Abstract
Objectives
Characterizing and evaluating the holistic value of innovative healthcare technologies (e.g. treatments, services) constitutes a crucial goal to maximize limited resources. However, the characteristics of innovation have not been well identified. This review aims to describe the characteristics of healthcare innovation.
Methods
We performed a comprehensive systematic search using PubMed, Embase, PsycINFO, and Econlit from inception to July 2022. Articles were included if they described innovation or the characteristics of innovation of the technologies in healthcare. Characteristics or definitions of innovation directly or indirectly described as innovation were extracted from the included articles. Two independent reviewers then conceptualized the identified characteristics of innovation to generate innovation attributes in healthcare.
Results
In total, 103 articles were included in this review. Eight attributes describing innovation, i.e. novelty, step change, substantial benefits, an improvement over existing technologies, convenience and/or adherence, added value, acceptable cost, and uncounted benefits, were conceptualized. Most of the identified innovation attributes were based on the researchers’ perspective.
Conclusions
This study conceptualized innovation attributes in healthcare based on the characteristics of healthcare innovation as defined in the literature. Further research is warranted to obtain a complete understanding of the perspectives of researchers and other stakeholders, including patients, healthcare providers, healthcare payers, and the pharmaceutical industry, on recognizing innovation in healthcare.
This is the first systematic review to conceptualize attributes of healthcare innovation.
We conceptualized eight attributes describing innovation, i.e. novelty, step change, substantial benefits, an improvement over existing technologies, convenience and/or adherence, added value, acceptable cost, and uncounted benefits based on the similar concept.
In existing literature, patients’ and caregivers’ perspectives were less frequently found to describe the innovation attributes.
Future research is needed to identify, measure, and value various stakeholders, including patients’ and caregivers’ perspectives on healthcare innovation.
KEY POINTS
1. Introduction
The term “innovation,” being ubiquitous in every industry, generally refers to the act or process of introducing something new ideas, devices, or methods or to the things themselves that are introduced [Citation1]. The WHO Health Innovation Group defines innovation as the development of “new or improved health policies, systems, products and technologies, and services and delivery methods that improve people’s health, with a special focus on the needs of vulnerable populations” [Citation2]. Healthcare innovation plays a critical role in maximizing limited available resources due to various key challenges, such as increasing aging populations and prevalence of chronic diseases, the advent of innovative health interventions, and higher demands for rapid access to innovation. Such problems urge innovative solutions in healthcare technology, its business models, and delivery to patients [Citation3]. To ensure equitable and prompt patient access to innovation to maximize health benefits, healthcare governing agencies have encountered unique issues in prioritizing interventions [Citation4,Citation5].
Policymakers are under ever-increasing pressure to obtain the highest value from their scarce resources. Policymakers have adopted health technology assessment (HTA) across countries to promote more equitable, efficient, and high-quality health care systems [Citation6]. Some countries like the UK, France, and the Netherlands have well-established national HTA agencies, and the quality-adjusted life-year (QALY) based economic evaluations are extensively used [Citation7]. In the absence of country-specific HTA, value assessment frameworks such as the American Society of Clinical Oncology (ASCO) framework and the National Comprehensive Cancer Network (NCCN) guidelines in the United States have emerged in the last few years to inform decision-makers when making critical judgments related to the reimbursement of healthcare services and treatments [Citation8]. However, it appeared to be a lack of an agreed-on overarching definition of innovation across HTA frameworks. A clear and comprehensive understanding of the characteristics of innovation would help prioritize healthcare resources and bring innovation to clinical practice [Citation9,Citation10]. Therefore, in this systematic review, we performed a comprehensive search for definitions of innovation in healthcare, medicine, or pharmacy to understand what characteristics, features, or definition of innovation were described in the literature and conceptualized them into innovation attributes.
2. Methods
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [Citation11] [see PRISMA checklist in the electronic supplementary material (ESM 1)], following a predetermined protocol (PROSPERO 2021 CRD42021233799).
2.1. Searching strategy
To capture peer-reviewed articles that discussed innovation, we performed a systematic search in four different bibliographic databases, including PubMed, EMBASE, PsycINFO, and EconLit. Our search strategies included MeSH terms and relevant keywords (ESM 2). Each of the databases was searched from inception to July 2022. Manual searches of the reference lists of eligible articles were performed to identify additional studies that were not retrieved through search strategies. Detailed search strategies are provided in the ESM 2. All retrieved references were managed with EndNote version 20 (Clarivate Analytics) and Microsoft Excel (Microsoft Corporation).
2.2. Study selection
Using the EndNote software, we combined all the search hits and removed duplicate references. Additional articles were excluded while manually screening titles and abstracts to finalize articles for full-text reading. Articles were included if they described innovation or the characteristics of innovation of the technologies in healthcare. Characteristics or definitions of innovation directly or indirectly described as innovation were extracted from the included articles. Those articles discussed community innovation or innovative ideas in healthcare, and innovations in education for health professionals were excluded from this review. Screening was independently performed by two reviewers (SS and NP), and any disagreements were referred to a third reviewer (NC) for resolution.
2.3. Data extraction and analysis
Features, characteristics, or definitions of innovation from different perspectives, including regulatory bodies, the public, or authors, were extracted from all included studies. We developed a customized data extraction table to extract the following information: the first author, year of publication, the original quotation from studies, innovation attributes, perspective, and type of study. We extracted innovation characteristics by categorizing them into two groups – explicit and implicit characteristics. The explicit characteristics were the characteristics of innovation that were clearly described in the articles. However, some innovation characteristics that were not directly claimed or described but inferred from the documents were grouped as implicit characteristics. Data extraction was independently performed by two reviewers (SS and NP), and any disagreements were referred to a third reviewer (NC) for resolution.
2.4. Qualitative synthesis
We summarized all innovation definitions or characteristics and conceptualized them to describe the innovation attributes. In the process of deriving attributes of innovation, similar concepts or terms were conceptualized as attributes of innovation. One article might have provided more than one innovation attribute in the conceptualization process. Since this study aimed to capture only the characteristics of healthcare innovation described without determining the internal validity of the findings, we decided not to perform a methodological quality assessment of studies included in this systematic review.
3. Results
3.1. Study selection
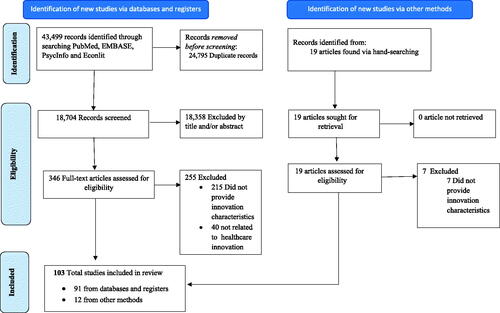
Searches in four bibliographic databases yielded 43,499 articles. After excluding 24,795 duplicate records, titles, and abstracts of 18,704 unique articles were screened. We identified 346 articles through the titles and abstracts screening process and obtained full texts. Following the full-text review, we excluded 255 articles in total that were not related to innovation in healthcare or did not provide innovation definitions or features. Of the 103 finally selected studies, 13 articles were identified through manual citation searching. The study selection process is depicted in .
3.2. Study characteristics
Of the included articles, most of them (70/103) were review articles, and seven were qualitative studies [Citation12–18], six reports [Citation5,Citation19–23], two mixed-method studies [Citation24,Citation25], and two editorials [Citation26,Citation27]. The rest of the articles were either cross-sectional surveys, perspective papers, short communication, dissertation paper, or cost-utility study. Most of the included articles were from Europe and North America, including the UK, Germany, Italy, Spain, The Netherland, the USA, and Canada. Different perspectives may have been offered in a single article. Out of 103 articles, 40 described innovations from the perspective of researchers, followed by 18 articles from payers, 14 articles from industry, and eight from regulatory agencies. The remaining articles were from providers, patients, the public, or societal perspectives.
3.3. Overall description of attributes of innovation
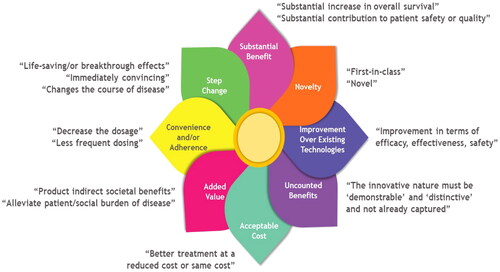
We extracted definitions, characteristics, or innovation features from 103 included articles in this review and utilized to conceptualize eight attributes of innovation (details in supplementary Table 1 in ESM 3). Innovation definitions or characteristics were described from the researcher’s perspective, regulatory bodies, HTA committees, expert opinions, physicians, patients, and the public. shows the innovation attributes, including novelty, step change, substantial benefits, improvements over existing technologies, convenience and/or adherence, added value, acceptable cost, uncounted benefits, and their detailed descriptions. It is noteworthy that these attributes could be overlapped. Therefore, shows an innovation flower in healthcare developed to depict each attribute overlapping at the flower’s receptacle. We also provided examples for each attribute in this figure. The newly approved drug, first-in-class therapy, was counted as a novelty. CAR-T (chimeric antigen receptor) cell therapies, for example, in oncology, are examples of novelty drugs where T cells are genetically engineered to fight tumor cells-a mechanism that has not been seen before [Citation63,Citation64]. Drugs that brought radical changes in the current standard medical practice or introduced breakthrough technology were considered a step change. Examples of step change can be found in cystic fibrosis management, where current gene therapies have enabled us to address the fundamental defect of cystic fibrosis, resulting in remarkable improvements in health outcomes [Citation65]. Substantial benefit is the attribute of innovation that might overlap with novelty and step change; in developing this attribute, we relied on a clear statement from the included articles regarding drugs’ substantial impacts on disease. Calcitonin gene-related peptide (CGRP) antagonist has demonstrated substantial benefit in preventive migraine treatment [Citation66]. An improvement over existing technologies was referred to as the new drug that did not necessarily provide novel or “ground-breaking” benefits but rather a modification and improvement over an existing drug. Newer generations of proton pump inhibitors offer several advantages over older agents, particularly in terms of the management of gastro-esophageal reflux disease but did not provide ground-breaking benefits are examples of improvement over existing technologies [Citation67]. Less dosage, administration frequency, and aid to enhance adherence to medical products were considered a convenience and/or adherence attribute of innovation. Bortezomib in the subcutaneous route, for example, has demonstrated equivalent efficacy with a significantly lower incidence of adverse events compared to the intravenous route, demonstrating the convenience and/or adherence attribute [Citation68]. Alleviating the social burden on a concerning disease, improving the population’s health, or reducing inequalities in healthcare would be added value to the healthcare system and society overall. For example, it was recognized that the COVID-19 vaccine generated a broader added value to society by not only safeguarding the vaccinated individuals but also avoiding the costs of treating patients in hospitals [Citation69]. Delivering better healthcare with cost-effective treatment was considered an acceptable cost attribute of innovation. Low-cost innovations, such as the use of mosquito net mesh to repair hernias, the use of sterilizable bags to replace the need for expensive-sterilizable surgical equipment, and the use of mobile-phone platforms to integrate decision-making support systems for community healthcare workers are a few examples of acceptable cost attribute of innovation [Citation70]. Finally, the demonstrable and distinctive nature of technology not already captured in the technology’s economic evaluation was referred to as uncounted benefits of innovation. The UK’s National Institute for Health and Care Excellence (NICE) awarded additional rewards for the uncounted benefits for the technologies that increase survival in in end-of-life situations [Citation4]. The ISPOR Special Task Force (STF) has identified other novel value elements to better recognize and incorporate uncounted benefits elements in healthcare decision-making [Citation71].
Table 1. Innovation attributes related to healthcare.
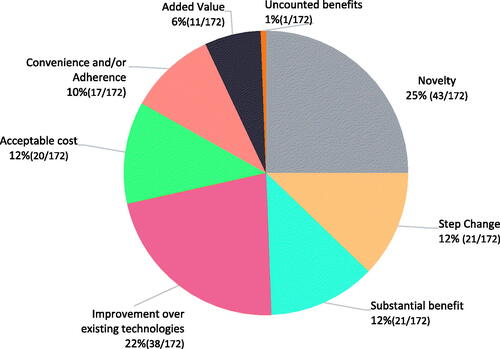
shows the proportion of the innovation attributes identified from the included articles for healthcare. One-fourth of the innovation attributes were a novelty, while approximately another one-fourth belonged to the improvement of existing technologies. Step change, substantial benefits, acceptable cost, and convenience and/or adherence were shared in approximately equal portions. Added value and uncounted benefits were the two least mentioned innovation attributes. Frequencies of innovation attributes based on direct or indirectly mentioned innovation definitions are illustrated in supplementary Figure 1 in ESM 3. In all these eight innovation attributes, at least half of them were derived from the explicit description of innovation.
4. Discussion
In this Systematic review, we focused on addressing the current knowledge gaps in the characteristics of healthcare innovation that currently exist in the literature. Our findings are generally consistent with other reviews that summarized the characteristics of healthcare innovation [Citation10,Citation72–75]. The uniqueness of this review was that we were able to conceptualize healthcare innovation into eight attributes, including novelty, step change, substantial benefits, an improvement over existing technologies, convenience and/or adherence, added value, acceptable cost, and uncounted benefits.
The novelty was the most frequently cited attribute of innovative technologies by analyzing the identified innovation characteristics. It was also the one that was most frequently and explicitly stated. It could be identified in different formats, notably first-in-class drugs [Citation17,Citation20,Citation28,Citation29] to fulfill the pressing unmet needs [Citation10,Citation31–34]. This result was intuitive because, among all identified attributes, novelty probably has the closest meaning to innovation. Similarly, the improvement over existing technologies, the second most frequently cited attribute, appeared intuitive because any innovation is anticipated to contain this attribute. However, whether any technology that improved over existing technologies was an innovation was more controversial since it was possible that the level of improvement might be marginal and/or span across one or more areas beyond efficacy and safety such as patient’s tolerability/quality of life (QoL), caregivers’ QoL, and reduction in health care resource utilization.
Among the other identified innovation attributes, the step change, substantial benefit, and acceptable cost appeared in a similar frequency. The step change was mentioned under several definitions, including an important innovative attribute that delivers breakthrough, lifesaving effects [Citation9,Citation22,Citation38,Citation40,Citation41] that can cure [Citation14], prevent [Citation14], or changes the course of disease states [Citation18]. The technology resulting in a substantial increase in overall survival [Citation43], or patient safety or quality [Citation16] was conceptualized as a substantial benefit attribute of innovation. The step change would be more certain of being an innovation attribute than a substantial benefit, which likely depended on a perspective. Health care technology that offers better outcomes with an acceptable cost would also be considered an innovation. Few studies expected that new technology would be available at a reduced or same cost as the previous generation technology with a better outcome [Citation17,Citation33,Citation59–61]. Convenience, which can lead to improved adherence, was conceptualized. Lesser frequency [Citation55,Citation56] or preferred route of administration [Citation30,Citation53] could be two major examples of this attribute.
The added value of innovation is referred to as technologies that alleviate patient/social burden in a concerning disease [Citation26]. This value could be added to the system in several ways, including increased productivity, knowledge spillovers, reduction in healthcare resource utilization, and release capacity in the system by an innovative delivery system that would allow a more decentralized health system to improve healthcare access and reduce health disparities [Citation18,Citation56,Citation62]. However, this attribute was mentioned less frequently. Finally, a 2019 article identified uncounted benefits as an innovation attribute, which was defined by the National Institute for Health And Care Excellence (NICE) as producing a demonstrable and distinct benefit that might not be adequately captured in the technology’s incremented cost-effective ratio (ICER) calculation [Citation4]. For instance, any technology that was beneficial to patients and their caregivers would contain uncounted benefits because, typically, ICER does not account for caregivers’ benefits. This innovation attribute might have been based on a relatively new value assessment concept [Citation71]. These uncounted benefits cannot be captured by a narrow cost-utility analysis (e.g. cost-per-QALY metric) but must be recognized and considered fully when assessing the value of healthcare innovation. Additional research is warranted to ascertain how the best uncounted benefits should be identified and incorporated into HTA decision-making. Multiple Criteria Decision Analysis (MCDA) has been long argued to be a potential approach, but there is a lack of evidence on how this method would perform in practice [Citation35].
It is noteworthy that an innovative healthcare technology does not need to have all attributes that were conceptualized in this work to be qualified as innovative. For example, a previous study identified to have at least one of the three conditions of a technology: novelty, substantial benefits, and demonstrable, uncounted benefits to qualify as innovative [Citation4]. Also, the innovation attributes specified in this review could overlap. For instance, technology could be the first-in-class and step change at the same time if it was the first treatment that also cures an uncured disease. Also, these overlapping attributes could vary across studies and technologies and depended on the perspectives. In other words, there was no consensus on the innovation attributes in healthcare. In this context, our findings were congruent with previous literature that also reported that the globally accepted definition of healthcare innovation was missing [Citation74].
While various perspectives were used to describe the innovation attributes in existing literature, the patients’ and caregivers’ perspectives were less frequently included. There could be several reasons. For instance, traditionally, the patients were excluded from the healthcare decision-making [Citation76]. The decision-makers tended to think they were the agents of the patients and might be skeptical about the capability of the patients to determine the innovativeness of healthcare technology. Recently patients’ active roles in healthcare are emerging and recognized as a patient-centered care approach [Citation77]. Patients and caregivers held meaningful experiences and perspectives that could substantially improve clinical problems, and ultimately patients could achieve their desired health outcomes [Citation77]. Therefore, it is critical to identify their perspectives on healthcare innovation and devise a way to integrate into the decision-making.
Our study suffered from two major limitations. First, we identified innovation characteristics mentioned either directly or indirectly in literature to generate innovation attributes. Those implicit definitions of innovation were left to subjective interpretation. Second, many included studies did not state from whose perspectives those attributes of technologies were innovative; in these scenarios, it was assumed as a researchers’ perspective. Third, articles were selected if innovation or innovative terms were mentioned in the abstract. It is possible some articles were potentially excluded as they did not mention innovation or innovation in the abstract but characteristics of innovation were discussed later in the full text.
Our review provided a better lens to visualize healthcare innovation. The identified innovation attributes would help various stakeholders in the healthcare system to recognize and value innovation better. Hopefully, the recognition and valuation of innovation would lessen the dissatisfaction of innovators, such as pharmaceutical industries with research and development objectives. For instance, while the ISPOR Special Task Force recently identified new elements of value in healthcare [Citation7], the innovation was not explicitly included. If healthcare technology innovation remains unrecognized or unrewarded, patients and society could subsequently be deprived of the full benefits of innovation.
5. Conclusions
This systematic literature review identified eight attributes of innovation that can provide policymakers with an additional tool for recognizing innovation in healthcare. Given the broad array of innovative attributes, it is likely that innovations are undervalued in the decision-making process. Additionally, future research is required to identify the patient’s and caregiver’s perspectives on innovation. Comprehensive knowledge on healthcare innovation will guide the decision-makers and priority-setting bodies to inform decision-making.
Transparency
Declaration of funding
This research was supported by Bristol Myers Squibb. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Declaration of financial/other relationships
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
SS and NP reviewed literatures, extracted data for the study, synthesis, and conceptualization with input from NC, SN, and SV. SS, NP, SN, SV, and SS wrote the first drafts of the manuscript and all authors made substantial contribution. All authors contributed to the study design, interpretation of findings, and critical revision of the manuscript.
Supplemental Material
Download MS Word (83.7 KB)Acknowledgements
Authors are grateful to Alexandre Hikiji Watanabe, Anindit Chibber, Viji Chandran, and Musaina Thasneem for assistance in the study selection process.
Data availability statement
All data are available from the corresponding author upon request.
References
- Aronson JK. Something new every day: defining innovation and innovativeness in drug therapy. J Ambul Care Manage. 2008;31(1):65–68.
- WHO Health Innovation Group. How does WHIG define health. Scotpublichealth. 2020. Available from: https://scotpublichealth.com/2020/04/26/health-innovationand-covid-19-pandemic-defining-the-need-and-understanding-theresponse/
- Herzlinger RE. Why innovation in health care is so hard. Harv Bus Rev. 2006;84(5):58–66, 156.
- Charlton V, Rid A. Innovation as a value in healthcare priority-setting: the UK experience. Soc Justice Res. 2019;32(2):208–238.
- Fortinguerra F, Tafuri G, Trotta F, et al. Using GRADE methodology to assess innovation of new medicinal products in Italy. Br J Clin Pharmacol. 2020;86(1):93–105.
- Assessment ENfHT. What is Health Technology Assessment (HTA). Available from: https://www.eunethta.eu/services/submission-guidelines/pharmaceutical-submission/#ptfaq1
- Torbica A, Tarricone R, Drummond M. Does the approach to economic evaluation in health care depend on culture, values, and institutional context? Eur J Health Econ. 2018;19:769–774.
- Westrich K. Current landscape: value assessment frameworks. Washington (DC): National Pharmaceutical Council; 2016.
- Ciani O, Armeni P, Boscolo PR, et al. De innovatione: the concept of innovation for medical technologies and its implications for healthcare policy-making. Health Policy Technol. 2016;5(1):47–64.
- de Solà-Morales O, Cunningham D, Flume M, et al. Defining innovation with respect to new medicines: a systematic review from a payer perspective. Int J Technol Assess Health Care. 2018;34(3):224–240.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Joseph ML, Huber DL, Bair H, et al. A typology of innovations in nursing. J Nurs Adm. 2019;49(7–8):389–395.
- Karampli E, Souliotis K, Polyzos N, et al. Why do physicians prescribe new antidiabetic drugs? A qualitative study in the Greek healthcare setting. Health Policy Technol. 2020;9(2):166–173.
- NICE Citizens Council. NICE citizens council reports. Health innovation and value. London: National Institute for Health and Care Excellence (NICE) Copyright © 2009 National Institute for Health and Clinical Excellence; 2009.
- Billaux M, Borget I, Prognon P, et al. Innovative medical devices and hospital decision making: a study comparing the views of hospital pharmacists and physicians. Aust Health Rev. 2016;40(3):257–261.
- Sheard L, Jackson C, Lawton R. How is success achieved by individuals innovating for patient safety and quality in the NHS? BMC Health Serv Res. 2017;17(1):1–9.
- Nicod E, Brigham KB, Durand-Zaleski I, et al. Dealing with uncertainty and accounting for social value judgments in assessments of orphan drugs: evidence from four European countries. Value Health. 2017;20(7):919–926.
- McGloughlin EK, Anglim P, Keogh I, et al. Innovation for the future of Irish MedTech industry: retrospective qualitative review of impact of BioInnovate Ireland’s clinical fellows. BMJ Innov. 2018;4(1):32–38.
- Florindi F, De Lorenzo F, Apostolidis K, et al. Value of innovation in oncology: the position of European cancer patients on access to innovative treatments. Am Soc Clin Oncol. 2017;35(15_suppl):e18021–e18021.
- Jonker CJ, van den Berg HM, Kwa MS, et al. Registries supporting new drug applications. Pharmacoepidemiol Drug Saf. 2017;26(12):1451–1457.
- Lakdawalla D, Malani A, Reif J. The insurance value of medical innovation. J Public Econ. 2017;145:94–102.
- Hamilton BH, Hincapié A, Miller RA, et al. Innovation and diffusion of medical treatment. Cambridge (MA): National Bureau of Economic Research; 2018.
- Mestre-Ferrandiz JM, Sussex J. The many faces of innovation. A report for the ABPI by the Office of Health Economics. London (UK): Association of the British Pharmaceutical Industry; 2012. Available from: https://www.ohe.org/system/files/private/publications/373%20-%20Many_Faces_of_Innovation_Mestre-Ferrandiz_2012.pdf?download=1
- Dhar M, Griffin M, Hollin I, et al. Innovation spaces: six strategies to inform health care. Health Care Manag (Frederick). 2012;31(2):166–177.
- Ferlie E, Nicolini D, Ledger J, et al. NHS top managers, knowledge exchange and leadership: the early development of academic health science networks–a mixed-methods study. Health Serv Deliv Res. 2017;5(17):1–204.
- Monia M. Value of innovation for hematologic malignancies. J Med Econ. 2016;19(5):487–489.
- Lanzafame RJ. Responsibilities and innovation. New Rochelle (NY): Mary Ann Liebert, Inc.; 2010.
- Miller KL, Lanthier M. Trends in orphan new molecular entities, 1983–2014: half were first in class, and rare cancers were the most frequent target. Health Aff (Millwood). 2016;35(3):464–470.
- Swinney DC, Lee JA. Recent advances in phenotypic drug discovery. F1000Res. 2020;9:944.
- Baum RA, Baum S. Interventional radiology: a half century of innovation. Radiology. 2014;273(2 Suppl):S75–S91.
- Gashaw I, Ellinghaus P, Sommer A, et al. What makes a good drug target? Drug Discov Today. 2011;16(23–24):1037–1043.
- Petykó ZI, Inotai A, Holtorf A-P, et al. Barriers and facilitators of exploiting the potential of value-added medicines. Expert Rev Pharmacoecon Outcomes Res. 2020;20(3):229–236.
- Roubou I, Alexopoulou DK. The various shapes of innovation. Forum Clin Oncol. 2015;6(4):12–22.
- Bryan S, Lee H, Mitton C. ‘Innovation’ in health care coverage decisions: all talk and no substance? J Health Serv Res Policy. 2013;18(1):57–60.
- Angelis A, Kanavos P. Multiple criteria decision analysis (MCDA) for evaluating new medicines in health technology assessment and beyond: the advance value framework. Soc Sci Med. 2017;188:137–156.
- Consoli D, Mina A. An evolutionary perspective on health innovation systems. J Evol Econ. 2009;19(2):297–319.
- Ellner AL, Stout S, Sullivan EE, et al. Health systems innovation at academic health centers: Leading in a new era of health care delivery. Acad Med. 2015;90(7):872–880.
- Malik NN. Controlling the cost of innovative cancer therapeutics. Nat Rev Clin Oncol. 2009;6(9):550–552.
- Hübner U. What are complex eHealth innovations and how do you measure them? Methods Inf Med. 2015;54(4):319–327.
- Quinn C, Palmer S, Bruns J, editors. Innovation in oncology: why focusing only on breakthrough innovation may be counter-productive. Haematologica. Pavia (Italy): Ferrata Storti Foundation; 2015.
- Pammolli F, Riccaboni M. Market structure and drug innovation. Health Aff (Millwood). 2004;23(1):48–50.
- Ferner R, Hughes DA, Aronson J. NICE and new: appraising innovation. BMJ. 2010;340(jan05 2):b5493–b5493.
- Quinn C, Palmer S, Bruns J, et al. Progressive innovation in oncology: valuing outcomes beyond survival. Value in Health. 2015;18(7):A482.
- Garrett MD, Workman P. Discovering novel chemotherapeutic drugs for the third millennium. Eur J Cancer. 1999;35(14):2010–2030.
- Conti RM. Economic value of new treatments for depression. PhD dissertation. Harvard University; 2006. Available from: https://scholar.google.com/scholar?cluster=6395544026005408420&hl=en&as_sdt=5,45
- Costa LS. Innovation in healthcare services: notes on the limits of field research. Cad Saude Publica. 2016;32:e00151915.
- Chagpar AB. Innovation in surgery: from imagination to implementation. Am J Surg. 2011;202(6):641–645.
- Biddiscombe MF, Usmani OS. Is there room for further innovation in inhaled therapy for airways disease? Breathe (Sheff). 2018;14(3):216–224.
- Chan TY, Hamilton BH, Papageorge NW. Health, risky behaviour and the value of medical innovation for infectious disease. Rev Econ Stud. 2016;83(4):1465–1510.
- Bhatti Y, Taylor A, Harris M, et al. Global lessons in frugal innovation to improve health care delivery in the United States. Health Aff (Millwood). 2017;36(11):1912–1919.
- Papageorge NW. Why medical innovation is valuable: Health, human capital, and the labor market. Quant Econ. 2016;7(3):671–725.
- Zaim R, Tran L, Groen H, et al. ‘DE NOVO’Quantification of genotype-directed therapy with afatinib in metastatic lung cancer. Value Health. 2014;17(7):A641.
- Djupesland PG, Messina JC, Mahmoud RA. Breath powered nasal delivery: a new route to rapid headache relief. Headache. 2013;53(S2):72–84.
- Rezvani M, Hesari J, Peighambardoust SH, et al. Development and characterization of nanostructured pharmacosomal mesophases: an innovative delivery system for bioactive peptides. Adv Pharm Bull. 2018;8(4):609–615.
- Graziottin A. Safety, efficacy and patient acceptability of the combined estrogen and progestin transdermal contraceptive patch: a review. Patient Prefer Adherence. 2008;2:357–367.
- Stephanie W, Hill C, Ricks ML, et al. The scope and impact of mobile health clinics in the United States: a literature review. Int J Equity Health. 2017;16(1):1–12.
- Balducci AG, Magosso E, Colombo G, et al. From tablets to pharmaceutical nanotechnologies: Innovation in drug delivery strategies for the administration of antimalarial drugs. J Drug Delivery Sci Technol. 2016;32:167–173.
- Moreira M, Sarraguça M. How can oral paediatric formulations be improved? A challenge for the XXI century. Int J Pharm. 2020;590:119905.
- Belloso WH. On innovation. Ther Innov Regul Sci. 2020;54(5):1068–1075.
- Henshall C, Schuller T. Health technology assessment, value-based decision making, and innovation. Int J Technol Assess Health Care. 2013;29(4):353–359.
- Kelly CJ, Young AJ. Promoting innovation in healthcare. Future Healthc J. 2017;4(2):121–125.
- Toskin I, Blondeel K, Peeling RW, et al. Advancing point of care diagnostics for the control and prevention of STIs: the way forward. Sex Transm Infect. 2017;93(S4):S81–S88.
- Hartmann J, Schüßler‐Lenz M, Bondanza A, et al. Clinical development of CAR T cells—challenges and opportunities in translating innovative treatment concepts. EMBO Mol Med. 2017;9(9):1183–1197.
- Park TS, Rosenberg SA, Morgan RA. Treating cancer with genetically engineered T cells. Trends Biotechnol. 2011;29(11):550–557.
- Bell SC, Mall MA, Gutierrez H, et al. The future of cystic fibrosis care: a global perspective. Lancet Respir Med. 2020;8(1):65–124.
- Martin V, Samaan KH, Aurora S, et al. Efficacy and safety of galcanezumab for the preventive treatment of migraine: a narrative review. Adv Ther. 2020;37(5):2034–2049.
- Robinson M. New-generation proton pump inhibitors: overcoming the limitations of early-generation agents. Eur J Gastroenterol Hepatol. 2001;13:S43–S47.
- Ye Z, Chen J, Xuan Z, et al. Subcutaneous bortezomib might be standard of care for patients with multiple myeloma: a systematic review and meta-analysis. Drug Des Devel Ther. 2019;13:1707–1716.
- Bell E, Neri M, Steuten L. Towards a broader assessment of value in vaccines: the BRAVE way forward. Appl Health Econ Health Policy. 2022;20(1):105–117.
- Harris M, Bhatti Y, Prime M, et al. Low-cost innovation in healthcare: what you find depends on where you look. J R Soc Med. 2018;111(2):47–50.
- Lakdawalla DN, Doshi JA, Garrison LP, Jr, et al. Defining elements of value in health care- a health economics approach: an ISPOR Special Task Force report [3]. Value Health. 2018;21(2):131–139.
- Hofmann S, Branner J, Misra A, et al. A review of current approaches to defining and valuing innovation in health technology assessment. Value Health. 2021;24(12):1773–1783.
- Angelis A, Lange A, Kanavos P. Using health technology assessment to assess the value of new medicines: results of a systematic review and expert consultation across eight European countries. Eur J Health Econ. 2018;19(1):123–152.
- Tutone M, Villa F, Addis A, et al. How do drug regulatory bodies deal with potential innovative therapies? Ther Innov Regul Sci. 2020;54(1):195–199.
- Rejon-Parrilla JC, Espin J, Epstein D. How innovation can be defined, evaluated and rewarded in health technology assessment. Health Econ Rev. 2022;12(1):1–11.
- Basch E, Aronson N, Berg A, et al. Methodological standards and patient-centeredness in comparative effectiveness research: the PCORI perspective. JAMA. 2012;307(15):1636–1640.
- Frank L, Basch E, Selby JV, et al. The PCORI perspective on patient-centered outcomes research. Jama. 2014;312(15):1513–1514.