), incremental cost savings with rFVIIIFc were greater than in the base-case analysis (€125,352,125 for adolescents/adults and €105,872,727 for children) (). Wastage costs increased by 86% (adolescents/adults) and 106% (children) compared with the base-case analysis.
At maximal emicizumab wastage (bodyweight set at 81 kg for adolescents/adults and 41 kg for children), incremental cost savings with rFVIIIFc were €121,145,014 and €62,665,311, respectively ().
Discussion
To our knowledge, this the first study to compare the economic impact of rFVIIIFc with emicizumab when used as prophylactic treatment for hemophilia A without inhibitors in Europe. Results of the cost-minimization analysis demonstrate that based on emicizumab and rFVIIIFc list prices in the UK, France, Italy, Spain, and Germany, prophylactic treatment of hemophilia A is 69% to 94% more expensive with emicizumab than rFVIIIFc in adolescents and adults and 109% to 140% more expensive in children. Cost savings for 100 adolescents/adults treated with rFVIIIFc instead of emicizumab for 5 years range from €84,097,355 to €103,250,057; respective cost savings for 100 children range from €43,806,974 to €57,143,667. Clearly, the magnitude of the cost savings has important implications for healthcare resource allocation and budgets in general, particularly when one considers the lifelong nature of hemophilia A.
Most of the cost savings associated with rFVIIIFc reflect the difference in acquisition costs between the two products, but drug wastage also has an impact, particularly in children. At maximum wastage (when adolescents/adults bodyweight is set at 81 kg and children’s at 41 kg), cost savings for rFVIIIFc versus emicizumab increase from €103,250,057 to €121,145,014 and €57,143,667 to €62,665,311, respectively (UK data). Reducing the dosing frequency of emicizumab reduces the incremental costs, but the cost savings associated with using rFVIIIFc remain substantial (in excess of €92 million in adults and adolescents, and €32 million in children). Conversely, as expected, using a more convenient combination of vial sizes (rather than the combination associated with least wastage) to achieve the appropriate emicizumab dose is associated with greater costs savings for rFVIIIFc. The extent to which clinicians use the most convenient approach to dosing is not clear, but in view of the impact of wastage on costs, particularly in children, it should be avoided. It is acknowledged that up-titration of the emicizumab dose, as permitted in the HAVEN 3 studyCitation25, is not licensedCitation22. Excluding this cost from the model reduces the cost differential between rFVIIIFc and emicizumab, but the excess cost of the latter remains substantial.
The current study used cost-minimization modeling to evaluate the economic impact of rFVIIIFc, as it provides a simple analysis with few assumptions for drugs with equivalent/similar efficacyCitation27. Previous studies have used budget impactCitation38, cost-utilityCitation18, and cost-effectiveness analysesCitation19. The budget impact model evaluated the economic impact of introducing rFVIIIFc to the market in Italy over a 3-year time horizon. The results showed a potential cost saving of more than €13.3 million compared with the “market mix” at the time (commonly used recombinant or plasma-derived FVIII) for treating hemophilia ACitation38. The cost-utilityCitation18 and cost-effectivenessCitation19 studies evaluated life-long prophylaxis with rFVIIIFc versus rFVIII products for severe hemophilia A (from Swedish and Italian healthcare perspectives, respectively) and both showed rFVIIIFc to dominate rFVIII (i.e. it provided greater quality-adjusted life-years at a lower cost). The authors of the Italian study concluded that the cost-effectiveness of rFVIIIFc reflected the modeled lower frequency of bleeding, improved joint health, and reduced factor consumptionCitation19. Analyses of the economic impact of emicizumab have also been conducted, but these focus on its cost-effectiveness versus bypassing agents in patients with hemophilia A and inhibitorsCitation45–47 or the cost impact of delayed inhibitor development versus FVIII prophylaxis in patients with hemophilia ACitation39. The cost-effectiveness analyses showed that emicizumab dominated bypassing agents, and delayed inhibitor development with emicizumab was associated with cost savings versus FVIII products.
One of the assumptions underlying cost-minimization analyses is that the products being evaluated have equivalent efficacy and safety. Although there are no direct comparative trials of rFVIIIFc and emicizumab, rFVIIIFc has been shown to be at least as effective as emicizumab, as measured by ABR, in a matching-adjusted indirect comparisonCitation23, a validated method for comparing treatments in the absence of head-to-head studiesCitation48. However, it is acknowledged that the indirect comparison did not evaluate long-term outcomes, such as joint health. Other potential limitations of the current model include the generalizability of the results outside the five European countries evaluated, and the source of data on ABRs and additional factor required to treat bleeds and patients whose emicizumab dose requires up-titration; these came from clinical trials rather than clinical practice so data from the real-world setting are required to substantiate these cost drivers. Another limitation is that acquisition costs for rFVIIIFc and emicizumab in this analysis were based on the list price for each country, but, in practice, costs can vary according to national tenders or even regional agreements. In some countries or regions, there is an agreement between the manufacturer and the regulatory authorities ensuring the annual cost for emicizumab prophylaxis is equivalent to the average annual cost of prophylaxis with FVIII concentrates. As such, while there would still be a cost differential between emicizumab and rFVIIIFc, it is much less than that demonstrated by the model. Finally, it is worth noting that an additional factor may be required before physical activity in some emicizumab-treated patients, as shown in the HAVEN 4 trialCitation26, but this was not included in the model.
In conclusion, when making the decision to use rFVIIIFc or emicizumab for prophylaxis in people with hemophilia A without inhibitors, payers should consider both the acquisition and other costs associated with their use. Emicizumab has a much higher acquisition cost; this and other factors, such as wastage related to the vial sizes for emicizumab and dose up-titration in some patients, mean that there are substantial cost savings associated with using rFVIIIFc. Given that rFVIIIFc appears to be at least as effective as emicizumab, the additional cost of the latter may not be justified as payers need to consider good value for money more closely and the opportunity for potential cost savings. Over the 5-year period evaluated in the current cost-minimization model, these cost savings amount to €840,974 to €1,032,501 per patient for adolescents/adults and €438,070 to €571,437 per patient for children. Therefore, each year, an additional 300 children could be treated considering a twice weekly regimen with 1,000 IU/infusion rFVIIIFc, and an additional 100 adolescents/adults with a twice weekly regimen of 3,000 IU/infusion. Scaling these costs up to the population level has significant cost implications for healthcare systems in Europe. As well as cost differences, the practicalities of using emicizumab in terms of wastage, particularly in children, are an important consideration. These results may help inform resource allocation decisions to improve overall health outcomes of individuals with hemophilia A without inhibitors in Europe.
Transparency
Declaration of funding
The study was funded by Swedish Orphan Biovitrum AB.
Declaration of financial/other interests
MEM has acted as a paid consultant/speaker/advisor for Bayer Healthcare, Biomarin, CSL Behring, Catalyst Bioscience, Grifols, Kedrion, LFB, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, Spark Therapeutic, Sobi, Takeda, and UniQure.
GC has acted as a paid consultant/speaker/advisor for Alexion, Bayer Healthcare, Biomarin, CSL Behring, Grifols, Kedrion, LFB, Novo Nordisk, Roche, Sanofi, Takeda, Werfen, and UniQure.
MP, SA, and AD are employees of Creativ-Ceutical, a consultancy company that received funding from Swedish Orphan Biovitrum for this research.
ZH and JN are employees of Swedish Orphan Biovitrum AB.
FF reports grant/research support from Swedish Orphan Biovitrum AB.
Author contributions
All authors were involved in the conception and design of the study. MP, SA, and AD were involved in analyzing the data associated with the study. All authors were involved with drafting and revising the manuscript, and for the final approval of the manuscript. All authors agree to be accountable for all aspects of the work.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Supplemental Material
Download MS Word (61.3 KB)Acknowledgements
Medical writing support was provided by Nicky French PhD of Bioscript Group, Macclesfield, UK and was funded by Swedish Orphan Biovitrum AB.
Data availability statement
The data that support the findings of this study are available from the corresponding author [GC], upon reasonable request.
References
- Gilbert GE. The evolving understanding of factor VIII binding sites and implications for the treatment of hemophilia A. Blood Rev. 2019;33:1–5.
- National Hemophilia Foundation. Fast facts. 2020. [cited 2020 May]. Available from: https://www.hemophilia.org/About-Us/Fast-Facts.
- Berntorp E. Future of haemophilia outcome assessment: registries are key to optimized treatment. J Intern Med. 2016;279(6):498–501.
- O'Hara J, Hughes D, Camp C, et al. The cost of severe haemophilia in Europe: the CHESS study. Orphanet J Rare Dis. 2017;12(1):106.
- World Federation of Hemophilia. Complications of hemophilia. 2012. [cited 2020 April]; Available from: http://www1.wfh.org/publications/files/pdf-1499.pdf.
- Buckner TW, Batt K, Quon D, et al. Assessments of pain, functional impairment, anxiety, and depression in US adults with hemophilia across patient-reported outcome instruments in the pain, functional impairment, and quality of life (P-FiQ) study. Eur J Haematol. 2018;100(Suppl 1):5–13.
- Cavazza M, Kodra Y, Armeni P, et al. Social/economic costs and quality of life in patients with haemophilia in Europe. Eur J Health Econ. 2016;17(Suppl 1):53–65.
- Poon J-L, Zhou Z-Y, Doctor JN, et al. Quality of life in haemophilia A: hemophilia utilization group study Va (HUGS-Va). Haemophilia. 2012;18(5):699–707.
- Darby SC, Kan SW, Spooner RJ, et al. Mortality rates, life expectancy, and causes of death in people with hemophilia A or B in the United Kingdom who were not infected with HIV. Blood. 2007;110(3):815–825.
- Lovdahl S, et al. Incidence, mortality rates and causes of deaths in haemophilia patients in Sweden. Haemophilia. 2013;19(3):362–369.
- Aledort L, Mannucci PM, Schramm W, et al. Factor VIII replacement is still the standard of care in haemophilia A. Blood Transfus. 2019;17(6):479–486.
- Efmoroctocog alfa for haemophilia A. Aust Prescr. 2018;41(6):200.
- European Medicines Agency. Elocta (rFVIIIFc) summary of product characteristics. 2019. [cited 2020 May]. Available from: https://www.ema.europa.eu/en/documents/product-information/elocta-epar-product-information_en.pdf.
- Mahlangu J, Powell JS, Ragni MV, et al. Phase 3 study of recombinant factor VIII Fc fusion protein in severe hemophilia A. Blood. 2014;123(3):317–325.
- Nolan B, Mahlangu J, Pabinger I, et al. Recombinant factor VIII Fc fusion protein for the treatment of severe haemophilia A: final results from the ASPIRE extension study. Haemophilia. 2020;26(3):494–502.
- Young G, Mahlangu J, Kulkarni R, et al. Recombinant factor VIII Fc fusion protein for the prevention and treatment of bleeding in children with severe hemophilia A. J Thromb Haemost. 2015;13(6):967–977.
- Hakimi Z, Santagostino E, Postma MJ, et al. Recombinant FVIIIFc versus Bay 94-9027 for treatment of patients with haemophilia A: comparative efficacy using a matching adjusted indirect comparison. Adv Ther. 2021;38(2):1263–1274.
- Henry N, Jovanović J, Schlueter M, et al. Cost-utility analysis of life-long prophylaxis with recombinant factor VIIIFc vs recombinant factor VIII for the management of severe hemophilia A in Sweden. J Med Econ. 2018;21(4):318–325.
- Bullement A, McMordie ST, Hatswell AJ, et al. Cost-effectiveness analysis of recombinant factor VIII Fc-fusion protein (rFVIIIFc) for the treatment of severe hemophilia A in Italy incorporating real-world dosing and joint health data. Pharmacoecon Open. 2020;4(1):133–142.
- Blair HA. Emicizumab: a review in haemophilia A. Drugs. 2019;79(15):1697–1707.
- Knight T, Callaghan MU. The role of emicizumab, a bispecific factor IXa- and factor X-directed antibody, for the prevention of bleeding episodes in patients with hemophilia A. Ther Adv Hematol. 2018;9(10):319–334.
- Roche Pharma AG. Hemlibra® summary of product characteristics. 2019. [cited 2020 March]. Available from: https://www.ema.europa.eu/en/documents/product-information/hemlibra-epar-product-information_en.pdf.
- Klamroth R, Wojciechowski P, Aballéa S, et al. Efficacy of rFVIIIFc versus emicizumab for the treatment of patients with hemophilia A without inhibitors: Matching-adjusted indirect comparison of A-LONG and HAVEN trials. J Blood Med. 2021;12:115–122.
- Genentech. Hemlibra dosing calculator. 2020. [cited 2020 March]. Available from: https://www.hemlibra.com/hcp/dosing-administration/dosing-calculator.html.
- Mahlangu J, Oldenburg J, Paz-Priel I, et al. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. 2018;379(9):811–822.
- Pipe SW, Shima M, Lehle M, et al. Efficacy, safety, and pharmacokinetics of emicizumab prophylaxis given every 4 weeks in people with haemophilia A (HAVEN 4): a multicentre, open-label, non-randomised phase 3 study. Lancet Haematol. 2019;6(6):e295–e305.
- KL R. Essentials of pharmacoeconomics. Chapter 4. Philadelphia (PA): Lippincott Williams & Wilkins; 2009.
- Buckley B, Dreyfus J, Prasad M, et al. Burden of illness and costs among paediatric haemophilia patients with and without central venous access devices treated in US hospitals. Haemophilia. 2018;24(3):e93–e102.
- Cafe A, Carvalho M, Crato M, et al. Haemophilia A: health and economic burden of a rare disease in Portugal. Orphanet J Rare Dis. 2019;14(1):211.
- Carlos-Rivera F, Gasca-Pineda R, Majluf-Cruz A, et al. Economic impact of hemophilia type A and B in Mexico. Gac Med Mex. 2016;152(1):19–29.
- Castro Jaramillo HE, Viscaya MM, Mejia AE. Cost-utility analysis of primary prophylaxis, compared with on-demand treatment, for patients with severe hemophilia type A in Colombia. Int J Technol Assess Health Care. 2016;32(5):337–347.
- Colombo GL, Di Matteo S, Mancuso ME, et al. Cost-utility analysis of prophylaxis versus treatment on demand in severe hemophilia A. Clinicoecon Outcomes Res. 2011;3:55–61.
- Coppola A, D'Ausilio A, Aiello A, et al. Cost-effectiveness analysis of late prophylaxis vs. on-demand treatment for severe haemophilia A in Italy. Haemophilia. 2017;23(3):422–429.
- Cortesi PA, D'Angiolella LS, Lafranconi A, et al. Modern treatments of haemophilia: review of cost-effectiveness analyses and future directions. Pharmacoeconomics. 2018;36(3):263–284.
- Gharibnaseri Z, Davari M, Cheraghali A, et al. Health care resource utilization and cost of care for haemophilia A and B patients in Iran. Transfus Apher Sci. 2016;54(1):122–126.
- Jadhav U, Mukherjee K. Assessment of healthcare measures, healthcare resource use, and cost of care among severe hemophilia A patients in Mumbai region of India. J Postgrad Med. 2018;64(3):138–144.
- Kodra Y, Cavazza M, Schieppati A, et al. The social burden and quality of life of patients with haemophilia in Italy. Blood Transfus. 2014;12(Suppl 3):s567–s575.
- Lorenzoni V, Triulzi I, Turchetti G. Budget impact analysis of the use of extended half-life recombinant factor VIII (efmoroctocog alfa) for the treatment of congenital haemophilia A: the Italian national health system perspective. BMC Health Serv Res. 2018;18(1):596.
- Patel AM, Corman SL, Chaplin S, et al. Economic impact model of delayed inhibitor development in patients with hemophilia A receiving emicizumab for the prevention of bleeding events. J Med Econ. 2019;22(12):1328–1337.
- Pattanaprateep O, Chuansumrit A, Kongsakon R. Cost-utility analysis of home-based care for treatment of Thai hemophilia A and B. Value Health Reg Issues. 2014;3:73–78.
- Polinski JM, Kowal MK, Gagnon M, et al. Home infusion: safe, clinically effective, patient preferred, and cost saving. Healthcare. 2017;5(1–2):68–80.
- Shrestha A, Eldar-Lissai A, Hou N, et al. Real-world resource use and costs of haemophilia A-related bleeding. Haemophilia. 2017;23(4):e267–e275.
- Thorat T, Neumann PJ, Chambers JD. Hemophilia burden of disease: a systematic review of the cost-utility literature for hemophilia. JMCP. 2018;24(7):632–642.
- Agency EM. Advate summary of product characteristics. 2018. [cited 2022 June]. Available from: https://www.ema.europa.eu/en/documents/product-information/advate-epar-product-information_en.pdf.
- Cortesi PA, Castaman G, Trifirò G, et al. Cost-effectiveness and budget impact of emicizumab prophylaxis in haemophilia A patients with inhibitors. Thromb Haemost. 2020;120(2):216–228.
- Lee H, Cho H, Han JW, et al. Cost-utility analysis of emicizumab prophylaxis in haemophilia A patients with factor VIII inhibitors in Korea. Haemophilia. 2021;27(1):e12–e21.
- Polack B, Trossaërt M, Cousin M, et al. Cost-effectiveness of emicizumab vs bypassing agents in the prevention of bleeding episodes in haemophilia A patients with anti-FVIII inhibitors in France. Haemophilia. 2021;27(1):e1–e11.
- Signorovitch JE, Sikirica V, Erder MH, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15(6):940–947.
