Abstract
Aims
A budget impact analysis (BIA) comparing bioprosthetic valves with RESILIA tissue and mechanical valves in aortic stenosis (AS) patients > 65 years in the public and private sectors of Saudi Arabia.
Materials and methods
A decision-tree with a partitioned survival model was adapted to estimate the financial consequences of either a RESILIA tissue valve or a mechanical valve in aortic valve replacement (AVR) procedures up to 5 years. The budget impact of resource consumption for both valve types was compared and included disabling strokes, reoperations, minor thromboembolic events, major bleeding, endocarditis, anticoagulation treatment and monitoring, and echocardiogram assessments. One-way sensitivity analyses (OWSA) were performed on cost and probability inputs.
Results
RESILIA tissue valves versus mechanical valves are overall budget saving commencing in Year 1 and savings gradually increase year-on-year. The higher costs of the initial procedure, reoperation, and additional monitoring (echocardiogram tests and visits) associated with RESILIA tissue valves are offset by savings in warfarin use, disabling strokes, major bleeding, and anticoagulation complications. The cost per initial procedure per patient is SAR795 higher for a RESILIA tissue valve reflecting the higher valve acquisition cost, which is partially offset by a shorter hospital stay. The OWSA suggests that total procedure costs of each valve, including the hospital stay, are the main cost drivers in the model.
Limitations
The variability of cost inputs and the presence of multiple payers with multiple costing data is a key challenge in Saudi Arabia. Budget impact results may, therefore, change if repeated per AVR center and may also be impacted by the long-term durability of RESILIA tissue valves.
Conclusions
An AVR in patients > 65 years with a RESILIA tissue valve is budget-saving from the first year in Saudi Arabia. Patients, payers, providers and policymakers may benefit economically from increased implantation of RESILIA tissue valves.
Introduction
The prevalence of aortic stenosis (AS) among patients > 60 years is 2–7% and is expected to continue increasing with an aging population and longer life expectancyCitation1–4. Globally, the population > 60 years will increase 300% between 2000 and 2025, peaking in Arab countries by 2050Citation5–7. In Saudi Arabia, demographic indicators also suggest steady increases in life expectancy, with the age group > 65 years expanding from approximately 1.2 million in 2025 to 10 million by 2050Citation8. As a result, the number of patients with AS is anticipated to increase, leading to higher mortality rates, lower quality-of-life, and additional healthcare costsCitation9–14.
Bioprosthetic and mechanical valves for aortic valve replacement (AVR) are frequently used for AS patients. Both valve types are acceptable options in AS patients > 65 years under guidelines by the European Society of Cardiology and American Heart Association/American College of Cardiology (AHA/ACC)Citation15–17. Novel bioprosthetic valves include RESILIAFootnotei tissue, made with bovine pericardium, which blocks residual aldehyde groups from binding to calcium, preventing calcification linked to structural valve deterioration (SVD) and improvements in hemodynamic performanceCitation18–20.
Two prospective, multicenter, single-arm, and observational studies evaluated at 2- and 5-years the long-term safety and hemodynamic performance of bioprosthetic valves with RESILIA tissueCitation18,Citation21. A long-term study compared bioprosthetic and mechanical valves and highlighted a differential impact on rates for reoperation, endocarditis, pacemaker implantation, mortality, stroke, and bleedingCitation22. Similarly, clinical practice guidelines and studies suggest that bioprosthetic valves require long-term echocardiogram monitoring, fewer in-hospital days, and avoid long-term anticoagulation associated with mechanical valvesCitation16–18,Citation23. RESILIA tissue valves are implanted in AS patients in Saudi Arabia at a higher acquisition cost compared to mechanical valvesCitation24. However, no studies are available on the budget impact comparing RESILIA tissue valves and mechanical valves, accounting for potential cost offsets associated with the outcomes in Saudi Arabia.
In Saudi Arabia, the emerging role of health technology assessment (HTA) and value assessment approaches in addition to the major shift to value-based health care driven by Saudi Arabia’s ambitious Vision 2030, have made budget impact analyses (BIA) a valuable tool to inform decision-makers on the affordability dimension of health technologies including medical devices. BIA is a core component of HTA dossier submissions in Saudi ArabiaCitation25,Citation26. Presently, there are no published studies on BIA to guide healthcare funders, hospitals (public or private), and decision-makers on the financial impact of AVRs with RESILIA tissue valves and mechanical valves in Saudi Arabia. To this end, we set out to analyze the budget impact of RESILIA tissue valves and mechanical valves among AS patients > 65 years in Saudi Arabia from the perspective of the public and private healthcare funders.
Methods
The financial impact of implanting a bioprosthetic valve with RESILIA tissue or a mechanical valve is calculated over a 5-year time horizon consistent with guidelines by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) for BIACitation27.
Model structure
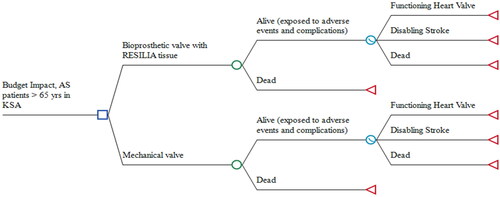
A decision-analytic model available in Microsoft ExcelCitation28 was adapted to compare RESILIA tissue valves with mechanical valves among AS patients who are > 65 years in Saudi Arabia. The model includes two components (): (i) a decision tree structure that captures outcomes related to both valve types during the initial 30 days after valve implantation, and (ii) a partitioned survival model that calculates the impact of long-term, annual outcomes over 5 years. The outcomes under the decision tree structure (: chance nodes) included disabling strokes, reoperations, minor thromboembolic events, major bleeding, endocarditis, and dead. The structure of the partitioned survival model (: survival curve node) included health states for a functioning heart valve, disabling stroke, and dead. Cycles of 1 month and probabilities per event determine how AS patients move between states over 5 years.
Patient population and clinical outcomes
All eligible AS patients entered the model at the time of AVR and were exposed to adverse events and complications during an initial 30-day period. Eligible patients were adults in Saudi Arabia > 65 years estimated to require first-time surgery for replacement of the aortic valveCitation29–31. All alive patients then entered the partitioned survival model in the functioning heart valve state until a disabling stroke or death. The starting age of patients was 65 years for both valve types (), consistent with published guidelinesCitation17.
Table 1. Model parameters – patient population and clinical outcomes (base case parameters).
Mortality
Patients were at risk of death during the initial 30-day period and for the duration of the model. Survival curves for mechanical valves were estimated using parametric survival analysis of reconstructed Kaplan-Meir curvesCitation15, and included an assumption that the mortality was no different for patients with a RESILIA tissue valveCitation35. Mortality rates in Saudi Arabia based on population life tables were used to adjust survival curves if transition probabilities dropped below the population averageCitation36.
The clinical outcomes covering adverse events and complications for both valve types were extracted from the COMMENCE trial and published evidenceCitation15,Citation22,Citation32,Citation33,Citation37,Citation38. The pragmatic literature search also targeted studies with generic tissue or generic mechanical valves (e.g. matched observational studies), and studies that reported results by age group for specific tissue valves. Clinical outcomes were extracted, summarized, and integrated in the decision-analytical model.
Thromboembolic events and disabling stroke
Patients implanted with either valve type are at risk of thromboembolic events or disabling stroke. Transition probabilities were estimated using parametric survival analysis derived from published clinical evidence among AS patientsCitation39. The large observational propensity score matched study reported a hazard ratio () favouring the generic bioprosthetic valve over the mechanical valve among patients > 65 years. We assumed a fixed proportion of thromboembolic events resulted in disabling stroke based on AS patients experiencing permanent neurological deficitsCitation32.
Reoperation
Both valve types are associated with reoperation resulting in additional healthcare resource consumption. We analyzed reoperation rates obtained from published evidenceCitation22,Citation32,Citation33,Citation37,Citation38,Citation40 and modeled their 5-year budget impact. Reoperation rates were higher among RESILIA tissue valves during the initial 30-day period and thereafter for all patients ().
Major bleeding
Major bleeding events require rehospitalization and may lead to strokeCitation15,Citation22, contributing to the overall budget impact of both valve types. We integrated estimates from published evidenceCitation15 where major bleeding events were defined as acute hemorrhagic events of the gastrointestinal, cerebral, cardiac, reproductive, and respiratory vasculature. Such events may require surgical exploration and a longer hospital length of stay. Major bleeding rates were lower among RESILIA tissue valves during the initial 30-day period and thereafter for all patients ().
Endocarditis
Infective endocarditis events are associated with lengthy antibiotic treatment, and high mortality rates, and may require reoperationCitation32,Citation34,Citation38,Citation41,Citation42. We compared endocarditis rates during the initial 30-day period and for the duration of the model. Both valve types are associated with the same event rate () during the initial 30-day periodCitation32,Citation38 and an estimated annual rate of 0.3–1.2%Citation34. A midpoint of the annual rate was applied for the duration of the model ().
Anticoagulation complications
AS patients implanted with a mechanical valve require lifetime anticoagulation, increasing the risk of anticoagulation complications, including major bleeding and thromboembolic eventsCitation15,Citation17,Citation21,Citation23,Citation33. We analyzed the impact of estimates from published evidenceCitation23 to account for patients requiring re-admission to treat subtherapeutic International Normalized Ratio (INR), INR checks, and anticoagulation dosage adjustments. Only mechanical valves were associated with event rates for anticoagulation complications and were estimated at 6 weeks post-procedure for readmission and additional monitoring (). The anticoagulation complication at 6 weeks was a one-time cost, and the long-term modeling of major bleeding is related to anticoagulation treatment and not to the valves itself.
Pacemaker implantation
Data are limited on new permanent pacemaker implantation among AS patients with either valve type. Data available on file suggest that 5.3% of patients receive a pacemaker applicable to both RESILIA tissue valves and mechanical valvesCitation33. We excluded the impact of new permanent pacemaker implantation given that event rates and costs are the same for both valve types and are, therefore, budget neutral.
Costs and resource consumption
Direct medical costs are modeled from both government and private sector institutions in 2022 Saudi Riyal (SAR). Costs for both valve types were categorized into in-hospital phases covering pre-procedural, periprocedural, and postprocedural resource consumption. In the outpatient setting, long-term costs associated with complications were included, such as major bleeding, endocarditis, minor thromboembolic event, and disabling stroke. Similarly, in the outpatient setting, recurring resource consumption linked to continuous monitoring of the implanted heart valve was also included. Costs during the in-hospital stay were calculated per procedure, while costs in the outpatient setting were calculated per event or per day and may include an in-hospital stay for major bleeding, endocarditis, or major stroke. The remaining cost elements associated with recurring resource consumption were calculated per annum.
In-hospital procedure costs
A patient’s in-hospital stay was organized into pre-procedural, periprocedural, and postprocedural phases covering the costs of consultants, laboratory tests, bioprosthetic valve with RESILIA tissue, mechanical valve, procedure duration, human resource utilization, length of stay, and drug costs (). Cost inputs (in 2022 SAR) representing billable costs per input were sourced from five institutions where AVR procedures are undertaken in Saudi Arabia. Inputs were tabulated, compared, and outliers were removed using Tukey fences with upper and lower limits set at 1.5-times the interquartile rangeCitation43,Citation44. This approach excluded potential cost input errors or a significantly higher/lower cost estimate due to a single institution. The mean of the remaining institutional cost inputs was calculated, included in the base case analysis, and applied to both RESILIA tissue and mechanical valves, other than the cost of each valve type and dental clearance.
Table 2. Model parameters – costs in SAR (base case parameters).
Dental clearance is required of all AS patients before an AVR. Dental staff evaluate the health of the oral cavity (teeth, mouth, and gums) to determine if an underlying infection (e.g., cavities or periodontal disease) may increase the risk of surgical site infection or post-procedure complications. A treatment plan is initiated depending on the severity of the issues. AS patients scheduled for a mechanical valve implantation require more dental staff visits and are associated with an additional length of stay. Approximately 20% of all AVR involve a dental evaluation after admission, based on Expert Opinion, suggesting a longer length of stay among mechanical valve patients. A weighted average of 0.4 additional days was included in the mechanical valve length of stay input as a one-time cost ().
The human resource utilization per procedure was based on an average duration of surgery of 150 min, based on Expert Opinion, and comparable to published evidence of 146 minCitation45. It covered the anesthesiologist, perfusionist, cardiac surgeon, surgical assistant, operating department practitioner, and nursing staff required for AVR. We assumed that human resource utilization was the same between procedures with a RESILIA tissue valve or a mechanical valve. The heart valve costs, provided by Edwards Lifesciences, are the acquisition cost of INSPIRIS RESILIA (SAR19,688) and the Masters Series Mechanical Valve (SAR6,200) by government institutions in Saudi Arabia.
Outpatient complication costs
The model accounts for long-term complication costs incurred after a patient’s discharge. It is challenging in Saudi Arabia to determine the budget impact of RESILIA tissue valves versus mechanical valves, given the presence of a multiple payer system with different data on the cost of healthcare interventions. We used published evidence available on the cost per event for disabling strokeCitation46. Where no evidence was available, we identified the main cost elements (consultations, duration of surgery, length of stay, medication, and laboratory) and estimated the total resource use per event for major bleeding and endocarditis. We also estimated the cost of a thromboembolic event based on the United Kingdom and Saudi Arabia purchasing power parity (PPP) adjustments to cost inputsCitation47.
AS patients with a RESILIA tissue valve require an annual echocardiogram after implantation consistent with Expert Opinion in Saudi Arabia and ESC/EACTS GuidelinesCitation48. Both RESILIA tissue valves and mechanical valves were associated with anticoagulation treatment, but only 3 months postprocedural among the former and for the duration of the budget impact model among the latterCitation49. The cost of the lowest priced warfarin sodium 5 mg (pack of 28 s – SAR26.20)Citation50 and general practitioner visits to monitor INR levels were included. We compared the final costs per event to published evidence on related cardiovascular interventions in Saudi ArabiaCitation51–55 as an additional check on their internal consistency and face validity. Cost inputs remained undiscounted as recommended by ISPOR Guidelines for BIACitation27, and were reported in 2022 SAR (3.75 SAR per United States Dollar (USD))Citation56.
Sensitivity analysis
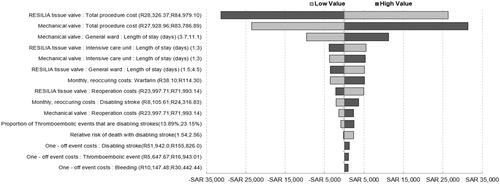
One-way sensitivity analyses (OWSA) of cost estimates with 50% lower and upper values were applied given the observed variability among the five institutions where AVR procedures are undertaken. Inputs for the length of stay were also adjusted with broad 50% lower and upper values to account for patient-level variability. All other inputs, including complication event rates, were varied within 25% of the base case. OWSA results were organized in a tornado diagram to enable comparison across all input categories.
Results
Overall budget impact results are presented in and include an incremental budget impact and disaggregated results for the cost of the initial procedure. We reported results for the initial 30-day period and annually thereafter consistent with guidelines by ISPOR for BIA. The guidelines recommend reporting results aligned with the budgeting period of the budget holderCitation27. Most budget holders in Saudi Arabia work with annual budgets.
Table 3. Budget impact results (in 2022 SAR) by year.
RESILIA tissue valves versus mechanical valves are overall budget saving commencing in Year 1 and savings gradually increase year-on-year as more AS patients are implanted with RESILIA tissue valves. The higher costs of the initial procedure, reoperation, and additional monitoring (echocardiogram tests and visits) associated with RESILIA tissue valves are offset by savings in all other cost categories, notably savings in warfarin use applicable to mechanical valves and reduced disabling strokes, major bleeding, and anticoagulation complications. Additional monitoring costs increase with expanded implantation of RESILIA tissue valves and, similarly, warfarin costs decrease as mechanical valves are displaced. The cost per initial procedure per patient is SAR795 higher for RESILIA tissue valves versus mechanical valves. This reflects the higher periprocedural costs (i.e. valve acquisition cost) for RESILIA tissue valves, which is partially offset by lower postprocedural costs (i.e. shorter hospital length of stay). The absolute cost per initial procedure per patient remained constant for the duration of the model.
Sensitivity analysis
Results of the OWSA comparing the impact of costs and probabilities on overall budget impact results () suggests that the total procedure costs of both valve types, including the hospital length of stay, are the main cost drivers in the model. Lower total procedure costs for RESILIA tissue valves and higher total procedure costs for mechanical valves result in greater budget savings. Higher monthly recurring costs for anticoagulation treatment results in greater budget savings in favor of RESILIA tissue valves.
Discussion
An AVR in patients > 65 years with RESILIA tissue valves is budget-saving from the first year, despite the higher acquisition cost in Saudi Arabia. Most long-term savings are realized in reduced warfarin use, disabling strokes, major bleeding, and anticoagulation complications. There is little published evidence to compare the above results. Given the novelty of this research, we highlight the only available international study and focus on the implications of our findings from the perspective of the public and private sectors in Saudi Arabia.
A recent study modeled the difference in long-term costs related to tissue and mechanical valves in the United StatesCitation57. They estimated annual expenditures up to 25 years across different age groups, including a group of patients > 65 years. Tissue valves were budget saving (USD 16,008) relative to mechanical valves in the latter group, and the additional reoperation costs were offset by savings from anticoagulation treatment and monitoring. Important differences limit the generalizability of the US results. In contrast to our analysis, the US study included patient costs, applied discounting, and excluded disabling stroke. Despite all these differences, the US study reported cost savings from year 1 (USD1,269) gradually increasing to year 5 (USD6,764), thus corroborating our results.
AVRs between the public and private sectors in Saudi Arabia may differ in important ways. The reasons for such differences are beyond the scope of this study; however, we assume that these are related to clinical practices as exemplified in dental clearance per institution or the number of human resources and duration per procedure. Similarly, systemic differences between each sector may depend on the distribution of AVR centers, availability of skilled operators, and the efficiency of each AVR center. The differences probably explain the variability in cost inputs from the five institutions where AVR procedures are undertaken. Such differences were accommodated by adopting a national perspective comprising both the public and private sectors, and by varying cost inputs by 50% and probabilities by 25% in the sensitivity analysis.
Given the lack of evidence to compare these results, an examination of only cost inputs suggests our approach is comparable to other analysesCitation14,Citation46,Citation52–55. Carapinha et al.Citation46 reported an ICU cost per day of SAR5,400 based on Expert Opinion versus a SAR4,599 cost per day in this research based on inputs from five institutions. Similarly, Alharthy and KarakitsosCitation53 reported a cost of SAR1.46 per minute for intensive care nursing services, comparable to the SAR1.70 per minute for operating room nursing services applied in this research. There are also differences in cost inputs, such as the cost of the general ward per day of SAR1,417 reported by Al-Senani et al.Citation52, and the mean estimate of SAR2,863 from five AVR centers in this research. This highlights, again, the variability of cost inputs between institutions and the possibility that results will change if the budget impact analysis is performed per AVR center.
This is the first budget impact analysis of its kind, but also the first to integrate the financial consequences of dental clearance. Dental clearance is required of all AS patients prior to an AVR procedure, but uncertainty remains on the trends of dental work done in the in-patient or out-patient setting. If a treatment plan requires extensive work to restore the health of the oral cavity while a patient is in-hospital, then total costs increase due to the additional length of stay, especially for mechanical valve patients on anticoagulation treatment. We found a single AVR center where, due to internal protocol, dental work was done on an in-patient basis. The results of future research on dental clearance may change the financial consequences of AVR procedures with either valve type, particularly the costs associated with required dental work and additional length of stay.
Limitations
The variability of cost inputs and the presence of a multiple payer system with different costing data are key challenges in Saudi ArabiaCitation46. As noted above, the methodological step of excluding specific cost inputs, namely cost inputs outside the Tukey fences, may bias results. We considered this a reasonable approach given the alternative of: (i) using the lowest cost estimate from the five AVR centers, or (ii) using the highest cost estimate. The former approach may result in conservative estimates unrepresentative of variable clinical settings among the AVR centers. The latter approach may similarly bias results and inadvertently include cost input errors from a single institution. We calculated a mean of the remaining cost inputs after removing outliers. This is still an imperfect substitute for a micro-costing analysisCitation58 or time-dependent activity costingCitation59 of AVR procedures across public and private institutions.
The relevance of these budget impact results beyond 5 years depends on the durability of RESILIA tissue valves – the main concern in nonelderly patientsCitation22,Citation40,Citation48. Results of the COMMENCE trial are encouraging for the hypothesized durability of RESILIA tissue valvesCitation33, but longer follow-up periods are required. To this end, the RESILIENCE trial will evaluate the long-term durability of RESILIA tissue valves in patients < 65 years at 7, 9, and 11 years with the primary outcome of re-intervention or death related to structural valve deteriorationCitation60.
Despite employing broader upper and lower values in the sensitivity analysis, parameter uncertainty remains in some inputs because of limited data. For instance, dental clearance and length of stay estimates were based on expert opinion given that no published data are available on either variable. Four cardiac surgeons from different institutions where AVRs are performed (among the group of co-authors) were consulted to validate the final estimates for both variables. Similarly, our study focused on patients > 65 years; however, data from younger patient cohorts were used when event rates were unavailable. The clinical outcomes covering adverse events and complications were extracted mainly from the COMMENCE trial (mean age: 66.9 years). We also sourced event rates (reported in ) from Brennan et al.Citation22 (mean age: 74 years), Glaser et al.Citation15 (mean age: 59.9 years for mechanical valves), and Lopez-Marco et al.Citation23 (mean age: 54.6 years for mechanical valves). Very few inputs were obtained where the age profile favored mechanical valves (younger patients).
Despite the budget savings demonstrated in this analysis, the financial consequences from a payer’s perspective in Saudi Arabia may change if the structural deterioration of the bioprosthetic valve leads to reoperation in the sixth year, soon after the end of the 5-year budget impact analysis. This is increasingly important as patients become “younger” via improved life expectancy in Saudi Arabia and Arab countriesCitation1–7. However, budget savings reported here are likely to persist with the proven long-term durability of RESILIA tissue valves. Results should, therefore, be interpreted within the scope of these limitations and the final choice of AVR valve should be individualized and decision-making shared with the patientCitation16,Citation48.
Conclusion
An AVR in patients > 65 years with RESILIA tissue valves is budget-saving from the first year in Saudi Arabia. Resource efficient procedures for RESILIA tissue valves, or in-hospital post-operative complications for AVR with mechanical valves and longer lengths of stay, are likely to amplify the budget savings in favor of RESILIA tissue valves. Patients, payers, providers, and policymakers in Saudi Arabia may benefit economically from increased implantation of RESILIA tissue valves.
Transparency
Declaration of financial/other interests
JLC is a consultant for Edwards Lifesciences. HAA, UA, TA, and AA are supported by their employing institutions. BM is an employee at Edwards Lifesciences.
Author contributions
JLC and BM: Study concept and design.
JLC, HAA, UA, TA, AA, KA, and BM: Data collection and data interpretation.
JLC primarily wrote the manuscript along with PC, HAA, UA, TA, AA, KA, and BM.
JLC, HAA, UA, TA, AA, KA, and BM revised the manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Acknowledgements
None reported.
Additional information
Funding
Notes
i RESILIA is a registered trademark of Edwards Lifesciences Corporation, Irvine, CA, USA.
References
- Iung B, Vahanian A. Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol. 2011;8(3):162–172.
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. The Lancet. 2006;368(9540):1005–1011.
- De Sciscio P, Brubert J, De Sciscio M, et al. Quantifying the shift toward transcatheter aortic valve replacement in low-risk patients: a meta-analysis. Circ Cardiovasc Qual Outcomes. 2017;10:e003287.
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24(13):1231–1243.
- Khraif RM, Salam AA, Elsegaey I, et al. Changing age structures and ageing scenario of the arab world. Soc Indic Res. 2015;121(3):763–785.
- Karlin NJ, Weil J, Felmban W. Aging in Saudi Arabia. Gerontol Geriatr Med. 2016;2:2333721415623911.
- Gire J. Cultural variations in perceptions of aging. In: Cross-cultural psychology, contemporary themes and perspectives. Chichester (UK): Wiley-Blackwell; 2011. p. 110–130.
- United Nations Department of Economic and Social Affairs. Population by broad age groups [Internet]. World Population Prospects 2022 - Population Division. 2022. [cited 2022 Jul 12]. Available from: https://population.un.org/wpp/Graphs/DemographicProfiles/Line/682.
- Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245.
- Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics - 2010 update: a report from the American heart association. Circulation. 2010;121(7):948–954.
- Varadarajan P, Kapoor N, Bansal RC, et al. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg. 2006;82(6):2111–2115.
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):2440–2492.
- Coylewright M, Palmer R, O'Neill ES, et al. Patient-defined goals for the treatment of severe aortic stenosis: a qualitative analysis. Health Expect. 2016;19(5):1036–1043.
- Alatawi FO, Abuelatta RA, AlAhmedi AB, et al. Clinical outcomes with transcatheter aortic valve implantation at a single cardiac center in Saudi Arabia. Ann Saudi Med. 2018;38(3):167–173.
- Glaser N, Jackson V, Holzmann MJ, et al. Aortic valve replacement with mechanical vs. biological prostheses in patients aged 50–69 years. Eur Heart J. 2016;37(34):2658–2667.
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2021;143(5):e72–e227.
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561–632.
- Puskas JD, Bavaria JE, Svensson LG, et al. The COMMENCE trial: 2-year outcomes with an aortic bioprosthesis with RESILIA tissue. Eur J Cardiothorac Surg. 2017;52(3):432–439.
- Flameng W, Hermans H, Verbeken E, et al. A randomized assessment of an advanced tissue preservation technology in the juvenile sheep model. J Thorac Cardiovasc Surg. 2015;149(1):340–345.
- De La Fuente AB, Wright GA, Olin JM, et al. Advanced integrity preservation technology reduces bioprosthesis calcification while preserving performance and safety. J Heart Valve Dis. 2015;24:101–109.
- Bartus K, Litwinowicz R, Bilewska A, et al. Final 5-year outcomes following aortic valve replacement with a RESILIATM tissue bioprosthesis. Eur J Cardiothorac Surg. 2021;59(2):434–441.
- Brennan JM, Edwards FH, Zhao Y, et al. Long-term safety and effectiveness of mechanical versus biologic aortic valve prostheses in older patients: results from the Society of Thoracic Surgeons Adult Cardiac Surgery National Database. Circulation. 2013;127(16):1647–1655.
- Lopez-Marco A, Grant SW, Mohamed S, et al. Impact of Mechanical Aortic Prostheses in hospital stay and anticoagulation related complications. J Surg Res. 2021;04(02):187–196.
- Syenza Data on File. Aortic valve market research and price comparisons. Syenza; 2022.
- Al-Omar HA, Aljuffali IA, Solà-Morales O. Value drivers for pharmaceutical products in health technology assessment (HTA) in Saudi Arabia: results from a capacity building, Multi-Stakeholder workshop. Saudi Pharm J. 2021;29(9):946–954.
- Al-Omar HA, Attuwaijri AA, Aljuffali IA. Pharmaceutical companies’ views on a health technology assessment (HTA) entity in Saudi Arabia. Saudi Pharm J. 2020;28(6):662–668.
- Mauskopf JA, Sullivan SD, Annemans L, et al. Principles of good practice for budget impact analysis: report of the ISPOR Task Force on good research practices–budget impact analysis. Value Health. 2007;10(5):336–347.
- Microsoft Corporation. Microsoft Excel, Version 2208 [Internet]. 2022. Available from: https://office.microsoft.com/excel.
- General Authority for Statistics. Population estimates in the midyear of 2021 [Internet]. General Authority for Statistics; 2021. Available from: https://www.stats.gov.sa/en/43.
- Baghai M, Wendler O, Grant SW, et al. Aortic valve surgery in the UK, trends in activity and outcomes from a 15-year complete national series. Eur J Cardiothorac Surg. 2021;60(6):1353–1357.
- Andell P, Li X, Martinsson A, et al. Epidemiology of valvular heart disease in a Swedish nationwide hospital-based register study. Heart. 2017;103(21):1696–1703.
- Bourguignon T, Bouquiaux-Stablo A-L, Candolfi P, et al. Very long-term outcomes of the Carpentier-Edwards Perimount valve in aortic position. Ann Thorac Surg. 2015;99(3):831–837.
- Bavaria JE, Griffith B, Heimansohn DA, et al. Five-year outcomes of the COMMENCE trial investigating aortic valve replacement with RESILIA tissue. Ann Thorac Surg. 2022. DOI:10.1016/j.athoracsur.2021.12.058
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the european society of cardiology (ESC). endorsed by: European association for Cardio-Thoracic surgery (EACTS), the european association of nuclear medicine (EANM). Eur Heart J. 2015;36(44):3075–3128.
- Attia T, Yang Y, Svensson LG, et al. Similar long-term survival after isolated bioprosthetic versus mechanical aortic valve replacement: a propensity-matched analysis. J Thor Cardiovasc Surg. 2021. Available from: https://www.jtcvs.org/article/S0022-5223(21)00059-3/fulltext.
- World Health Organization. Global health observatory, life tables [Internet]. WHO. World Health Organization; [cited 2021 Sep 21]. Available from: https://apps.who.int/gho/data/view.main.61440?lang=en
- Zhao DF, Seco M, Wu JJ, et al. Mechanical versus bioprosthetic aortic valve replacement in middle-aged adults: a systematic review and meta-analysis. Ann Thorac Surg. 2016;102(1):315–327.
- Bourguignon T, Lhommet P, El Khoury R, et al. Very long-term outcomes of the Carpentier-Edwards Perimount aortic valve in patients aged 50–65 years. Eur J Cardiothorac Surg. 2016;49(5):1462–1468.
- Kytö V, Ahtela E, Sipilä J, et al. Mechanical versus biological valve prosthesis for surgical aortic valve replacement in patients with infective endocarditis. Interact Cardiovasc Thorac Surg. 2019;29(3):386–392.
- Etnel JRG, Huygens SA, Grashuis P, et al. Bioprosthetic aortic valve replacement in nonelderly adults: a systematic review, meta-analysis, microsimulation. Circ Cardiovasc Qual Outcomes. 2019;12:e005481.
- Tackling G, Lala V. Endocarditis antibiotic regimens. Treasure Island (FL): StatPearls Publishing; 2022. [cited 2022 Jun 24]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK542162/.
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: Diagnosis, antimicrobial therapy, and management of complications. Circulation. 2015;132(15):1435–1486.
- Tukey JW. Exploratory data analysis. Reading (MA): Addison-Wesley Pub. Co.; 1977. [cited 2022 Sep 12]. Available from: http://archive.org/details/exploratorydataa00tuke_0.
- InterQuartile Range (IQR) [Internet]. [cited 2022 Sep 12]. Available from: https://sphweb.bumc.bu.edu/otlt/MPH-Modules/BS/BS704_SummarizingData/BS704_SummarizingData7.html.
- Wilbring M, Tugtekin S-M, Alexiou K, et al. Transapical transcatheter aortic valve implantation vs conventional aortic valve replacement in high-risk patients with previous cardiac surgery: a propensity-score analysis. Eur J Cardiothorac Surg. 2013;44(1):42–47.
- Carapinha JL, Al-Omar HA, Alqoofi F, et al. Budget impact analysis of transcatheter aortic valve replacement in low, intermediate, and high-risk patients with severe aortic stenosis in Saudi Arabia. J Med Econ. 2022;25(1):77–86.
- International Comparison Program (ICP) [Internet]. World Bank. [cited 2022 Jul 14]. Available from: https://www.worldbank.org/en/programs/icp.
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease: Developed by the task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2022;43(7):561–632.
- Keeling D, Baglin T, Tait C, et al. Guidelines on oral anticoagulation with warfarin – fourth edition. Br J Haematol. 2011;154(3):311–324.
- Saudi Food and Drug Authority. Drugs List [Internet]. Saudi Food and Drug Authority; 2022. Available from: https://sfda.gov.sa/en/drugs-list.
- Hersi AS, Osenenko KM, Kherraf SA, et al. Cost-effectiveness of apixaban for stroke prevention in non-valvular atrial fibrillation in Saudi Arabia. Ann Saudi Med. 2019;39(4):265–278.
- Al-Senani F, Al-Johani M, Salawati M, et al. A national economic and clinical model for ischemic stroke care development in Saudi Arabia: a call for change. Int J Stroke. 2019;14(8):835–842.
- Alharthy A, Karakitsos D. King Saud Medical City Intensive Care Unit: a critical and cost-focused appraisal. Saudi Crit Care J. 2019;3(1):19–23.
- Altowaijri A, Alshehri N, Balkhi B, et al. PCV50 economic burden of major cardiovascular diseases and ischemic stroke in Saudi Arabia: a cost of illness study. Value Health. 2020;23:S495–S496.
- Johnston KM, Osenenko KM, Qatami L, et al. Health care resource utilization and costs in individuals with atrial fibrillation in United Arab Emirates and Kingdom of Saudi Arabia: a retrospective cohort study. Int J Int Med. 2015;4:17–25.
- 1 USD to SAR - US Dollars to Saudi Arabian Riyals Exchange Rate [Internet]. [cited 2022 Sep 12]. Available from: https://www.xe.com/currencyconverter/convert/?Amount=1&From=USD&To=SAR.
- Nguyen TC, Walker T, Gunnarsson C, et al. Long-term healthcare expenditures over time for tissue and mechanical aortic valve replacement. Ann Thorac Surg. 2021;112(2):526–531.
- Potter S, Davies C, Davies G, et al. The use of micro-costing in economic analyses of surgical interventions: a systematic review. Health Econ Rev. 2020;10(1):3.
- Bobade RA, Helmers RA, Jaeger TM, et al. Time-driven activity-based cost analysis for outpatient anticoagulation therapy: direct costs in a primary care setting with optimal performance. J Med Econ. 2019;22(5):471–477.
- Pibarot P, Borger MA, Clavel M-A, et al. Study design of the prospective non-randomized single-arm multicenter evaluation of the durability of aortic bioprosthetic valves with RESILIA tissue in subjects under 65 years old (RESILIENCE trial)*. Struct Heart. 2020;4(1):46–52.